Abstract
Background
Graves' ophthalmopathy (GO) significantly impairs the quality of life of affected individuals and the most severe cases can be sight threatening. Given the limited therapeutic options, a strong emphasis should be placed on disease prevention to diminish the significant morbidity associated with this disease.
Summary
GO is most prevalent in women and most severe in men. Although some genetic differences between GO patients and Graves' disease patients without ophthalmopathy have been identified, none of the polymorphisms identified to date impart a high enough risk of GO to justify genetic testing to guide therapy or preventive strategies. Poorly defined mechanical factors that appear also to play a role in GO susceptibility will likely be better elucidated with advances in imaging techniques. Tobacco smoking has been consistently linked to development or deterioration of GO. Smokers who receive radioactive iodine have the highest incidence of unfavorable GO outcome, which is proportional to the number of cigarettes smoked per day. Several studies have reported an association between radioactive iodine treatment for Graves' disease and worsening or development of GO. Observational studies suggest that the same appears to be true for thyroid dysfunction, including both hyper- and hypothyroidism. While thyrotropin receptor antibody levels appear to be useful in predicting the course of disease and response to therapy, it is not known whether they are predictive of GO development. The puzzling scenarios of euthyroid or clinically unilateral GO, the large number of nonsmoking GO patients, and the occasional development of GO years after thyroid dysfunction has been treated all underline the multifactorial etiology of this disorder in which no single factor determines the clinical outcome.
Conclusions
GO appears to have a complex genetic basis with multiple susceptibility alleles that act in combination with nongenetic factors to contribute to disease expression.
Introduction
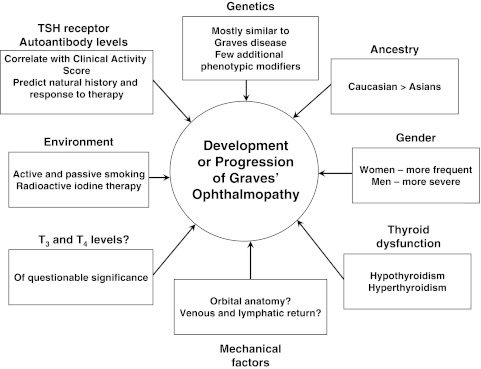
Graves' ophthalmopathy (GO) is a disease that significantly impairs quality of life, may be sight-threatening, and for which limited therapeutic options with variable effectiveness are available. It is therefore imperative that better disease prevention be achieved if the significant morbidity associated with this condition is to be limited. Since the first description of the disease about 200 years ago (1), a number of risk factors for the development or worsening of the condition have been studied. These include gender and ancestral group; genetic, environmental, and mechanical factors; and factors related to thyroid dysfunction (Fig. 1). We will discuss each of these in the context of our current understanding of the pathophysiology of the disease, touching only briefly on the impact of radioactive iodine (RAI) treatment for Graves' disease (GD) as the topic is discussed in a separate review in this series.
FIG. 1.
Risk factors for the development or progression of Graves' ophthalmopathy. TSH, thyrotropin; T3, triiodothyronine; T4, thyroxine.
Gender and Ancestry
Cultural norms lead to significant differences between genders in their environmental exposure, and both cultural norms and geography lead to differences in environmental exposure between ancestral groups. Yet, these populations are also likely to be dissimilar as regards GO development due to their different genetic profiles. Therefore, while we discuss gender and ancestry separately from genetics (see below), this separation is admittedly artificial.
Patients with GO are more likely to be women by a 2:1 ratio (2), following the usual predominance of autoimmunity in women. Yet, men with GD appear to be at the same if not higher risk of GO development, which is usually of a more severe form and occurs at a more advanced age than in their female counterparts (3,4). Differences in the prevalence of GO also appear to be present between ancestral groups, with Asians having a lower likelihood of developing the disease than Europeans (5). Confounding factors that should be considered in the interpretation of these data are the variability of smoking in different populations and between genders. In addition, normative data concerning proptosis in these different groups that show an increasing gradient from Asians to Caucasians to African-Americans (6), perhaps resulting in an over-estimation in the severity of proptosis in non-Asian GO patients.
Genetics
The concept that GO might be an autoimmune disease stems from its clinical association with GD, an associated condition known to be caused by anti-thyrotropin receptor antibodies (TRAb). Studies show that clinically apparent GO is present in 25%–50% of patients with Graves' hyperthyroidism, and that subclinical evidence of ocular involvement is detectable in most of these patients (7). Conversely, the presence of autoimmune thyroid disease appears to be necessary, but not sufficient, for the development of GO (8). That GO and GD might share a common etiology is further suggested by the close temporal relationship between the onset of GD and the onset of GO; regardless of which occurs first, the other develops within 18 months in 80% of affected patients (9).
On the basis of the clinical associations between GO and GD, it is reasonable to postulate that polymorphic variations in individual somatic genes or groups of genes known to be involved in thyroid autoimmunity might also predispose to GO. In addition, attempts have been made to distinguish from the pool of all patients with GD those who are most likely to develop GO. Studies have focused on immunomodulatory genes including human leukocyte antigen-DR3 (HLA-DR3); cytotoxic T lymphocyte antigen (CTLA-4); interleukin-1 (IL-1) family of cytokines; IL-23 receptor (IL-23R); CD40; protein tyrosine phosphatase, non-receptor type 22 (PTPN22); T-cell receptor β-chain (TCR-β); tumor necrosis factor-β (TNF-β); and various immunoglobulin heavy chain-associated genes. In addition, owing to evidence that both thyrotropin receptor (TSHR) expression and new fat cell development (adipogenesis) are enhanced in the GO orbit (10), the adipogenesis-related gene peroxisome proliferator–associated receptor-γ (PPAR-γ), and the TSHR gene have been investigated, as have genes encoding thyroglobulin and the glucocorticoid receptor.
As noted in a review by Jacobson and Tomer (11), most of the genes found to impact the risk for development of autoimmune thyroid disease are part of the immunological synapse (receptors, signaling pathway molecules, or presented peptides). Two IL-23R polymorphisms (rs10889677 and rs2201841) were shown to be associated with GO with odds ratios (OR) of 1.8 compared with GD patients not having GO (12). While an association between CTLA-4 A/G polymorphism at codon 17 and GO has been reported, with an OR directly related to the severity of the ophthalmopathy (13), that association was not confirmed in a larger cohort of patients with apparently similar background (14). More recently, it was noted that serum-soluble CTLA-4 concentration is higher in individuals with severe GO than in those with mild GO and that these levels are determined at least in part by the presence of the CTLA-4 gene polymorphisms Jo31 and CT60 (15). This finding was also disputed in an Asian population in which the CTLA-4 polymorphism CT60A/G described above, along with two other single-nucleotide polymorphisms (318C/T, and 49A/G), were not found to be associated with GO (16). However, the replication of these findings in different populations is not essential to their validity as the selective pressure of infectious agents likely promoted different immune-response polymorphisms in various ancestral groups during evolution. As a result, a particular autoimmune disorder can be associated with different susceptibility genes in different populations (17). Another recent report identifies IL-1 alpha 889C/T polymorphism (OR of 5.7 for TT genotype) and IL-1 receptor antagonist (IL-1RA) with Mspa-1 11100C/T polymorphism (OR of 6.7 for CC genotype) to be significantly more frequent among patients with GO than among GD patients without GO (18). The distribution of the Pro(12)Ala peroxisome proliferator–associated receptor-γ gene polymorphism has also been studied in terms of a genetic predisposition to adipogenesis in GO (19). While this polymorphism was found to be equally present in GD patients with and without GO, in those with GO this polymorphism was associated with disease that was milder and less active. While most of the other genes mentioned above have been shown to be associated with GD, with or without GO, the findings described above (e.g., IL-23, IL-1, and IL-1 RA genes) suggest that there might be a role for genetic factors in the development of the GO disease phenotype.
Overall, these findings suggest that GO has a complex genetic basis with multiple susceptibility alleles in combination with nongenetic factors that contribute to particular expression of GO in each affected individual. None of the polymorphisms identified to date, if validated at current OR, impart a high enough risk of GO to justify genetic testing to guide therapy or preventive strategies in patients with GD. Further, postgenetic mechanisms affecting the transcription and translation of these genes might be responsible for some of the risk of GO, as suggested with respect to TSHR variants (20).
Mechanical Factors
The bony confines of the orbit restrict the posterior expansion of the inflammatory tissues within the GO orbit. Therefore, the increased volume of the orbital fat and extraocular muscle may lead to anterior displacement of the globe, or proptosis. This serves as a natural orbital decompression to relieve the intraorbital pressures. On anatomical grounds the GO patients as a group appear to have a wider lateral orbital wall angle than their normal counterparts (21), a feature that is also present in a noninflammatory proptotic entity called nonsyndromic exorbitism. In addition to that, the presence of high myopia in a GO patient will lead to an exacerbation of the proptosis, even though in the general population myopia is not associated with noticeable exophthalmos (21). The authors of this report argued further that the association of GO with nonsyndromic exorbitism is likely to lead to a poor response to two- or three-wall orbital expansion, without any significant benefit from the resection of the wide-angle lateral wall. The importance of orbital anatomy is further emphasized by the significant prevalence of clinically unilateral GO in 6%–14% of all GO cases (22,23). Individual anatomic variations in the orbital contour or the venous or lymphatic vessels may make some patients with GD more prone to the development of clinically significant GO or might explain asymmetric eye involvement in some patients. Besides proptosis, other signs and symptoms of GO are attributable to mechanical pressures within the noncompliant bony orbit. These may impair venous and lymphatic outflow and cause pooling of proinflammatory cytokines within the orbital space leading to chemosis, periorbital edema, and inflammation.
Mechanical factors and trauma to soft tissues may be similarly involved in the dermal lesions associated with GO, termed pretibial dermopathy (PTD). Rapoport and colleagues reported the case of a Graves' patient having severe, elephantiasic PTD who suffered worsening coincident with increased periods of prolonged standing (24). The inferior limit of the PTD was just above the margin of this patient's shoes, with relative sparing of the feet. Dependent edema in the lower extremities with slower return of lymphatic fluid to the circulation might reduce the clearance and prolong the half-life of disease-related cytokines or chemokines within the affected tissues.
Environmental Factors
Tobacco smoking
Smoking has been the risk factor most consistently linked to either development or deterioration of GO. This combined outcome has been used by most trials, making the impact of smoking on the isolated development of new GO cases difficult to define. In a recent trial of patients with newly diagnosed GD treated with either RAI or antithyroid drugs (25), smoking was the dominant risk factor identified for the combined GO outcome with an OR of 5.2 (95% CI: 2.4–11.5). Upon post hoc analysis with a more strict set of criteria for outcome definition, smoking was found to have an even higher OR for the combined GO outcome at 9.8 (95% CI: 2.9–34.9). Of note is that in this study, the choice of therapy for Graves' hyperthyroidism in smokers did not impact GO outcome, a finding at odds with previous studies showing smoking to be additive to the radioiodine-induced risk of GO development (26). Given that the smokers who received radioiodine had the highest overall probability of GO at 3 years (∼50%), it is possible that the study was underpowered to detect a difference in GO outcome in this subset of patients. Overall, more than 40% of smokers either developed or worsened GO, which was almost double the rate of nonsmokers. In a similar therapeutic trial (26) of patients with established GO, smokers treated with radioiodine had a relative risk of development/progression of GO of 3.9 (95% CI: 1.4–11) compared with nonsmokers. In this study, smoking imparted additional risk for GO over that conferred by radioiodine alone and GO progression was prevented when radioiodine therapy was combined with steroids in 63.8% of nonsmokers compared with 14.9% of smokers.
These clinical studies suggest that the GO risk related to active smoking is proportional to the number of cigarettes smoked per day, and former smokers have significantly lower risk than current smokers, even after adjusting for lifetime cigarette consumption. The relationship of second-hand smoking or passive smoking with GO has been only indirectly investigated through a questionnaire looking at childhood GO in European countries (27). The authors identified a very high (52%) proportion of GO occurring in children <10 years old (presumably only exposed to second-hand smoking) in countries with smoking prevalence at >25% of the population. In contrast, in countries where the smoking prevalence is lower, the proportion of GO in children <10 years old is less (19%), which is similar to the overall prevalence of GO in children in these countries. Despite the absence of an intervention study demonstrating benefit of smoking cessation on GO outcome, we continue to advise and support (28) our GO patients in this endeavor based on the consistent clinical associations along with the multiple additional benefits of smoking cessation.
Mechanisms underlying the relationship between smoking and GO are unclear. Smoking has been associated with other autoimmune diseases, such as rheumatoid arthritis (29) and Crohn's disease (30), suggesting that smoking increases the risk for immune self-attack with subsequent development of autoimmune conditions. While patients with GO have higher levels of circulating IL-6 receptor than do GD patients without clinical GO, levels do not differ between smokers and nonsmokers (31). In vitro studies have shown that both cigarette smoke extract (32) and hypoxia (33) increase production by orbital fibroblasts of glycosaminoglycans, hydrophilic macromolecules that accumulate in GO orbital tissues. In addition, cigarette smoke extract synergized with IL-1 treatment (32) to enhance adipogenesis in these cells, also suggesting a direct and immediate effect of smoking on the development of GO.
Therapy for GD with RAI
Several retrospective cohorts and randomized trials have identified the risk of GO development or progression after RAI therapy for hyperthyroidism to be between 15% and 39%. After a number of observational studies, randomized controlled trials found that risk at 23/150 (15%) for radioiodine compared with 4/148 (3%) for antithyroid drugs (26); 13/39 (33%) for radioioactive iodine compared with 4/38 (10%) for antithyroid drugs and 6/37 (16%) for surgery (34); and 63/163 (38.7%) for RAI compared with 32/150 (21.3%) for antithyroid drugs (25). The majority of patients developing GO after RAI treatment had mild and transient disease requiring no treatment. Although these trials used slightly different parameters to define development of GO and some lacked the evaluation of disease activity, they are overall consistent in supporting the hypothesis that RAI treatment of Graves' hyperthyroidism may negatively impact the eyes. Whether particular subsets of Graves' patients might be especially vulnerable to the effect is yet to be determined, although the most recent of the trials described above identified a trend for smoking as an additive risk factor to RAI for GO development (25).
Biochemical Factors
Thyroid dysfunction
Both hyper- and hypothyroidism have been shown in multiple reports to be associated with increased risk for development or deterioration of GO. DeGroot et al. (35) in a cohort of 264 patients, found an OR of 2.8 for development or worsening of preexistent GO in patients requiring more than one dose of RAI to control hyperthyroidism, compared with patients requiring only a single dose. The confounding issue not addressed in that study was the independent impact of multiple doses of RAI on GO outcome, independent of thyroid dysfunction. Prummel et al. (36) stratified 90 GO patients in a referral population using a severity index and found both hypothyroidism and hyperthyroidism to be associated with an increased risk for severe GO, with an OR of 2.8 (95% CI: 1.2–6.8) for patients with more severe GO having current thyroid dysfunction compared to patients with milder GO. The duration of GD and GO was the same in euthyroid and dysthyroid individuals in this study. The caveat is that 37 referred GO patients without a history of hyperthyroidism (i.e., euthyroid GO or GO associated with hypothyroidism alone) were excluded from the study and that patients with subclinical hyperthyroidism were considered to be euthyroid.
The studies described above and others (37) have led some clinicians to a change in practice toward cautious avoidance of hypothyroidism after radioiodine therapy for GD. One group studied the early initiation of levothyroxine (at 2 week after the treatment) to avoid hypothyroidism, comparing GO outcome in that group with their historical cohort of patients who did not receive levothroxine until they were documented to be hypothyroid (permissive hypothyroidism) (38). Results identified the relative risk of GO development or worsening at 1.64 (95% CI: 1.1–2.6) in the permissive hypothyroidism group compared with the early treatment group. The study also documented an increase in the severity of GO in the group with permissive hypothyroidism, as measured by the number of patients requiring specific therapy for their eye disease [relative risk (RR) 2.3 with 95% CI: 1.2–4.6]. This association between development of hypothyroidism after RAI therapy and development or worsening of GO was also confirmed in a prospective manner by Kung et al. (39) in a cohort of 114 patients followed for 2 years. On the basis of these studies, it has became widely accepted that hypothyroidism after radioactive therapy for Graves' hyperthyroidism is a GO risk factor warranting active prevention of hypothyroidism in clinical practice and in research protocols (25,26,40,41).
TSHR antibodies
Stimulatory autoantibodies in GD activate TSHR on thyroid follicular cells, leading to thyroid hyperplasia and unregulated thyroid hormone production and secretion. The close clinical relationship between Graves' hyperthyroidism and GO has long suggested that immunoreactivity against TSHR present in both the thyroid and orbit underlies both conditions. The concept that orbital adipose tissue may contain TSHR evolved from early studies showing TSH binding to guinea pig retro-orbital adipose tissues, or to porcine orbital connective tissue membranes (42,43). A prerequisite for involvement of TSHR as an autoantigen in GO is that it be expressed in affected orbital tissues. Studies aimed at identifying TSHR in these tissues have been performed by several laboratories using many methodologies. Results of these studies are in general agreement and demonstrate that TSHR mRNA and protein are present in both GO and normal orbital adipose tissues and derivative cultures (10). Further, TSHR expression has been shown to be higher in GO orbital fat compared with normal orbital adipose tissues (44), and there exists a positive correlation between TSHR mRNA levels in individual GO orbital adipose tissue specimens and the patient's clinical activity score (CAS) (45).
Currently, two different methods of assessing antibodies directed against TSHR (TRAb) are being used clinically and in research studies. TBII (thyrotropin binding inhibitory immunoglobulin) quantifies the titer of patient's immunoglobulins that inhibit the binding of TSH to purified or recombinant TSHR. It thus measures both thyroid-stimulating antibodies (TSI) and thyroid-blocking antibodies targeting the receptor. The second method is a bioassay that can distinguish between TSHR-stimulating and -blocking antibodies through their effect on cyclic adenosine monophosphate (cAMP) production in a cell line stably transfected with the receptor; neither assay can identify neutral TSHR antibodies. While the physiologic relevance to thyroid function of TSHR-stimulating and -blocking antibodies is understood, how these antibodies, or even neutral TSHR antibodies, impact TSHR signaling in orbital or dermal fibroblasts is less clear. It may be that a subset or subsets of TSHR-directed antibodies, not yet specifically measureable and perhaps using pathways other than or in addition to adenylyl cyclase, are involved in the development of the various extrathyroidal manifestations of GD, including not only GO but also thyroid dermopathy and acropathy.
The association of TBII and TSI values with GO development was reported by Khoo et al. (46) in 100 consecutive patients with GD. In this series of nonsmokers, they found a prevalence of 20%, 36%, 52%, and 64% for GO in the first, second, third, and fourth quartiles of TSI, respectively. The OR of GO when TSI levels were above the median level was 3.6 (1.5–8.0). No relationship between TBII levels and GO was found in that group. Noh et al. (47) and Goh et al. (48) reported similar results with TSI being strongly associated with GO in GD patients compared with those having only clinical hyperthyroidism without ocular features. A study reported by Kung et al. (39) was at odds with these data, reporting that the development of new GO (and deterioration of preexistent GO) after RAI therapy did not correlate with TRAb titers. Initial studies to determine whether TRAb titers might be useful in the clinical evaluation of established GO were not encouraging (49–51). However, these studies used first-generation TBII assays or long-acting thyroid stimulator assays, now known to be quite insensitive. Subsequent studies measured TSI directly and found significant correlations with disease severity (52).
The concept of measurable GO disease activity, the CAS, was introduced by Mourits et al. (53) and evaluated in relation to TRAb by Gerding et al. (54). These investigators studied GD patients rendered euthyroid who had untreated GO of moderate severity. They found strong and direct correlations between both TBII levels and CAS and TSI levels and CAS (r = 0.54 for both). They also found similar associations between proptosis (as a severity marker) and levels of both types of antibodies. Later investigations focused on using TRAb levels to predict or assess response to anti-inflammatory therapy. Eckstein et al. (55) found that TRAb levels remained detectable in most nonresponders (93%) while they converted to undetectable in the majority of responders. Kahaly et al. (56) noted a similar decrease in TRAb after intravenous glucocorticoid therapy that correlated with a decrease in CAS. Thereafter, Eckstein et al. (57) followed 159 GO patients for 12–24 months, recording their TBII levels every 3 months. While accounting for other GO risk factors, they were able to predict the GO progression in a dichotomous fashion (mild or severe) in 50% of patients. It remains to be determined whether the presence of high TBII levels represents a risk for GO after RAI therapy.
Thyroxine and triiodothyronine levels
As discussed above, both persistent hyperthyroidism and hypothyroidism after treatment for GD appears to predispose to development or worsening of GO. In addition, studies have suggested that circulating triiodothyronine (T3) or thyroxine (T4) may also be associated with GO. Tallstedt et al. (34) performed a post hoc analysis of patients with pretreatment T3 values of ≥5 nmol/L and found the relative risk for GO development or worsening to be 12.7 (95% CI: 1.7–92.7) for patients whose hyperthyroidism was treated with thyroidectomy or antithyroid drugs and 5.8 (95% CI: 1.5–22.8) for patients treated with RAI, as compared with the patients having T3 values < 5 nmol/L. The same group later confirmed higher T3 values in patients in whom GO developed or deteriorated after RAI therapy, although a significant overlap was found between the groups (means of 4.4 with standard deviation 1.6 vs. 4.8 with standard deviation of 1.6). Another trial in an Asian population failed to identify any relationship between T3 or T4 levels and GO (39). A recent prospective study examining the outcome of the three modes of therapy for GD with regard to GO development (25) found that higher free T4 levels, but not elevated free T3 levels, minimally increased the risk for GO with an OR of 1.03 (95% CI: 1.01–1.04). However, the patient data and T4 values studied were collected from different medical centers that used various T4 assays and reference range intervals. A recent retrospective study by Vannucchi et al. (58) examined GO development, deterioration, or reactivation after RAI in a cohort in which prophylactic steroids were used for patients considered at risk for GO progression. They found that T3 levels were not associated with GO outcomes (development, reactivation of inactive disease, or deterioration of active disease) and similarly noted that smoking, TRAb levels, and duration of hypothyroidism after radioiodine bore no relation to GO outcome. However, these results are difficult to interpret owing to methodological issues, including the retrospective nature of the study, the heterogeneity of patients enrolled, and the various steroid doses and regimens used. Taken together, the lack of consistency in results along with the low OR reported in these studies suggest that T4 and T3 levels do not have clinical utility as predictors of GO progression.
In conclusion, while genetic factors involved in the development of GD and GO suggest avenues for research concerning potential therapeutic targets, the biochemical and environmental factors discussed in this review are of immediate clinical applicability. Smoking cessation should be encouraged and aided in all patients with GD, especially those having clinical evidence of GO. In addition, euthyroidism should be achieved expeditiously in hyperthyroid patients and hypothyroidism should be avoided. TRAb levels are useful in GO risk assessment and may be helpful in decisions regarding whether to use prophylactic glucocorticoids when radioiodine is employed for treatment of hyperthyroidism in patients with GO. Finally, mechanical factors have not to date received adequate attention and are likely to be more widely studied using detailed imaging techniques now available. The puzzling scenarios of euthyroid or clinically unilateral GO, the large number of nonsmoking GO patients, and the occasional development of GO years after thyroid dysfunction has been treated all underline the multifactorial etiology of this disorder in which no single factor determines the clinical outcome.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Parry CH. Collections from the Unpublished Papers of the Late Caleb Hillier Parry. Underwood; London: 1825. Enlargement of the thyroid gland in connection with enlargement or palpitation of the heart; pp. 111–129. [Google Scholar]
- 2.Burch HB. Wartofsky L. Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747–793. doi: 10.1210/edrv-14-6-747. [DOI] [PubMed] [Google Scholar]
- 3.Kendler DL. Lippa J. Rootman J. The initial clinical characteristics of Graves' orbitopathy vary with age and sex. Arch Ophthalmol. 1993;111:197–201. doi: 10.1001/archopht.1993.01090020051022. [DOI] [PubMed] [Google Scholar]
- 4.Lim SL. Lim AK. Mumtaz M. Hussein E. Wan Bebakar WM. Khir AS. Prevalence, risk factors, and clinical features of thyroid-associated ophthalmopathy in multiethnic Malaysian patients with Graves' disease. Thyroid. 2008;18:1297–1301. doi: 10.1089/thy.2008.0044. [DOI] [PubMed] [Google Scholar]
- 5.Tellez M. Cooper J. Edmonds C. Graves' ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf) 1992;36:291–294. doi: 10.1111/j.1365-2265.1992.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CC. Kau HC. Kao SC. Hsu WM. Exophthalmos of patients with Graves' disease in Chinese of Taiwan. Eye. 2006;20:569–573. doi: 10.1038/sj.eye.6701925. [DOI] [PubMed] [Google Scholar]
- 7.Wiersinga WM. Bartalena L. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002;12:855–860. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]
- 8.Salvi M. Zhang ZG. Haegert D. Woo M. Liberman A. Cadarso L. Wall JR. Patients with endocrine ophthalmopathy not associated with overt thyroid disease have multiple thyroid immunological abnormalities. J Clin Endocrinol Metab. 1990;70:89–94. doi: 10.1210/jcem-70-1-89. [DOI] [PubMed] [Google Scholar]
- 9.Wiersinga WM. Smit T. van der Gaag R. Koornneef L. Temporal relationship between onset of Graves' ophthalmopathy and onset of thyroidal Graves' disease. J Endocrinol Invest. 1988;11:615–619. doi: 10.1007/BF03350193. [DOI] [PubMed] [Google Scholar]
- 10.Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson EM. Tomer Y. The genetic basis of thyroid autoimmunity. Thyroid. 2007;17:949–961. doi: 10.1089/thy.2007.0153. [DOI] [PubMed] [Google Scholar]
- 12.Huber AK. Jacobson EM. Jazdzewski K. Concepcion ES. Tomer Y. Interleukin (IL)-23 receptor is a major susceptibility gene for Graves' ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. J Clin Endocrinol Metab. 2008;93:1077–1081. doi: 10.1210/jc.2007-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaidya B. Imrie H. Perros P. Dickinson J. McCarthy MI. Kendall-Taylor P. Pearce SH. Cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphism confers susceptibility to thyroid associated orbitopathy. Lancet. 1999;354:743–744. doi: 10.1016/S0140-6736(99)01465-8. [DOI] [PubMed] [Google Scholar]
- 14.Allahabadia A. Heward JM. Nithiyananthan R. Gibson SM. Reuser TT. Dodson PM. Franklyn JA. Gough SC. MHC class II region, CTLA4 gene, and ophthalmopathy in patients with Graves' disease. Lancet. 2001;358:984–985. doi: 10.1016/s0140-6736(01)06125-6. [DOI] [PubMed] [Google Scholar]
- 15.Daroszewski J. Pawlak E. Karabon L. Frydecka I. Jonkisz A. Slowik M. Bolanowski M. Soluble CTLA-4 receptor an immunological marker of Graves' disease and severity of ophthalmopathy is associated with CTLA-4 Jo31 and CT60 gene polymorphisms. Eur J Endocrinol/Eur Fed Endocr Soc. 2009;161:787–793. doi: 10.1530/EJE-09-0600. [DOI] [PubMed] [Google Scholar]
- 16.Chong KK. Chiang SW. Wong GW. Tam PO. Ng TK. Hu YJ. Yam GH. Lam DS. Pang CP. Association of CTLA-4 and IL-13 gene polymorphisms with Graves' disease and ophthalmopathy in Chinese children. Invest Ophthalmol Vis Sci. 2008;49:2409–2415. doi: 10.1167/iovs.07-1433. [DOI] [PubMed] [Google Scholar]
- 17.Pearce SH. Merriman TR. Genetic progress towards the molecular basis of autoimmunity. Trends Mol Med. 2006;12:90–98. doi: 10.1016/j.molmed.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Khalilzadeh O. Anvari M. Esteghamati A. Mahmoudi M. Tahvildari M. Rashidi A. Khosravi F. Amirzargar A. Graves' ophthalmopathy and gene polymorphisms in interleukin-1alpha, interleukin-1beta, interleukin-1 receptor and interleukin-1 receptor antagonist. Clin Exp Ophthalmol. 2009;37:614–619. doi: 10.1111/j.1442-9071.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 19.Alevizaki M. Mantzou E. Cimponeriu A. Saltiki K. Philippou G. Wiersinga W. The Pro12Ala PPARgamma gene polymorphism: possible modifier of the activity and severity of thyroid-associated orbitopathy (TAO) Clin Endocrinol (Oxf) 2009;70:464–468. doi: 10.1111/j.1365-2265.2008.03343.x. [DOI] [PubMed] [Google Scholar]
- 20.Brand OJ. Gough SC. Genetics of thyroid autoimmunity and the role of the TSHR. Mol Cell Endocrinol. 2010;322:135–143. doi: 10.1016/j.mce.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Baujat B. Krastinova D. Bach CA. Coquille F. Chabolle F. 2006 Orbital morphology in exophthalmos, exorbitism. Plast Reconstr Surg. 117:542–550. doi: 10.1097/01.prs.0000200773.00268.56. discussion 551–542. [DOI] [PubMed] [Google Scholar]
- 22.Enzmann DR. Donaldson SS. Kriss JP. Appearance of Graves' disease on orbital computed tomography. J Comput Assist Tomogr. 1979;3:815–819. [PubMed] [Google Scholar]
- 23.Wiersinga WM. Smit T. van der Gaag R. Mourits M. Koornneef L. Clinical presentation of Graves' ophthalmopathy. Ophthalmic Res. 1989;21:73–82. doi: 10.1159/000266782. [DOI] [PubMed] [Google Scholar]
- 24.Rapoport B. Alsabeh R. Aftergood D. McLachlan SM. Elephantiasic pretibial myxedema: insight into and a hypothesis regarding the pathogenesis of the extrathyroidal manifestations of Graves' disease. Thyroid. 2000;10:685–692. doi: 10.1089/10507250050137761. [DOI] [PubMed] [Google Scholar]
- 25.Traisk F. Tallstedt L. Abraham-Nordling M. Andersson T. Berg G. Calissendorff J. Hallengren B. Hedner P. Lantz M. Nystrom E. Ponjavic V. Taube A. Torring O. Wallin G. Asman P. Lundell G. Thyroid-associated ophthalmopathy after treatment for Graves' hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700–3707. doi: 10.1210/jc.2009-0747. [DOI] [PubMed] [Google Scholar]
- 26.Bartalena L. Marcocci C. Bogazzi F. Manetti L. Tanda ML. Dell'Unto E. Bruno-Bossio G. Nardi M. Bartolomei MP. Lepri A. Rossi G. Martino E. Pinchera A. Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med. 1998;338:73–78. doi: 10.1056/NEJM199801083380201. [see comment] [DOI] [PubMed] [Google Scholar]
- 27.Krassas GE. Segni M. Wiersinga WM. Childhood Graves' ophthalmopathy: results of a European questionnaire study. Eur J Endocrinology/Eur Fed Endocr Soc. 2005;153:515–521. doi: 10.1530/eje.1.01991. [DOI] [PubMed] [Google Scholar]
- 28.Fiore MC. Jaen CR. A clinical blueprint to accelerate the elimination of tobacco use. JAMA. 2008;299:2083–2085. doi: 10.1001/jama.299.17.2083. [DOI] [PubMed] [Google Scholar]
- 29.Bang SY. Lee KH. Cho SK. Lee HS. Lee KW. Bae SC. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of rheumatoid factor or anti-cyclic citrullinated peptide antibody status. Arthritis Rheum. 2010;62:369–377. doi: 10.1002/art.27272. [DOI] [PubMed] [Google Scholar]
- 30.van der Heide F. Dijkstra A. Weersma RK. Albersnagel FA. van der Logt EM. Faber KN. Sluiter WJ. Kleibeuker JH. Dijkstra G. Effects of active and passive smoking on disease course of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2009;15:1199–1207. doi: 10.1002/ibd.20884. [DOI] [PubMed] [Google Scholar]
- 31.Salvi M. Pedrazzoni M. Girasole G. Giuliani N. Minelli R. Wall JR. Roti E. Serum concentrations of proinflammatory cytokines in Graves' disease: effect of treatment, thyroid function, ophthalmopathy and cigarette smoking. Eur J Endocrinol/Eur Fed Endocr Soc. 2000;143:197–202. doi: 10.1530/eje.0.1430197. [DOI] [PubMed] [Google Scholar]
- 32.Cawood TJ. Moriarty P. O'Farrelly C. O'Shea D. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92:59–64. doi: 10.1210/jc.2006-1824. [DOI] [PubMed] [Google Scholar]
- 33.Metcalfe RA. Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: possible role in thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf) 1994;40:67–72. doi: 10.1111/j.1365-2265.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 34.Tallstedt L. Lundell G. Torring O. Wallin G. Ljunggren JG. Blomgren H. Taube A. Sjoberg HE. Saaf M. Thoren M. Alinder I. Farnebo LO. Hamberger B. Hall P. Tengroth B. Lowhagen T. Norberg R. Curstedt T. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. N Engl J Med. 1992;326:1733–1738. doi: 10.1056/NEJM199206253262603. [DOI] [PubMed] [Google Scholar]
- 35.DeGroot LJ. Mangklabruks A. McCormick M. Comparison of RA 131I treatment protocols for Graves' disease. J Endocrinol Invest. 1990;13:111–118. doi: 10.1007/BF03349519. [DOI] [PubMed] [Google Scholar]
- 36.Prummel MF. Wiersinga WM. Mourits MP. Koornneef L. Berghout A. van der Gaag R. Effect of abnormal thyroid function on the severity of Graves' ophthalmopathy. Arch Intern Med. 1990;150:1098–1101. [see comment] [PubMed] [Google Scholar]
- 37.Almqvist S. Algvere P. Hypothyroidism in progressive ophthalmopathy of Graves' disease. Acta Ophthalmol (Copenh) 1972;50:761–770. doi: 10.1111/j.1755-3768.1972.tb06615.x. [DOI] [PubMed] [Google Scholar]
- 38.Tallstedt L. Lundell G. Blomgren H. Bring J. Does early administration of thyroxine reduce the development of Graves' ophthalmopathy after radioiodine treatment? Eur J Endocrinol/Eur Fed Endocr Soc. 1994;130:494–497. doi: 10.1530/eje.0.1300494. [DOI] [PubMed] [Google Scholar]
- 39.Kung AW. Yau CC. Cheng A. The incidence of ophthalmopathy after radioiodine therapy for Graves' disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994;79:542–546. doi: 10.1210/jcem.79.2.7913934. [DOI] [PubMed] [Google Scholar]
- 40.Wiersinga WM. Preventing Graves' ophthalmopathy. N Engl J Med. 1998;338:121–122. doi: 10.1056/NEJM199801083380209. [DOI] [PubMed] [Google Scholar]
- 41.Perros P. Kendall-Taylor P. Neoh C. Frewin S. Dickinson J. A prospective study of the effects of radioiodine therapy for hyperthyroidism in patients with minimally active graves' ophthalmopathy. J Clin Endocrinol Metab. 2005;90:5321–5323. doi: 10.1210/jc.2005-0507. [DOI] [PubMed] [Google Scholar]
- 42.Davies TF. Teng CS. McLachlan SM. Smith BR. Hall R. Thyrotropin receptors in adipose tissue, retro-orbital tissue and lymphocytes. Mol Cell Endocrinol. 1978;9:303–310. doi: 10.1016/0303-7207(78)90072-2. [DOI] [PubMed] [Google Scholar]
- 43.Perros P. Kendall-Taylor P. Demonstration of thyrotropin binding sites in orbital connective tissue: possible role in the pathogenesis of thyroid-associated ophthalmopathy. J Endocrinol Invest. 1994;17:163–170. doi: 10.1007/BF03347708. [DOI] [PubMed] [Google Scholar]
- 44.Bahn RS. Dutton CM. Natt N. Joba W. Spitzweg C. Heufelder AE. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 45.Wakelkamp IM. Bakker O. Baldeschi L. Wiersinga WM. Prummel MF. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves' ophthalmopathy patients. Clin Endocrinol (Oxf) 2003;58:280–287. doi: 10.1046/j.1365-2265.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 46.Khoo DH. Ho SC. Seah LL. Fong KS. Tai ES. Chee SP. Eng PH. Aw SE. Fok AC. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves' disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid. 1999;9:1175–1180. doi: 10.1089/thy.1999.9.1175. [DOI] [PubMed] [Google Scholar]
- 47.Noh JY. Hamada N. Inoue Y. Abe Y. Ito K. Thyroid-stimulating antibody is related to graves' ophthalmopathy, but thyrotropin-binding inhibitor immunoglobulin is related to hyperthyroidism in patients with Graves' disease. Thyroid. 2000;10:809–813. doi: 10.1089/thy.2000.10.809. [DOI] [PubMed] [Google Scholar]
- 48.Goh SY. Ho SC. Seah LL. Fong KS. Khoo DH. Thyroid autoantibody profiles in ophthalmic dominant and thyroid dominant Graves' disease differ and suggest ophthalmopathy is a multiantigenic disease. Clin Endocrinol (Oxf) 2004;60:600–607. doi: 10.1111/j.1365-2265.2004.02033.x. [DOI] [PubMed] [Google Scholar]
- 49.Feldt-Rasmussen U. Kemp A. Bech K. Madsen SN. Date J. Serum thyroglobulin, its autoantibody and thyroid stimulating antibodies in the endocrine exophthalmos. Acta Endocrinol (Copenh) 1981;96:192–198. doi: 10.1530/acta.0.0960192. [DOI] [PubMed] [Google Scholar]
- 50.Teng CS. Smith BR. Clayton B. Evered DC. Clark F. Hall R. Thyroid-stimulating immunoglobulins in ophthalmic Graves' disease. Clin Endocrinol (Oxf) 1977;6:207–211. doi: 10.1111/j.1365-2265.1977.tb03316.x. [DOI] [PubMed] [Google Scholar]
- 51.Wall JR. Strakosch CR. Fang SL. Ingbar SH. Braverman LE. Thyroid binding antibodies and other immunological abnormalities in patients with Graves' ophthalmopathy: effect of treatment with cyclophosphamide. Clin Endocrinol (Oxf) 1979;10:79–91. doi: 10.1111/j.1365-2265.1979.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 52.Morris JC., 3rd Hay ID. Nelson RE. Jiang NS. Clinical utility of thyrotropin-receptor antibody assays: comparison of radioreceptor and bioassay methods. Mayo Clinic Proc. 1988;63:707–717. doi: 10.1016/s0025-6196(12)65533-5. [DOI] [PubMed] [Google Scholar]
- 53.Mourits MP. Koornneef L. Wiersinga WM. Prummel MF. Berghout A. van der Gaag R. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerding MN. van der Meer JW. Broenink M. Bakker O. Wiersinga WM. Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2000;52:267–271. doi: 10.1046/j.1365-2265.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 55.Eckstein AK. Plicht M. Lax H. Hirche H. Quadbeck B. Mann K. Steuhl KP. Esser J. Morgenthaler NG. Clinical results of anti-inflammatory therapy in Graves' ophthalmopathy and association with thyroidal autoantibodies. Clin Endocrinol (Oxf) 2004;61:612–618. doi: 10.1111/j.1365-2265.2004.02143.x. [DOI] [PubMed] [Google Scholar]
- 56.Kahaly GJ. Pitz S. Hommel G. Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves' orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–5240. doi: 10.1210/jc.2005-0148. [see comment] [DOI] [PubMed] [Google Scholar]
- 57.Eckstein AK. Plicht M. Lax H. Neuhauser M. Mann K. Lederbogen S. Heckmann C. Esser J. Morgenthaler NG. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–3470. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- 58.Vannucchi G. Campi I. Covelli D. Dazzi D. Curro N. Simonetta S. Ratiglia R. Beck-Peccoz P. Salvi M. Graves' orbitopathy activation after radioactive iodine therapy with and without steroid prophylaxis. J Clin Endocrinol Metab. 2009;94:3381–3386. doi: 10.1210/jc.2009-0506. [DOI] [PubMed] [Google Scholar]