Abstract
The class I histone deacetylases HDAC1 and HDAC2 belong to a family of 11 zinc-dependent human HDACs and are overexpressed in many cancers. Inhibitors of these HDACs now in clinical trials show activity against several types of cancers. This review is focuse on recent advances in both clinical and preclinical efforts to understand the basis for HDACi actions, with an emphasis on implications for rational combinations with conventional or other targeted agents. We will address new perspectives on the molecular mechanisms by which HDACs act and how these actions relate to cancer. We will also review new evidence demonstrating that HDACs are direct intracellular targets of the potent sphingolipid mediator sphingosine-1-phosphate (S1P), the first identified endogenous nuclear regulator of these enzymes, linking sphingolipid metabolism in the nucleus to remodeling of chromatin and epigenetic regulation of gene expression. Understanding how endogenous molecules regulate HDAC activity in vivo may facilitate the search for safer and more effective anti-cancer drugs capable of interfering with HDAC functions in a highly specific manner.
Keywords: histone deacetylase, histone deacetylase inhibitor, apoptosis, sphingosine-1-phosphate, cancer
1. Background on histone deacetylases
Acetylations and deacetylations of histones have emerged as a critical component of an epigenetic indexing system demarcating transcriptionally active chromatin domains. This dynamic balance is regulated by two important families of enzymes, histone acetyltransferases (HATs) and histone deacetylases (HDACs) (Clayton et al 2006). HATs catalyze acetylation of lysine residues, neutralizing positive charges, relaxing chromatin structure and increasing accessibility of the transcription machinery. HDACs remove acetyl groups from histones (and other nuclear proteins), thereby inducing chromatin condensation and transcriptional repression (Haberland et al 2009, Yang and Seto 2008) and have emerged as key targets to reverse aberrant epigenetic changes associated with cancer (Glozak and Seto 2007, Marks 2010, Minucci and Pelicci 2006).
Recent data provide evidence that both HATs and HDACs are targeted to transcribed regions of active genes and that the majority of HDACs in the human genome function to reset chromatin by removing acetylation of histones at active genes (Wang et al 2009). Moreover, inhibition of HDACs revealed that they have two major roles: First, to remove acetyl groups at active genes added by HATs during transcriptional initiation and elongation in order to maintain a sufficient level of acetylation to enable specific transcriptional elongation and prevent promiscuous initiation, and second, to remove acetyl groups added by transient binding of HATs at inactive gene promoters to maintain a reduced level of acetylation and to prevent Pol II from binding. This work also provided an example of how the order of implementation of histone modifications can affect transcription (Wang et al 2009). They showed that treatment of cells with HDAC inhibitors (HDACis) does not lead to productive transcription, despite the presence of H3K4 methylation and Pol II recruitment (Wang et al 2009). This, together with other data, led to the suggestion that patterns of histone modifications cannot simply be “read” but instead have distinct effects depending on the cellular context and upstream signaling events (Lee et al 2010b).
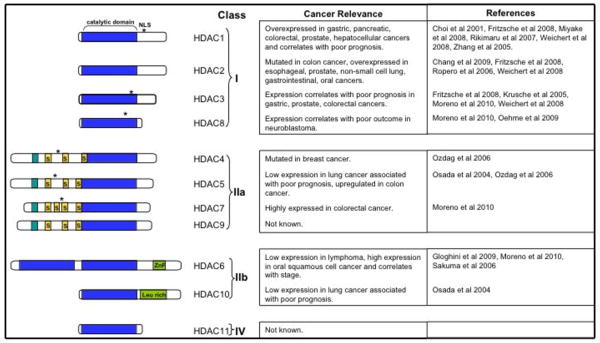
HDACs are expressed in all eukaryotic cells and histone deacetylase activity is essential for cell proliferation, differentiation, and homeostasis. Eighteen HDACs have been identified in humans that are classified based on their homology to yeast HDACs (Figure 1). Eleven of these HDACs contain highly conserved deacetylase domains and are zinc-dependent: class I (HDAC1, 2, 3, and 8 have homology to yeast RPD3); class IIa (HDACs 4, 5, 7, and 9 have homology to yeast HDA1); class IIb (HDACs 6 and 10 have two catalytic sites); and class IV (HDAC11, has conserved residues shared with both class I and class II deacetylases). The different classes differ in structure, enzymatic activity, localization, and expression pattern. In contrast to class I HDACs, Class IIa have low enzymatic activity and it has been suggested that they may have evolved to maintain low basal activities with acetyl-lysines and to efficiently process restricted sets of specific, unknown natural substrates (Lahm et al 2007). Class IIb HDACs have primarily non-epigenetic functions and regulate protein folding and turnover (Boyault et al 2007). In addition to these classical HDACs, another group of deacetylases, known as class III or sirtuins, has been recognized. This seven-member family requires NAD for enzymatic activity, is not homologous to the classical HDAC superfamily, and will not be discussed here.
Figure 1. Human HDAC superfamily showing domain organization and cancer relevance.
HDACs are grouped into different classes according to sequence similarity. Blue indicates the conserved catalytic domain; asterisks indicate nuclear localization signals; ZnF, zinc finger, myocyte enhancer factor-2 (MEF2)-binding motifs are depicted as short turquoise cylinders; and 14-3-3 chaperone binding motifs are shown as short yellow cylinders labeled with ‘S’ (for serine phosphorylation sites).
Class I HDACs are expressed in all tissues, have significant histone deacetylase activity, and are primarily localized in the nucleus where they are present in multi-protein complexes with transcription factors and co-repressors that are targeted to specific genetic loci crucial for transcriptional repression and epigenetic landscaping. HDACs form the catalytic core of megadalton complexes involved in chromatin modification and gene repression. The HDAC1-HDAC2 dimer is in the CoREST, NuRD and Sin3 complexes, while HDAC3 has been found in the NCoR complex (Yang and Seto 2008). The roles of these four molecular complexes are diverse and often cell-type specific.
Overxpression of specific HDACs has been observed in many types of cancer and often correlates with poor prognosis (Figure 1). For example, HDAC1 is overexpressed in gastric, pancreatic, colorectal, prostate, hepatocellular cancers and correlates with poor prognosis (Choi et al 2001, Fritzsche et al 2008, Miyake et al 2008, Rikimaru et al 2007, Weichert et al 2008, Zhang et al 2005). HDAC2 is mutated in colon cancer, and is overexpressed in esophageal, prostate, non-small cell lung, gastrointestinal, and oral cancers (Chang et al 2009, Fritzsche et al 2008, Ropero et al 2006, Weichert et al 2008). Poor prognosis in gastric, prostate, colorectal cancers, CLL correlates with HDAC3 expression (Fritzsche et al 2008, Krusche et al 2005, Moreno et al 2010, Weichert et al 2008). HDAC8 is overexpressed in CLL and correlates with poor outcome in neuroblastoma (Moreno et al 2010, Oehme et al 2009). While low expression of HDAC5 in lung cancer is associated with poor prognosis, its expression is upregulated in colon cancer (Osada et al 2004, Ozdag et al 2006). Although HDAC6 is expressed at low levels in lymphoma, there are high levels in CLL and expression in oral squamous cell cancer correlates with stage (Gloghini et al 2009, Moreno et al 2010, Sakuma et al 2006). Much less is known of the relationship of the other HDACs to cancer. Because abundant evidence indicates that HDAC1 and HDAC2 are overexpressed in many cancers (Figure 1) and their expression correlates with poor prognosis in many cases, our remarks will mainly be focused on these two HDACs. Studies of HDAC knockout mice and inhibition of HDACs with class I-selective inhibitors indicate that class I HDACs are important for cell survival and proliferation (Marks 2010). Of interest, transcriptional regulation of the cyclin-dependent kinase inhibitor 1 (p21) is one of the most extensively studied targets of class I HDACs (Ocker and Schneider-Stock 2007). Disruption of hdac1 causes early embryonic lethality due to decreased proliferation resulting from increased expression of p21 (Lagger et al 2002). Likewise, inactivation of HDAC2 increases p21 expression and HDAC2 overexpression correlates with reduced p21 expression (Huang et al 2005). Deletion of HDAC3 also delayed cell cycle progression and induced DNA damage and apoptosis (Bhaskara et al 2008). It is well known that class I HDACs can regulate the transcription of many other genes encoding proteins involved in control of cell growth, apoptosis, tumorigenesis, and angiogenesis. However, over the past decade, it has become apparent that in addition to histones HDACs can deacetylate numerous non-histone proteins that regulate cellular functions (Glozak and Seto 2007). Of this steadily growing list of non-histone targets, the most important are transcription factors which are often considered to be “master immune regulators”, the signal transducers and activators of transcription, Stat1 and Stat3, and NF-κB subunits, whose functions are regulated by acetylation/deacetylation and are known be important in inflammation and tumorigenesis. For example, N-terminal acetylation of Stat3 has been suggested to be important for its nuclear localization, dimerization, and transcriptional activity (Ray et al 2008, Yuan et al 2005). In contrast, HDAC1, HDAC2, and HDAC3 are necessary for Stat1-dependent gene activation, as silencing of these HDACs or their inhibition blocks the induction of IFN-stimulated Stat1 target gene expression (Nusinzon and Horvath 2003). p65 acetylation/deacetylation may be functionally important as endogenous p65 is acetylated in response to several stimuli, and deacetylation of specific lysine residues on p65 by HDAC1, HDAC3, or SIRT1 has been proposed to be involved in termination of NF-kB responses by decreased transcriptional activity and/or its nuclear export (Calao et al 2008). Another important example is ∝-tubulin, which is effectively deacetylated by class IIb HDAC6 (Hubbert et al 2002). The ability of class II HDACs to shuttle between the nucleus and the cytoplasm may be related to this important cytoplasmic function. Interestingly, many proteins post-translationally modified by acetylation that are deacetylated by HDACs play key roles in oncogenesis and tumorigenesis. As noted previously, histone deacetylases might not be the most accurate name for all of these enzymes, and they should more appropriately be referred to as acetyllysine deacetylases (Walkinshaw et al 2008).
2. Histone deacetylase inhibitors in cancer therapy
Because HDACs are frequently dysregulated in transformed cells (Marks 2010), the development of HDAC inhibitors ( HDACis) has become the subject of intense interest, and many of these agents have now entered the clinical arena. Multiple classes of HDACis have been developed, and members of these classes differ substantially in their potency and target specificity. Hydroxamic acid HDACis, which include vorinostat, LBH-589, belinostat, and PCI24781, among others, are pan-HDACis that are active against Class I and IIa/b HDACs. These agents are generally active in the low to intermediate nanomolar concentration range. Short-chain fatty acid HDACis, including sodium butyrate, valproic acid, and OSU-HDAC42, are active against Class I and IIa HDACs, but not Class IIb HDACs. They are the least potent of the HDACis, requiring millimolar concentrations to achieve their effects. Benzamide HDACis include SNDX-275, and MGCDO101, are primarily active against Class I HDACs, and show activity at intermediate nanomolar concentrations. Cyclic peptides include romidepsin and apicidin, also largely target Class I HDACs, although the possibility exists that they may also inhibit Class II HDACs at higher concentrations. These compounds are very potent, and exert their effects in the low nanomolar range. Finally, several newer classes of HDACis have been identified, including thiolates, non-hydroxamic acid carboxamides, and oxadiazoles, which exhibit varying specificities and potencies.
Critical questions remaining to be resolved are whether HDACi isoform specificity offers therapeutic advantages, or whether more broadly acting HDACis (i.e., pan-HDACis) will prove superior in the clinic. The theoretical advantage of isoform-specific inhibitors is that they have the capacity to target selectively those HDACs dysregulated in a particular cancer type, thus avoiding possible host toxicity stemming from inhibition of other HDACs. On the other hand, pan-HDACis, and particularly those that inhibit Class IIb HDACis (Hubbert et al 2002, Valenzuela-Fernandez et al 2008), have the advantage of targeting multiple cellular processes, including those involved in protein disposition (see below). For example, the ability of class IIb HDACis to disrupt aggresome function (Bali et al 2005) may be particularly important in interactions between such compounds and other targeted agents e.g., tyrosine kinase inhibitors that target mutant oncoproteins (Fiskus et al 2006). The development of selective HDACis with anti-cancer activities remains challenging in part due to the difficulty of probing the interaction of small molecules with megadalton protein complexes. A recent study utilized “chemoproteomics”, a combination of affinity capture and quantitative mass spectrometry profiling, to reveal selective targeting of HDAC complexes by known HDACis (Bantscheff et al 2011). This group demonstrated that HDACis with distinct profiles have different effects on downstream targets, suggesting that evaluation of the selectivity of HDAC inhibitors in the context of HDAC complexes, and not only with purified catalytic subunits, is important for development of new selective HDACis (Bantscheff et al 2011).
It is still not completely understood why HDAC inhibitors are relatively more toxic to transformed cells than to normal cells. This phenomenon may reflect the genetic dysregulation characteristic of malignant cells and/or an impaired capacity of such cells to respond to noxious stimuli compared to their normal counterparts. For example, it has long been recognized that HDACis can kill cells through oxidative injury and induction of reactive oxygen species (ROS) (Rosato et al 2003b, Ruefli et al 2001). It has also been shown that upon exposure to HDACis, transformed cells display a very limited ability to induce cytoprotective antioxidant proteins, such as thioredoxin, compared to normal cells, and consequently generate significantly more ROS (Ungerstedt et al 2005). More recently, it has been shown that following HDACi treatment, transformed cells display reductions in DNA repair proteins (e.g., RAD50, MRE) and are selectively impaired in their ability to repair DNA damage (Lee et al 2010a). It is therefore possible that the selectivity HDACis exert toward transformed cells represent the combined consequences of increased ROS generation in neoplastic cells in conjunction with disruption of DNA repair processes. Whether these events are responsible for or contribute to the therapeutic index of HDACis remains to be determined.
To date, two HDACis have been approved for the treatment of cancer i.e., the pan- HDACi vorinostat and the cyclic peptide romidepsin have been approved for the treatment of refractory cutaneous T-cell lymphoma (CTCL) (Grant et al 2007). Clinical trials involving these and additional HDACis are underway in other lymphomas i.e., peripheral T-cell lymphoma, and initial results appear promising. The underlying basis for the unique sensitivity of these malignancies to HDACis remains to be fully elucidated, but clues bearing on this question have recently emerged. These studies suggest that expression of HR23B, a gene whose protein product has been implicated in shuttling ubiquitinated proteins to the proteasome for degradation, may represent a useful biomarker for responses to HDACis in CTCL (Fotheringham et al 2009, Khan et al 2010). Whether HR23B in involved in regulating the responses of other tumor cell types to HDACis remains to be determined.
Because HDACis act through multiple mechanisms, they are particularly well suited to rational combination strategies involving either conventional cytotoxic agents or other targeted agents. In the case of the former, the ability of HDACis to induce chromatin relaxation has been invoked to explain synergism with certain DNA-interactive agents e.g., topoisomerase II inhibitors (Marchion et al 2004). In the case of the latter, attention has focused on strategies combining HDACis with proteasome inhibitors. Such interactions may involve multiple mechanisms including interference (i.e., by proteasome inhibitors) with HDACi-mediated NF-κB activation (Dai et al 2005, Dai et al 2008b), as well as disruption of aggresome function and induction of proteotoxic stress (Hideshima et al 2005). Notably, early results of trials combining HDAC with proteasome inhibitors have shown promise in patients with refractory multiple myeloma (Badros et al 2009), and such efforts are being expanded in other malignancies. In preclinical studies, synergistic interactions between HDACis and TRAIL have been attributed to up-regulation of death receptors (Insinga et al 2005) and/or to simultaneous interruption of the intrinsic and extrinsic apoptotic pathways (Rosato et al 2003a). In addition, pan-HDACis that prevent tubulin and Hsp90 deacetylation have been shown to interact synergistically with tyrosine kinase inhibitors targeting oncogenic proteins requiring chaperone function for their survival e.g., Bcr/Abl (Bali et al 2005, Yu et al 2003). HDACis have also been reported to increase the sensitivity of lung cancer cells by up-regulating E-cadherin (Lagger et al 2010, Witta et al 2006), and, as a consequence of their actions as anti-angiogenic agents, to potentiate the activity of angiogenesis inhibitors in prostate and breast carcinoma cells (Qian et al 2004). The ability of HDACis to up-regulate the pro-apoptotic protein Bim has been invoked to account for synergism between these agents and BH3-mimetics such as ABT-737 in human leukemia cells (Chen et al 2009). Finally, considerable attention has focused on coordinated strategies designed to promote re-expression of silenced tumor-suppressor genes by simultaneously increasing histone acetylation (by HDACis) and diminishing methylation of CpG islands (i.e., by DNA-methyltransferase inhibitors) (Cameron et al 1999, Gore et al 2006, Luszczek et al 2010).
A recent study by Sharma et al on development of tolerance to anti-cancer drugs by cancer cells demonstrated that it is a transient phenotype acquired at low frequency with hypersensitivity to HDAC inhibition, altered chromatin, and an intrinsic ability to tolerate drug exposure (Sharma et al 2010). While the in vivo significance of the cell culture findings needs to be established, this hypersensitivity to HDAC inhibitors has the potential to provide a therapeutic opportunity to prevent the development of drug resistance.
3. Mechanisms of action of histone deacetylase inhibitors: new perspectives
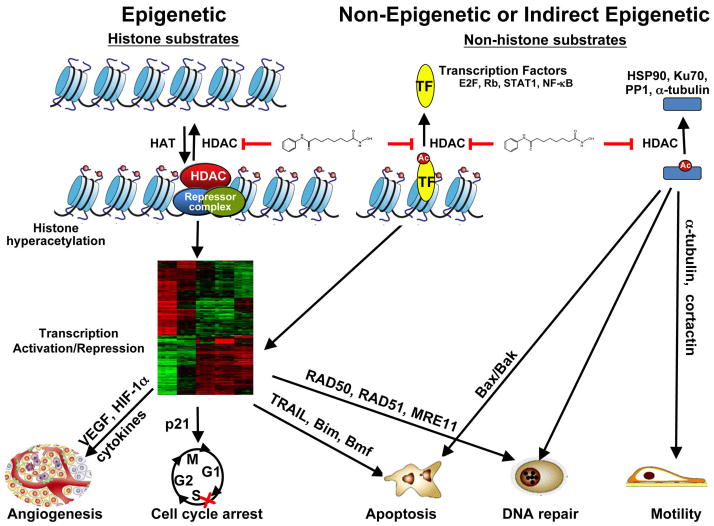
The mechanism(s) by which HDACis exert their anti-neoplastic effects remains the subject of considerable debate, but there is now general agreement that these agents most likely act through multiple interacting processes. In the most general sense, HDACis influence transformed cell behavior through five interrelated mechanisms. First, HDACis modify the acetylation status of gene promoters, which regulates gene expression (Bolden et al 2006) (Figure 2). Second, by neutralizing the acidic charges on histone tails, HDACis promote chromatin relaxation, which is generally more conducive to gene transcription (Marks 2010). However, it is important to recognize that the effects of HDACis do not always favor gene activation, as down-regulation of certain genes may also occur in response to these agents (Mitsiades et al 2004). Besides their direct effects, HDACis also prevent deacetylation of multiple transcription factors, including NF-κB, STAT3, and YY1, among numerous others, thereby controlling their activity (Chen et al 2002). Consequently, HDACis may regulate gene expression directly, by preventing or reversing deacetylation of promoters, or indirectly, by altering chromatin structure and/or by influencing transcription factor function (Figure 2). In addition to transcription factors, HDACis also promote acetylation of a large number of non-histone proteins, which may influence their function. These include chaperone proteins (e.g., Hsp90), DNA repair proteins (e.g., Ku70), tubulin, nuclear receptors, and HIF1α, among numerous others (Buchwald et al 2009) (Figure 2). It is not hard to envision that such direct and indirect actions of HDACis might cooperate to determine cell fate. For example, HDACis induce down-regulation of DNA repair genes and acetylation of DNA repair proteins; induce cell-cycle arrest at the G1/S boundary by upregulation of p21 and/or downregulation of cyclins; suppress angiogenesis by decreased expression of pro-angiogenic factors (VEGF, hypoxia-inducible factor 1α and cytokines (TNF-α, IL-1). Finally, HDACis may act, at least in part, by disrupting interactions between HDACs and co-repressors implicated in transformation (Cerchietti et al 2010).
Figure 2. Epigenetic, non-epigenetic or indirect epigenetic effects of HDACis.
HDACis, such as vorinostat, prevent histone deacetylations leading to transcription activation or repression. HDACis also can affect the acetylation status of transcription factors (E2F, NF-κB, Stats) and other proteins with important biological functions (α-tubulin, Ku70, Hsp90). See text for more detailed information.
With this background in mind, mechanisms of HDACi lethality can be grouped into several broad categories, summarized below. A particular emphasis has been placed on emerging insights into the role of ROS generation and DNA damage as determinants of HDACi lethality.
A) Modulation of the expression/function of Bcl-2 family members
HDACis have been reported to up-regulate the expression of pro-apoptotic proteins, and to down-regulate the expression of anti-apoptotic proteins through activation of the intrinsic, mitochondrial apoptotic pathway. For example, HDACis up-regulate Bim through an E2F-dependent mechanism (Zhao et al 2005). HDACi-mediated up-regulation of Noxa and Puma have also been described (Chen et al 2007). Conversely, HDACis have been shown to down-regulate the expression of Bcl-2, Bcl-xL, XIAP, and Mcl-1 (Gillespie et al 2006).
As noted previously, HDACis may also activate the extrinsic, receptor-mediated apoptotic pathway by up-regulating death receptor expression (DR4, DR5, Fas) (Insinga et al 2005, Nebbioso et al 2005). Such a mechanism may help to explain the ability of HDACis to activate Bid (Ruefli et al 2001). In agreement, valproic acid synergized with TRAIL to induce apoptosis of pancreatic cancer cells by a mechanism involving increased expression of TRAIL receptor 1 (DR5), accelerated processing of caspase 8, pronounced cleavage of the BH3-only protein Bid, and increased effector caspase activation suggesting a strategy to overcome TRAIL resistance in pancreatic cancer (Schuler et al 2010).
B) Disruption of cell cycle checkpoints
Various cell cycle checkpoints are known to be dysregulated in transformed cells (Kastan and Bartek 2004). It has been postulated that in normal cells, HDACis trigger a G2-phase checkpoint, leading to cell cycle arrest, whereas in their transformed counterparts, HDACis cause cells to enter an aberrant mitosis, culminating in cell death (Warrener et al 2003). Aberrant checkpoint responses, including that of the mitotic spindle checkpoint, to HDACis in transformed cells may also contribute to increased susceptibility to DNA damage (see below). HDACis increase acetylation of the promoter of the cyclin-dependent inhibitor p21CIP1/WAF1, one of the most commonly induced genes by HDACis, and potently induce its expression in diverse transformed cell types independently of p53, leading to cell cycle arrest (Ocker and Schneider-Stock 2007, Zupkovitz et al 2010). Decreased levels of cyclins by HDACis may contribute to cell cycle arrest by causing dephosphorylation of Rb and inhibition of E2F. Interestingly, disruption of p21CIP1/WAF1 induction by either genetic or pharmacologic means markedly increases HDACi lethality (Almenara et al 2002). A recent study demonstrated that induction of p21 by HDACi was linked to simultaneous acetylation and phosphorylation of histone H3 (Simboeck et al 2010). Association of the phospho-binding protein 14-3-3ζ to this H3S10phK14ac dual modification mark at the activated p21 promoter protects it from being processed by PP2A. This work revealed a new type of cross-talk between reversible phosphorylation and acetylation signals that controls the activation of p21 by HDACis (Simboeck et al 2010).
C) Disruption of chaperone function
Specific inhibitors of HDAC6, whose major substrate is α-tubulin, also increase acetylation of heat shock proteins such as Hsp90 (Neckers and Ivy 2003). Hsp90 is an abundant cellular chaperone whose overexpression in tumor cells correlates with resistance to chemotherapy (Whitesell and Lindquist 2005). Hsp90 is in a multi-protein complex that includes Hsp70, binds many different client proteins, including key oncogenic and anti-apoptotic proteins, and prevents their ubiquitinylation and proteasomal degradation. Thus, inhibiting HDAC6 leads to the proteasomal degradation of its “client proteins” including HER2/neu, ERBB1, ERBB2, Akt, c-Raf, Bcr/Abl and FLT3, among others in transformed cells (Bali et al 2004, Fiskus et al 2006) leading to blockade of many cancer-causing pathways and the antagonism of the hallmark traits of malignancy (Workman et al 2007). Specific inhibitors of HDAC6 such as tubacin have been shown to interact synergistically with proteasome inhibitors such as bortezomib to kill multiple myeloma and Burkitt’s lymphoma cells (Kawada et al 2009). The pan-histone deacetylase inhibitor panobinostat also increased acetylation of the Hsp90 and reduced the chaperone association between CXCR4 and hsp90, directing CXCR4 to degradation by the proteasome. In agreement, cotreatment with panobinostat and a CXCR4 antagonist synergistically induced apoptosis of AML cells, supporting the rationale for testing their efficacy against AML (Mandawat et al 2010).
D) Activation of stress-related signaling cascades and ceramide generation
HDACis, acting through multiple mechanisms, including the induction of oxidative injury, can signal through the stress-related JNK pathway to trigger apoptosis in transformed cells (Dai et al 2005). HDACis can also inhibit cytoprotective signaling pathways, including those related to MEK1/2/ERK1/2 and AKT (Rahmani et al 2003), thereby shifting the balance away from cell survival and toward cell death. The lethality of HDACis, administered either alone, or in combination with nucleoside analogs such as fludarabine or with inhibitors of AKT, have been attributed to the acid sphingomyelinase-dependent generation of the pro-apoptotic sphingolipid metabolite ceramide (Rahmani et al 2005). Similarly, low doses of the multi-kinase inhibitor sorafenib and vorinostat interact in a synergistic fashion to kill carcinoma cells by causing an increase in ceramide formation. Ceramide in turn permits CD95 plasma membrane localization and activation leading to the induction of at least three immediate downstream survival regulatory signals: activation of pro-caspase 8 (death); activation of PERK-eIF2α-ER stress (death); activation of PERK-ATG5-autophagy (survival) (Park et al 2008). In this regard, recent findings demonstrated that elevation of cytosolic calcium in gastrointestinal tumor cells by sorafenib and vorinostat is a primary event that increases dihydroceramide levels, which is essential for ROS generation and activation of PP2A that promotes CD95 activation (Park et al 2010). Although cell killing by this targeted therapy correlated with ceramide synthase 6 (Cers6) expression (Park et al 2010, Walker et al 2009), interestingly, the generation of C18-ceramide via the expression of Cers1, and not C16-ceramide by Cers5 or Cers6 expression, resulted in repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by HDAC1 in A549 human lung adenocarcinoma cells (Wooten-Blanks et al 2007). Mechanistically, increased generation of C18-ceramide by CerS1 expression, mediated the association and recruitment of the deacetylated Sp3/HDAC1 complex to the telomerase reverse transcriptase promoter, resulting in local histone H3 deacetylation and repression of the promoter (Wooten-Blanks et al 2007).
E) Induction of oxidative injury
While it has long been known that HDACis trigger the generation of ROS in transformed cells (Ruefli et al 2001), the mechanism by which this phenomenon occurs remains to be elucidated. It has been proposed that the selective toxicity that HDACis exert toward transformed cells stems from differential effects on antioxidant proteins, including MnSOD2, and particularly thioredoxin (Ungerstedt et al 2005). Notably, administration of antioxidants (e.g, TBAP, a cell permeable superoxide dismutase mimic) has been shown to protect cells from both HDACi-induced DNA damage and lethality (Dai et al 2005). Addition of phenylethyl isothiocyanate, a natural compound capable of depleting cellular glutathione, significantly enhanced the cytotoxicity of vorinostat in leukemia by inhibiting the cytoprotective antioxidant response. These results suggest that that combination with a redox-modulating compound increases sensitivity to HDACis to overcome resistance (Hu et al 2010). Despite strong evidence that ROS are responsible for DNA damage in transformed cells exposed to HDACis (Rosato et al 2008), the precise mechanism by which HDACis induce ROS as well as the source of the ROS remain to be defined.
F) Perturbations in NF-κB
There is accumulating evidence that the NF-κB transcription factor family plays a key role in integrating oxidative stress, DNA damage, and cell death responses to HDACis. HDACis, like TNFα, activate RelA, but in contrast to the TNFα responses, which are terminated by resynthesis of the NF-κB-inhibitory protein IκBα, HDACi-mediated NF-κB activation tends to be sustained (Chen and Greene 2004). This most likely reflects acetylation and phosphorylation of RelA, which promote its binding to DNA while inhibiting its nuclear export (Chen et al 2001, Chen et al 2005). Significantly, activation of NF-κB initiates events that protect cells from HDACi lethality, including induction of MnSOD, which scavenges ROS, and up-regulation of anti-apoptotic proteins such as Bcl-xL and XIAP, which attenuate apoptosis (Dai et al 2005). A corollary of this model is that agents that block activation of NF-κB by HDACis would be predicted to enhance lethality. This has been shown to be case in studies involving proteasome inhibitors, which block IκBα degradation (Dai et al 2008a) or by IKK inhibitors, which block IκBα phosphorylation and ubiquitination (Dai et al 2005, Dai et al 2010).
Until recently, the mechanism by which HDACis triggered NF-κB activation remained obscure. However, recent evidence suggests that this most likely occurs through the atypical, “inside out” ATM/NEMO DNA damage-related NF-κB activation pathway (Figure 3). In this pathway, DNA damage induces SUMOylation of NEMO, the IKK regulatory subunit, which traps it in the nucleus. Concurrently, The kinase ataxia telangiectasia mutated (ATM) is activated, leading to phosphorylation of the complex, removal of SUMO residues, and export of the NEMO complex to the cytoplasm, where it activates IKK, in a manner dependent on another IKK regulator, a protein rich in glutamate, leucine, lysine, and serine (ELKS), resulting in IκBα phosphorylation and proteasomal degradation (Wu et al 2006) (Figure 3). Two reports recently suggested that after activation, ATM is exported from the nucleus and stimulates the ubiquitin ligase activity of TRAF6 or XIAP, causing polyubiquitination of TRAF6 and the IKK adaptor ELKS (Hinz et al 2010, Wu et al 2010). The ubiquitination promotes the recruitment of the kinase TAK1 and activation of the cytosolic IKK complex, which then phosphorylates IκBα. This leads in turn to the release and nuclear translocation of p65/RelA and transcriptional activation of multiple NF-κB-dependent genes, including the ROS scavenger Mn-SOD2 which eliminates ROS and limits further DNA damage and cell death. Recently, it has been shown that HDACis, by inducing ROS and DNA damage in leukemia cells, activate ATM which leads to nuclear export of NEMO followed by NF-κB induction, up-regulation of MnSOD2, and elimination of ROS (Rosato et al 2010) (Figure 3). Notably, knock-down of ATM or transfection of cells with vectors expressing a mutant form of NEMO lacking SUMOylation sites substantially reduced HDACi-mediated NF-κB activation, associated with increased ROS generation, DNA damage induction, reflected by increased expression of phosphorylated histone H2AX (γH2AX), a marker of DNA double strand breaks, and cell death. Therefore, the initial induction of ROS by HDACIs and the resulting DNA damage are critical for NF-κB activation, which, through induction of Mn-SOD2 and ROS elimination, limits further genotoxic stress and lethality. A corollary of this notion is that interruption of HDACi-mediated NF-κB activation and potentiation of lethality may occur by two mechanisms: interference with IKK activation and/or RelA acetylation; and disruption of the ATM/NEMO DNA damage-related pathway (Rosato et al 2010). The implication of these findings is that the ATM/NEMO/SUMOylation-related NF-κB activation pathway may play a key role in integrating oxidative injury, DNA damage, and cell death responses in transformed cells exposed to HDACis and suggest that blocking NF-κB activation via the atypical ATM/NEMO nuclear pathway can enhance HDACi lethality.
Figure 3. HDACis induce “Inside out” signaling involving ATM/NEMO axis and NF-kB activation.
HDACis, by inducing ROS and double strand DNA breaks, induce SUMOylation of NEMO and activate ATM. This leads to phosphorylation of NEMO by ATM and subsequent translocation of NEMO with ATM from the nucleus. NEMO is then capable of binding and activating the IKK complex culminating in NF-κB activation and expression of survival genes.
G) DNA damage responses
The preceding findings, as well as the results of several other recent studies, suggest that among their many actions, modulation of DNA damage responses play an important role in the biologic effects of HDACis in transformed cells. For example, the rapid induction of DNA damage, manifested by γH2A.X formation and ATM phosphorylation, has been invoked to explain the preferential induction of cell death in leukemia vs. normal cells (Gaymes et al 2006). As noted above, HDACi-mediated DNA damage most likely stems from induction of oxidative injury (Dai et al 2005, Rosato et al 2008). In addition to triggering DNA damage through ROS generation, evidence is now accumulating that HDACis may also act by disrupting DNA repair processes at multiple levels. For example, as noted previously, HDACis may prevent deacetylation and disrupt the function of DNA repair proteins such as Ku70 by preventing it from suppressing Bax-mediated apoptosis (Cohen et al 2004, Subramanian et al 2005). Second, HDACis may act through a transcriptional mechanism to down-regulate various DNA repair proteins e.g., RAD50, RAD51, MRE11 (Lee et al 2010a, Rosato et al 2008). Furthermore, these events may occur preferentially in transformed cells (Lee et al 2010a). Indeed, in transformed cells, levels of phosphorylated γH2AX increased with continued culture with vorinostat, whereas this marker decreased with time in normal cells (Lee et al 2010a). Thus, HDACis like vorinostat can induce DNA damage that normal but not cancer cells can repair and explain, in part, the selectivity of vorinostat in induction of cancer cell death at concentrations that do not kill normal cells (Lee et al 2010a). In addition, there is evidence that HDACs participate in the DNA repair process. For example, deletion of HDAC3 has been shown to increase DNA damage and to disrupt the S-phase checkpoint in MEF cells (Bhaskara et al 2008). Finally, recent studies suggest that HDACs may play a role in both homologous and non-homologous end-joining DNA repair, events that may be disrupted by HDACis (Kachhap et al 2010, Miller et al 2010). Thus, HDACis may promote lethal DNA damage through multiple interactive mechanisms including the induction of ROS, down-regulation of antioxidant proteins, disabling and down-regulation of DNA repair proteins, and interference with proteins involved in homologous recombination and non-homologous end-joining repair.
4. Endogenous histone deacetylase inhibitors
Sodium butyrate, a short-chain fatty acid formed by fermentation of dietary fibers by the microbial flora of the colon, was the first natural product discovered to be an inhibitor of histone deacetylase activity (Candido et al 1978). It induces cell cycle arrest, differentiation and apoptosis in various types of cancer cells and inhibition of HDAC seems to play a central role in these responses. Butyrate was thus suggested to act as a chemopreventive metabolite that can prevent the occurrence of colorectal cancer. It was later discovered that the ubiquitous metabolite pyruvate is also an HDAC inhibitor (Thangaraju et al 2006). It has long been known that tumor cells depend on glycolysis but they convert pyruvate into lactate instead of oxidizing it in the mitochondria (Warburg 1956), a phenomenon termed the “Warburg effect”. Interestingly, pyruvate, but not lactate, inhibits HDACs and induces tumor cell-specific apoptosis (Thangaraju et al 2006). The tumor suppressor SLC5A8 (SMCT1), a Na+-coupled cotransporter for short-chain fatty acids (acetate, propionate, and butyrate), is downregulated by cancer cells as a complementary mechanism to evade pyruvate lethality (Thangaraju et al 2006). Surprisingly, however, despite the development of numerous short chain fatty acid analogues as drugs that inhibit HDACs that are now in clinical trials, little progress has been made in further understanding of the physiological regulators of HDAC activity. Recently, the potent sphingolipid metabolite sphingosine-1-phosphate (S1P) was shown to be produced in the nucleus and act as an endogenous HDAC inhibitor. Although sphingomyelin, the membrane sphingolipid precursor of S1P, has long been known to be a component of the nuclear matrix (Cocco et al 1980), the possibility that sphingolipids are also metabolized within the nucleus has only recently emerged from the observations that enzymes that metabolize sphingomyelin are present there. These include sphingomyelinase, which hydrolyzes sphingomyelin to ceramide, and ceramidase that cleaves ceramide to sphingosine (Albi et al 2008), which is then phosphorylated by sphingosine kinase 2 (SphK2) to S1P (Igarashi et al 2003, Sankala et al 2007).
S1P is a pleiotropic signaling lipid that regulates many cellular processes important for cancer progression including cell growth and survival, invasion, angiogenesis, lymphocyte trafficking, and inflammation, among others (Spiegel and Milstien 2003). S1P is produced inside cells by two closely related sphingosine kinases, SphK1 and SphK2 (Spiegel and Milstien 2007), and is exported out where it regulates many of these functions by binding to and signaling through a family of five G protein-coupled receptors, now designated S1P1–5, in a process known as “inside-out” signaling (Takabe et al 2008) (Figure 4). SphK1, which is mainly cytosolic and translocated to the plasma membrane after stimulation by growth factors and cytokines, but not SphK2, is involved in S1P export from cancer cells, mediated by the ATP-binding cassette transporters, ABCC1 and ABCG2 (Takabe et al 2010). There is no doubt that there are links between SphK1 and cancer. Overexpression of SphK1 enhances cell growth, survival, and resistance to chemotherapeutics, whereas overexpression of SphK2 reduces proliferation. SphK1 regulates motility and invasiveness of tumor cells and enhances tumorigenesis and angiogenesis. Moreover, expression and abundance of SphK1 is increased in many types of human cancers and there are correlations between its expression and poor prognosis (Shida et al 2008). Yet much less has been learned about the functions of SphK2, which is present in the nucleus of many cancer cells (Figure 4).
Figure 4. Inside out signaling by S1P.
Binding of growth factors (e.g. EGF) to their tyrosine kinase receptors activates ERK1, which then phosphorylates cytosolic SphK1 leading to its translocation to the plasma membrane where its substrate sphingosine resides. In some cells, SphK2 is at the plasma membrane and can also be activated by ERK1 phosphorylation. Once produced, S1P can be exported out of cells by ABC transporters to activate cell surface S1P receptors in an autocrine or paracrine manner, known as “inside out signaling by S1P”. This leads to activation of mutiple signals downstream of G proteins important for tumorigenesis, including growth, survival, motility, invasion and regulation of gene expression. In many types of cancer cells, SphK2 is predominantly in the nucleus where it produces S1P that inhibits class I HDACs.
Surprisingly, it was recently reported that the nucleus contains a large fraction of cellular sphingosine and dihydrosphingosine, which lacks the trans double bond at the 4 position, and that SphK2 expression significantly increased nuclear levels of S1P and dihydro-S1P. Expression of SphK2, but not catalytically inactive SphK2 or SphK1, increased acetylation of lysine 9 of histone H3, lysine 5 of histone H4, and lysine 12 of histone H2B without affecting acetylation of histone H2A. Conversely, depletion of SphK2, which specifically reduced levels of S1P and dihyrdro-S1P in the nucleus, decreased acetylation of the same histone lysine residues. In agreement, addition of S1P or dihydro-S1P to isolated nuclei further enhanced acetylation of these histone lysines (Hait et al 2009). Importantly, both S1P and dihydro-S1P potently inhibited the in vitro enzymatic activities of recombinant and purified HDAC1 and HDAC2, which explains their effects on enhancement of histone acetylations (Hait et al 2009). Several methods were used to demonstrate that HDAC1 and HDAC2 are indeed bona fide intracellular targets of S1P. First, endogenous HDAC1 and HDAC2 were pulled down specifically by S1P immobilized on affinity matrices. In contrast, neither class IIa (HDAC4, HDAC5, HDAC7) nor class IIb HDACs (HDAC6), or sirtuins bound to S1P matrices. Second, sphingolipid mass spectrometry revealed that of all of the sphingolipids present in the nucleus, only endogenous S1P was bound to nuclear HDAC1. Finally, S1P and dihydro-S1P, but not any other related lipids, specifically displaced bound S1P from HDAC1 and HDAC2. Similarly, both suberoylanilide hydroxamic acid (SAHA) and trichostatin A, potent inhibitors of HDACs, also competed with S1P for binding to HDAC1 and HDAC2, suggesting that they shared similar or overlapping binding sites. Indeed, molecular modeling of S1P into the active site of the HDAC1 homologue from the hyperthermophilic bacterium Aquifex aeolicus (HDLP) indicated that it docked as well as SAHA with estimated Ki values by AutoDock for S1P and SAHA of 9.47e-06 and 6.32e-06, respectively (Hait et al 2009). This conserved HDAC active site consists of a tubular pocket with a Zn binding site at the base, two Asp-His charge-relay systems, and a tyrosine that stabilizes the tetrahedral oxyanion necessary for catalysis (Finnin et al 1999, Hodawadekar and Marmorstein 2007). Interestingly, molecular modeling predicted that Tyr297 in HDLP, which is important for catalysis, and Arg27 (Arg34 in HDAC1), a highly conserved residue in close proximity to Tyr297 (Finnin et al 1999, Hodawadekar and Marmorstein 2007), form hydrogen bonds with the phosphate group of S1P (Hait et al 2009), providing a potential explanation of its inhibition of HDAC activity by S1P.
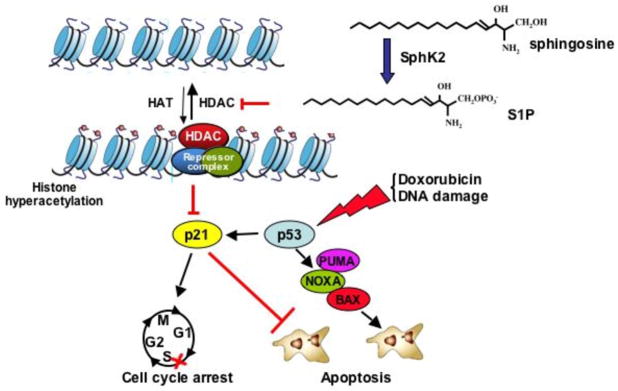
What then are the consequences of inhibition of HDAC1 and HDAC2 by S1P? As one of the common responses to inhibition of HDAC1 and HDAC2 is the induction of expression of p21, not surprisingly, SphK2 upregulates p21 expression in a p53-independent manner (Sankala et al 2007) (Figure 5). SphK2 associates with HDAC1/2 in repressor complexes and is selectively enriched at the proximal p21 promoter, where it enhances local histone H3-K9 acetylation leading to chromatin reorganization and enhanced gene transcription (Hait et al 2009). In agreement, down-regulation of SphK2 decreased basal and doxorubicin-induced expression of p21 without affecting increased expression of p53. Down-regulation of SphK2 also decreased G(2)-M arrest and markedly enhanced apoptosis induced by doxorubicin. Likewise, in human wild-type p53-expressing HCT116 colon carcinoma cells, as well as in p53-null counterparts, SphK2 depletion also markedly reduced p21 induction by doxorubicin and sensitized HCT116 cells to apoptosis induced by doxorubicin. These results further support the notion that endogenous SphK2 is important for p53-independent induction of p21 expression (Figure 5). Upregulation of p53 by doxorubicin is not only important for induction of p21 but also of several pro-apoptotic proteins, including PUMA and NOXA. However, these lead to apoptosis only in the absence of p21, which can suppress apoptosis (Seoane et al 2002). Downregulation of SphK2 expression represses p21 and switches the response from cell cycle arrest to apoptosis, suggesting that SphK2 may influence the balance between cytostasis and apoptosis of cancer cells (Figure 5).
Figure 5. Role of S1P formed in the nucleus by SphK2 in regulation of histone acetylation and p21 expression.
Activation of nuclear activation of SphK2 leads to formation of S1P, which inhibits HDAC1 and HDAC2, leading to increased histone acetylation p21 gene expression. In response to DNA damage (doxorubicin), p53 is upregulated, which leads to induction of BAX, NOXA, and PUMA (cell death mediators), and also induces p21, which suppresses apoptosis and induces cell cycle arrest. Downregulation of SphK2 prevents induction of p21 and removes p21-mediated protection against apoptosis, facilitating cell death.
Nuclear SphK2 and S1P also similarly regulated expression of c-fos, another well known HDAC1-regulated gene (Hait et al 2009). Interestingly, phorbol 12-myristate 13-acetate (PMA), which enhances ERK1-dependent phosphorylation of SphK2 and its catalytic activity (Hait et al 2007), transiently increased S1P in the nucleus and also rapidly enhanced colocalization of SphK2 with HDAC1 (Hait et al 2009). At later times, PMA induced protein kinase D-mediated phosphorylation of serine residues within the nuclear export signal of SphK2 (Ding et al 2007), resulting in its export from the nucleus to ensure transient inhibition of HDACs. Although there is still much more to be learned about physiological stimuli that regulate SphK2 and S1P synthesis in the nucleus, Hait et al provided the first demonstration that S1P formed by nuclear SphK2 in response to environmental signals influences the turnover of histone acetylation and the transcription of target genes (Hait et al 2009), linking S1P and sphingolipid metabolism in the nucleus to remodeling of chromatin and epigenetic regulation of gene expression.
5. Conclusions, questions, and future perspectives
Despite the widespread interest in HDACs, the environmental cues, signal transduction pathways regulating their activity, and endogenous regulators remain largely unknown. The finding that S1P and SphK2 are part of a corepressor complex that influences histone acetylation and gene expression (Hait et al 2009) suggests an intriguing paradigm for sphingolipid signaling in the nucleus and for HDAC regulation. Further studies may provide new clues to unresolved questions regarding specificity: How do distinct signaling pathways influence HDAC-dependent gene repression and determine the choice of acetylated targets? Deciphering the role of S1P in the regulation of histone acetylation is important as HDAC inhibitors represent a promising new therapeutic approach for cancer treatment. In this context, it will be particularly important to gain an improved understanding of how HDACs are regulated as well as the mechanisms by which HDACis exert their lethal effects toward tumor cells. To this end, a substantial number of questions remain to be answered. For example, an explanation for the selective ability of HDACis to kill transformed but not normal cells, at least in the preclinical setting, remains to be identified. Although initial clues suggest impaired antioxidant and DNA damage defenses in the former, this needs to be validated. In addition, the relative advantages of isoform-specific versus non-specific HDACis remains the subject of considerable debate. While it is possible that isoform-specific inhibitors may be relatively free of undesirable toxicities, it is also conceivable that agents that inhibit both nuclear (e.g., Class I) as well as cytoplasmic HDACs (e.g., Class IIb) may offer therapeutic advantages by simultaneously targeting multiple processes i.e., gene transcription as well as protein disposition. For the immediate future, important questions involving HDACis to be addressed include deciphering their precise relationship to DNA damage responses, their complex bi-directional interactions with transcription factors (e.g., NF-κB) and co-repressors, and the nature of their interactions with other agents that modify the epigenome e.g., DNMTIs, histone methyltransferases, and histone demethylases. Complicating these tasks is the likelihood that HDACis undoubtedly act simultaneously through multiple interrelated mechanisms to exert their biologic effects e.g., through alterations in chromatin structure and gene expression, induction of oxidative injury and DNA damage, modification of the expression and function of chaperone and repair proteins, interactions with transcription factors and co-repressor proteins. Despite the obvious challenges, answers to these questions will greatly facilitate the search for safer and more effective drugs capable of interfering with HDAC functions in a highly specific manner.
Acknowledgments
We apologize to authors whose work has not been cited here owing to space limitations. This work was supported by R01CA61774 (SS) R37GM043880 (SS), R01AI50094 (SS), 1U19AI077435 (SS),), R01AI50094 (SS), 1U19AI077435 (SS), RO1CA93738-05 (SG), CA100866 (SG), 1P50CA142509 (SG), RC2CA148431 (SG), 1P50CA130805 (SG), 1R21CA137823, Leukemia and Lymphoma Society of America 6181-10 (SG), the V Foundation, and the Multiple Myeloma Research Foundation (SG).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Albi E, Lazzarini R, Viola Magni M. Phosphatidylcholine/sphingomyelin metabolism crosstalk inside the nucleus. Biochem J. 2008;410:381–389. doi: 10.1042/BJ20070758. [DOI] [PubMed] [Google Scholar]
- Almenara J, Rosato R, Grant S. Synergistic induction of mitochondrial damage and apoptosis in human leukemia cells by flavopiridol and the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) Leukemia. 2002;16:1331–1343. doi: 10.1038/sj.leu.2402535. [DOI] [PubMed] [Google Scholar]
- Badros A, Burger AM, Philip S, Niesvizky R, Kolla SS, Goloubeva O, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15:5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali P, George P, Cohen P, Tao J, Guo F, Sigua C, et al. Superior activity of the combination of histone deacetylase inhibitor LAQ824 and the FLT-3 kinase inhibitor PKC412 against human acute myelogenous leukemia cells with mutant FLT-3. Clin Cancer Res. 2004;10:4991–4997. doi: 10.1158/1078-0432.CCR-04-0210. [DOI] [PubMed] [Google Scholar]
- Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- Bantscheff M, Hopf C, Savitski MM, Dittmann A, Grandi P, Michon AM, et al. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat Biotechnol. 2011;29:255–265. doi: 10.1038/nbt.1759. [DOI] [PubMed] [Google Scholar]
- Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell. 2008;30:61–72. doi: 10.1016/j.molcel.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- Buchwald M, Kramer OH, Heinzel T. HDACi--targets beyond chromatin. Cancer Lett. 2009;280:160–167. doi: 10.1016/j.canlet.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Calao M, Burny A, Quivy V, Dekoninck A, Van Lint C. A pervasive role of histone acetyltransferases and deacetylases in an NF-kappaB-signaling code. Trends Biochem Sci. 2008;33:339–439. doi: 10.1016/j.tibs.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Cerchietti LC, Hatzi K, Caldas-Lopes E, Yang SN, Figueroa ME, Morin RD, et al. BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J Clin Invest. 2011 doi: 10.1172/JCI42869. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Chiang CP, Hung HC, Lin CY, Deng YT, Kuo MY. Histone deacetylase 2 expression predicts poorer prognosis in oral cancer patients. Oral Oncol. 2009;45:610–614. doi: 10.1016/j.oraloncology.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, et al. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–6169. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Kwon HJ, Yoon BI, Kim JH, Han SU, Joo HJ, et al. Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res. 2001;92:1300–1304. doi: 10.1111/j.1349-7006.2001.tb02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289–296. doi: 10.1016/j.molcel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Cocco L, Maraldi NM, Manzoli FA, Gilmour RS, Lang A. Phospholipid interactions in rat liver nuclear matrix. Biochem Biophys Res Commun. 1980;96:890–898. doi: 10.1016/0006-291x(80)91439-4. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008a;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Rahmani M, Dent P, Grant S. Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-kappaB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol Cell Biol. 2005;25:5429–5444. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Chen S, Kramer LB, Funk VL, Dent P, Grant S. Interactions between bortezomib and romidepsin and belinostat in chronic lymphocytic leukemia cells. Clin Cancer Res. 2008b;14:549–558. doi: 10.1158/1078-0432.CCR-07-1934. [DOI] [PubMed] [Google Scholar]
- Dai Y, Guzman ML, Chen S, Wang L, Yeung SK, Pei XY, et al. The NF (Nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol. 2010;151:70–83. doi: 10.1111/j.1365-2141.2010.08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Sonoda H, Yu H, Kajimoto T, Goparaju SK, Jahangeer S, et al. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem. 2007;282:27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]
- Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- Fiskus W, Pranpat M, Balasis M, Bali P, Estrella V, Kumaraswamy S, et al. Cotreatment with vorinostat (suberoylanilide hydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinib mesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells. Clin Cancer Res. 2006;12:5869–5878. doi: 10.1158/1078-0432.CCR-06-0980. [DOI] [PubMed] [Google Scholar]
- Fotheringham S, Epping MT, Stimson L, Khan O, Wood V, Pezzella F, et al. Genome-wide loss-of-function screen reveals an important role for the proteasome in HDAC inhibitor-induced apoptosis. Cancer Cell. 2009;15:57–66. doi: 10.1016/j.ccr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Fritzsche FR, Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, et al. Class I histone deacetylases 1, 2 and 3 are highly expressed in renal cell cancer. BMC Cancer. 2008;8:381. doi: 10.1186/1471-2407-8-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymes TJ, Padua RA, Pla M, Orr S, Omidvar N, Chomienne C, et al. Histone deacetylase inhibitors (HDI) cause DNA damage in leukemia cells: a mechanism for leukemia-specific HDI-dependent apoptosis? Mol Cancer Res. 2006;4:563–573. doi: 10.1158/1541-7786.MCR-06-0111. [DOI] [PubMed] [Google Scholar]
- Gillespie S, Borrow J, Zhang XD, Hersey P. Bim plays a crucial role in synergistic induction of apoptosis by the histone deacetylase inhibitor SBHA and TRAIL in melanoma cells. Apoptosis. 2006;11:2251–2265. doi: 10.1007/s10495-006-0283-6. [DOI] [PubMed] [Google Scholar]
- Gloghini A, Buglio D, Khaskhely NM, Georgakis G, Orlowski RZ, Neelapu SS, et al. Expression of histone deacetylases in lymphoma: implication for the development of selective inhibitors. Br J Haematol. 2009;147:515–525. doi: 10.1111/j.1365-2141.2009.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- Grant S, Easley C, Kirkpatrick P. Vorinostat. Nat Rev Drug Discov. 2007;6:21–22. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Bradner JE, Wong J, Chauhan D, Richardson P, Schreiber SL, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci USA. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Hodawadekar SC, Marmorstein R. Chemistry of acetyl transfer by histone modifying enzymes: structure, mechanism and implications for effector design. Oncogene. 2007;26:5528–5540. doi: 10.1038/sj.onc.1210619. [DOI] [PubMed] [Google Scholar]
- Hu Y, Lu W, Chen G, Zhang H, Jia Y, Wei Y, et al. Overcoming resistance to histone deacetylase inhibitors in human leukemia with the redox modulating compound beta-phenylethyl isothiocyanate. Blood. 2010;116:2732–2741. doi: 10.1182/blood-2009-11-256354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BH, Laban M, Leung CH, Lee L, Lee CK, Salto-Tellez M, et al. Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ. 2005;12:395–404. doi: 10.1038/sj.cdd.4401567. [DOI] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura SI. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11:71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- Kachhap SK, Rosmus N, Collis SJ, Kortenhorst MS, Wissing MD, Hedayati M, et al. Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PLoS One. 2010;5:e11208. doi: 10.1371/journal.pone.0011208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kawada J, Zou P, Mazitschek R, Bradner JE, Cohen JI. Tubacin kills Epstein-Barr virus (EBV)-Burkitt lymphoma cells by inducing reactive oxygen species and EBV lymphoblastoid cells by inducing apoptosis. J Biol Chem. 2009;284:17102–17109. doi: 10.1074/jbc.M809090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O, Fotheringham S, Wood V, Stimson L, Zhang C, Pezzella F, et al. HR23B is a biomarker for tumor sensitivity to HDAC inhibitor-based therapy. Proc Natl Acad Sci USA. 2010;107:6532–6537. doi: 10.1073/pnas.0913912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusche CA, Wulfing P, Kersting C, Vloet A, Bocker W, Kiesel L, et al. Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat. 2005;90:15–23. doi: 10.1007/s10549-004-1668-2. [DOI] [PubMed] [Google Scholar]
- Lagger G, O’Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, et al. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagger S, Meunier D, Mikula M, Brunmeir R, Schlederer M, Artaker M, et al. Crucial function of histone deacetylase 1 for differentiation of teratomas in mice and humans. EMBO J. 2010;29:3992–4007. doi: 10.1038/emboj.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci USA. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci USA. 2010a;107:14639–14644. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Smith E, Shilatifard A. The language of histone crosstalk. Cell. 2010b;142:682–685. doi: 10.1016/j.cell.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszczek W, Cheriyath V, Mekhail TM, Borden EC. Combinations of DNA methyltransferase and histone deacetylase inhibitors induce DNA damage in small cell lung cancer cells: correlation of resistance with IFN-stimulated gene expression. Mol Cancer Ther. 2010;9:2309–2321. doi: 10.1158/1535-7163.MCT-10-0309. [DOI] [PubMed] [Google Scholar]
- Mandawat A, Fiskus W, Buckley KM, Robbins K, Rao R, Balusu R, et al. Pan-histone deacetylase inhibitor panobinostat depletes CXCR4 levels and signaling and exerts synergistic antimyeloid activity in combination with CXCR4 antagonists. Blood. 2010;116:5306–5315. doi: 10.1182/blood-2010-05-284414. [DOI] [PubMed] [Google Scholar]
- Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN. Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem. 2004;92:223–237. doi: 10.1002/jcb.20045. [DOI] [PubMed] [Google Scholar]
- Marks PA. The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin Investig Drugs. 2010;19:1049–1066. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;17:1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci USA. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Yoshizumi T, Imura S, Sugimoto K, Batmunkh E, Kanemura H, et al. Expression of hypoxia-inducible factor-1alpha, histone deacetylase 1, and metastasis-associated protein 1 in pancreatic carcinoma: correlation with poor prognosis with possible regulation. Pancreas. 2008;36:e1–9. doi: 10.1097/MPA.0b013e31815f2c2a. [DOI] [PubMed] [Google Scholar]
- Moreno DA, Scrideli CA, Cortez MA, de Paula Queiroz R, Valera ET, da Silva Silveira V, et al. Differential expression of HDAC3, HDAC7 and HDAC9 is associated with prognosis and survival in childhood acute lymphoblastic leukaemia. Br J Haematol. 2010;150:665–673. doi: 10.1111/j.1365-2141.2010.08301.x. [DOI] [PubMed] [Google Scholar]
- Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- Neckers L, Ivy SP. Heat shock protein 90. Curr Opin Oncol. 2003;15:419–424. doi: 10.1097/00001622-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Nusinzon I, Horvath CM. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc Natl Acad Sci USA. 2003;100:14742–14747. doi: 10.1073/pnas.2433987100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocker M, Schneider-Stock R. Histone deacetylase inhibitors: signalling towards p21cip1/waf1. Int J Biochem Cell Biol. 2007;39:1367–1374. doi: 10.1016/j.biocel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Oehme I, Deubzer HE, Lodrini M, Milde T, Witt O. Targeting of HDAC8 and investigational inhibitors in neuroblastoma. Expert Opin Investig Drugs. 2009;18:1605–1617. doi: 10.1517/14728220903241658. [DOI] [PubMed] [Google Scholar]
- Osada H, Tatematsu Y, Saito H, Yatabe Y, Mitsudomi T, Takahashi T. Reduced expression of class II histone deacetylase genes is associated with poor prognosis in lung cancer patients. Int J Cancer. 2004;112:26–32. doi: 10.1002/ijc.20395. [DOI] [PubMed] [Google Scholar]
- Ozdag H, Teschendorff AE, Ahmed AA, Hyland SJ, Blenkiron C, Bobrow L, et al. Differential expression of selected histone modifier genes in human solid cancers. BMC Genomics. 2006;7:90. doi: 10.1186/1471-2164-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Haussinger D, et al. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res. 2010;70:6313–6324. doi: 10.1158/0008-5472.CAN-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian DZ, Wang X, Kachhap SK, Kato Y, Wei Y, Zhang L, et al. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2004;64:6626–6634. doi: 10.1158/0008-5472.CAN-04-0540. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Yu C, Dai Y, Reese E, Ahmed W, Dent P, et al. Coadministration of the heat shock protein 90 antagonist 17-allylamino- 17-demethoxygeldanamycin with suberoylanilide hydroxamic acid or sodium butyrate synergistically induces apoptosis in human leukemia cells. Cancer Res. 2003;63:8420–8427. [PubMed] [Google Scholar]
- Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- Ray S, Lee C, Hou T, Boldogh I, Brasier AR. Requirement of histone deacetylase1 (HDAC1) in signal transducer and activator of transcription 3 (STAT3) nucleocytoplasmic distribution. Nucleic Acids Res. 2008;36:4510–4520. doi: 10.1093/nar/gkn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikimaru T, Taketomi A, Yamashita Y, Shirabe K, Hamatsu T, Shimada M, et al. Clinical significance of histone deacetylase 1 expression in patients with hepatocellular carcinoma. Oncology. 2007;72:69–74. doi: 10.1159/000111106. [DOI] [PubMed] [Google Scholar]
- Ropero S, Fraga MF, Ballestar E, Hamelin R, Yamamoto H, Boix-Chornet M, et al. A truncating mutation of HDAC2 in human cancers confers resistance to histone deacetylase inhibition. Nat Genet. 2006;38:566–569. doi: 10.1038/ng1773. [DOI] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther. 2003a;2:1273–1284. [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003b;63:3637–3645. [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Maggio SC, Coe S, Atadja P, Dent P, et al. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther. 2008;7:3285–3297. doi: 10.1158/1535-7163.MCT-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato RR, Kolla SS, Hock SK, Almenara JA, Patel A, Amin S, et al. Histone deacetylase inhibitors activate NF-kappaB in human leukemia cells through an ATM/NEMO-related pathway. J Biol Chem. 2010;285:10064–10077. doi: 10.1074/jbc.M109.095208. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Uzawa K, Onda T, Shiiba M, Yokoe H, Shibahara T, et al. Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma. Int J Oncol. 2006;29:117–124. [PubMed] [Google Scholar]
- Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- Schuler S, Fritsche P, Diersch S, Arlt A, Schmid RM, Saur D, et al. HDAC2 attenuates TRAIL-induced apoptosis of pancreatic cancer cells. Mol Cancer. 2010;9:80. doi: 10.1186/1476-4598-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simboeck E, Sawicka A, Zupkovitz G, Senese S, Winter S, Dequiedt F, et al. A phosphorylation switch regulates the transcriptional activation of cell cycle regulator p21 by histone deacetylase inhibitors. J Biol Chem. 2010;285:41062–41073. doi: 10.1074/jbc.M110.184481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- Subramanian C, Opipari AW, Jr, Bian X, Castle VP, Kwok RP. Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:4842–4847. doi: 10.1073/pnas.0408351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraju M, Gopal E, Martin PM, Ananth S, Smith SB, Prasad PD, et al. SLC5A8 triggers tumor cell apoptosis through pyruvate-dependent inhibition of histone deacetylases. Cancer Res. 2006;66:11560–11564. doi: 10.1158/0008-5472.CAN-06-1950. [DOI] [PubMed] [Google Scholar]
- Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Walker T, Mitchell C, Park MA, Yacoub A, Graf M, Rahmani M, et al. Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and -independent mechanisms. Mol Pharmacol. 2009;76:342–355. doi: 10.1124/mol.109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkinshaw DR, Tahmasebi S, Bertos NR, Yang XJ. Histone deacetylases as transducers and targets of nuclear signaling. J Cell Biochem. 2008;104:1541–1552. doi: 10.1002/jcb.21746. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warrener R, Beamish H, Burgess A, Waterhouse NJ, Giles N, Fairlie D, et al. Tumor cell-selective cytotoxicity by targeting cell cycle checkpoints. FASEB J. 2003;17:1550–1552. doi: 10.1096/fj.02-1003fje. [DOI] [PubMed] [Google Scholar]
- Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, Ravdel L, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. Faseb J. 2007;21:3386–3397. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann NY Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Wong ET, Shi Y, Niu J, Chen Z, Miyamoto S, et al. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol Cell. 2010;40:75–86. doi: 10.1016/j.molcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Rahmani M, Almenara J, Subler M, Krystal G, Conrad D, et al. Histone deacetylase inhibitors promote STI571-mediated apoptosis in STI571-sensitive and -resistant Bcr/Abl+ human myeloid leukemia cells. Cancer Res. 2003;63:2118–2126. [PubMed] [Google Scholar]
- Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yamashita H, Toyama T, Sugiura H, Ando Y, Mita K, et al. Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast*. Breast Cancer Res Treat. 2005;94:11–16. doi: 10.1007/s10549-005-6001-1. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci USA. 2005;102:16090–16095. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupkovitz G, Grausenburger R, Brunmeir R, Senese S, Tischler J, Jurkin J, et al. The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation. Mol Cell Biol. 2010;30:1171–1181. doi: 10.1128/MCB.01500-09. [DOI] [PMC free article] [PubMed] [Google Scholar]