Abstract
Mitochondria play a fundamental role in the maintenance of normal structure, function, and survival of tissues. There is considerable evidence for mitochondrial dysfunction in association with metabolic diseases including insulin resistance, obesity, diabetes, and the cardiorenal metabolic syndrome. The phenomenon of reactive oxygen species (ROS)-induced ROS release through interactions between cytosolic and mitochondrial oxidative stress contributes to a vicious cycle of enhanced oxidative stress and mitochondrial dysfunction. Activation of the cytosolic and mitochondrial NADPH oxidase system, impairment of the mitochondrial electron transport, activation of p66shc pathway-targeting mitochondria, endoplasmic reticular stress, and activation of the mammalian target of the rapamycin-S6 kinase pathway underlie dysregulation of mitochondrial dynamics and promote mitochondrial oxidative stress. These processes are further modulated by acetyltransferases including sirtuin 1 and sirtuin 3, the former regulating nuclear acetylation and the latter regulating mitochondrial acetylation. The regulation of mitochondrial functions by microRNAs forms an additional layer of molecular control of mitochondrial oxidative stress. Alcohol further exacerbates mitochondrial oxidative stress induced by overnutrition and promotes the development of metabolic diseases.
Key Words: Cardiorenal syndrome, Overnutrition, Mitochondria, NADPH oxidase, Angiotensin II, MicroRNA, Alcoholic cardiomyopathy
Introduction
Mitochondria are critical subcellular components that play a crucial role in energy generation, intermediary metabolism, and cell death. Accordingly, mitochondrial dysfunction is an important mechanism underlying human disease states including cardiac diseases, diabetes, and the cardiorenal metabolic syndrome (CRS) [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. Although genetic disease is a primary cause for mitochondrial dysfunction, oxidative stress is linked to mitochondrial dysfunction either as a cause or as a result in pathological states. Mitochondria are major sources of reactive oxygen species (ROS) such as superoxide within the cell. Yet, they are very susceptible to oxidative damage, thereby contributing to mitochondrial dysfunction in a range of diseases. This review summarizes data that link oxidative stress to mitochondrial dysfunction in the pathogenesis of organ damage and dysfunction in the CRS.
Maintenance of Normal Mitochondrial Structure and Function
Mitochondria normally play a fundamental role in the maintenance of normal structure, function, and survival of various tissues. Aerobic organisms consume oxygen to produce energy from nutrients, and energy production, mostly in the form of adenosine triphosphate (ATP), is controlled by mitochondria that link oxidative respiration with the metabolism of nutrients (fig. 1) [1, 2, 3, 4, 5, 6]. In particular, almost all (>95%) of ATP produced in the heart comes from mitochondrial oxidative phosphorylation (OXPHOS), with the reminder derived from glycolysis. Mitochondria are compartmentalized by outer and inner membranes, and the mitochondrial respiratory chain is located in the inner membrane. The generation of ATP requires two major steps: the oxidation of nicotinamide adenine dinucleotide, its reduced form (NADH), or flavine adenine dinucleotide, its hydroquinone form (FADH2), and the phosphorylation of ADP to form ATP (OXPHOS). These two reactions are coupled in mitochondria, and OXPHOS is an efficient and energy-conserving mechanism of producing energy. NADH or FADH2 are generated during glucose metabolism via glycolysis (tricarboxylic acid cycle) or β-oxidation of fatty acids. NADH or FADH2 are oxidized to NAD+ or FAD while protons are pumped to the intermitochondrial membrane space through respiratory complexes I, III, and IV. Electrons from NADH or FADH2 are then transferred through a series of respiratory chain complexes to O2, which finally generates H2O. A proton gradient across the membrane is the driving force of F0F1-ATPase (ATP synthase) to produce ATP from ADP. ATP is transported to the cytosol by exchanging ADP through an adenine nucleotide translocator and is key for various biological events that require energy. On the other hand, mitochondria generate heat by a mechanism called ‘proton leak’. Proton leak from the intermembrane space to the matrix (uncoupling) reduces proton-motive force and generates heat instead of ATP. Uncoupling proteins (UCPs) play a major role in reducing the proton gradient. UCP1 is expressed almost exclusively in brown adipose tissue, UCP2 is ubiquitously expressed, and UCP3 is expressed in skeletal muscle. UCP1, which comprises up to 10% of membrane protein, regulates adaptive thermogenesis, whereas UCP2 and UCP3 do not appear to play a major role in thermogenesis. It was reported that mice with genetic ablation of UCP2 and UCP3 display a normal response to cold, normal basal proton conductance, and normal body weight [6]. In this regard, overexpression of UCP2 or UCP3 lowers ROS production, stimulates the metabolic rate, and protects against weight gain and associated insulin resistance. Collectively, these results suggest that UCPs play an important role in mitochondrial function by regulating both heat and ROS generation.
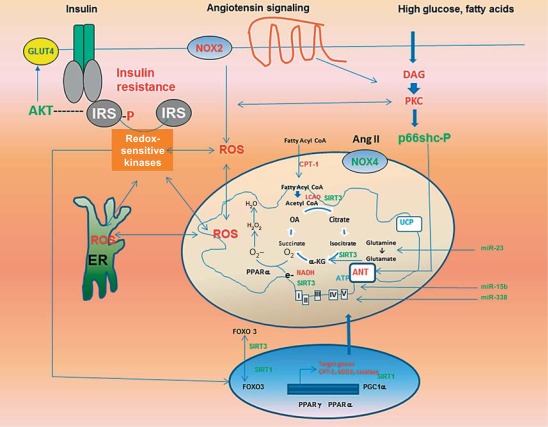
Fig. 1.
Sources and consequences of mitochondrial oxidative stress and its relationship to insulin resistance in the CRS. ROS-induced ROS release results from several pathways, including hyperglycemia and lipid-induced activation of PKC, Ang II-mediated activation of cytosolic (NOX2) and mitochondrial (NOX) NADPH oxidase, activation of p66shc, ER stress, and dysregulation of nuclear and mitochondrial transcriptional response. The activation of redox-sensitive kinases induces insulin resistance through increased phosphorylation of serine residues in IRS proteins, which in turn suppresses insulin metabolic signaling.
Mitochondrial Dysfunction in the CRS and Diabetes
There is considerable evidence for mitochondrial dysfunction in association with insulin resistance, obesity, and diabetes [1, 6]. This is especially relevant considering mitochondrial dysfunction compromises glucose-stimulated pancreatic insulin secretion as well as insulin-stimulated skeletal muscle glucose utilization [6]. Mitochondrial dysfunction has been shown to compromise insulin signaling through serine phosphorylation of insulin receptor substrate (IRS)-1 [32]. The contribution of mitochondrial dysfunction to impairments in insulin metabolic signaling is also suggested by gene array analysis showing that reductions in the expression of genes regulating mitochondrial ATP production are associated with insulin resistance [1] and type 2 diabetes mellitus [1, 2, 3, 4, 5, 6]. Moreover, reductions in the oxidative capacity of the mitochondrial electron transport chain are manifested in obese, insulin-resistant persons as well as diabetic patients [1, 6]. Genetic and environmental factors, oxidative stress, and alterations in mitochondrial biogenesis can adversely affect mitochondrial function, leading to insulin resistance and various pathological conditions such as the CRS and type 2 diabetes [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35].
Mitochondrial proteins are encoded by both nuclear and mitochondrial genes. Mitochondrial genes encode 13 protein subunits of the OXPHOS complex as well as mitochondrial-specific ribosomal and transfer (t) RNAs [1, 6, 26]. The oxidative capacity of mitochondria is determined by the expression level of OXPHOS subunits and by the number and size of mitochondria [6]. Because mitochondrial dysfunction and gene expression of mitochondrial OXPHOS genes are related to insulin resistance, mutations in mitochondrial genes caused by metabolic and/or oxidative stress conditions may be one of the mechanisms underlying insulin resistance and other features of the CRS.
The mitochondrial genome appears to be more susceptible to various mutagenic stressors because mitochondrial genes are more proximal to the ROS source and are not protected by histones [26, 27]. In this regard, the mitochondrial genome constitutes only coding sequences, whereas nuclear DNA contains non-coding sequences. Indeed, a naturally occurring thymidine-to-cytidine mutation in the mitochondrial tRNA gene is associated with hypertension, hypercholesterolemia, and other components of the CRS [26]. Another mutation, A3243G, on mitochondrial DNA that encodes tRNA (LeuUUR) causes impaired insulin secretion [27]. In addition, patients with defects in acyl-coenzyme A (CoA) dehydrogenase have phenotypes of cardiomyopathy and liver dysfunction [28]. Polymorphisms in the promoter of UCP2 are associated with a decreased incidence of obesity, reduced insulin secretion, and a high prevalence of diabetes [29, 30]. Alterations of nuclear genes encoding mitochondrial proteins are also associated with insulin resistance [31]. Thus, genetic factors that are inherited through nuclear genes that code for mitochondrial proteins or mitochondrial genes can influence the pathogenesis of the CRS through impairment of the mitochondrial function [6].
There have been reports of alterations in morphology and numbers of mitochondria in skeletal muscle, heart, and the liver in insulin-resistant, obese, and/or diabetic animals and patients [1, 6, 32, 34, 51]. The number and size of mitochondria are related to the mitochondrial oxidative capacity [1, 6]. In this regard, decreased mitochondrial oxidative capacity accompanies the reduction in expression of mitochondrial proteins encoded by both the mitochondrial genome (cytochrome c oxidase 1) and nucleus (succinate dehydrogenase and pyruvate dehydrogenase) [6, 32]. The molecular mechanism of mitochondrial biogenesis is driven, in part, through peroxisome proliferator-activated receptor (PPAR) co-activator (PGC)-1α. PGC-1α has been known as an inducible integrator of transcriptional circuits controlling mitochondrial biogenesis and function in a variety of tissue and cell types. The expression of PGC-1αis increased on cellular ATP demand, including exercise, cold exposure, and fasting [35, 36, 37, 38]. PGC-1α is a co-activator of nuclear transcription factors, including nuclear respiratory factor (NRF)-1, PPAR-γ and PPAR-α [38, 39]. NRF-1, in turn, regulates the expression of many mitochondrial genes, including OXPHOS genes and mitochondrial transcription factor A, that are crucial for mitochondrial gene expression and replication of the mitochondrial genome [1, 6]. Expression of PGC-1α is decreased in insulin-resistant and diabetic humans, and NRF-1 expression is reduced in diabetic persons [40]. The reduction of PGC-1α expression is age dependent [41], and PGC-1α null mice exhibit serious defects in contractility in both skeletal and cardiac muscles [39, 40, 41, 42, 43]. Expression of PGC-1α is also regulated by the endothelial nitric oxide (NO) synthase (eNOS)/NO/cGMP. The activation of eNOS plays an important role in mitochondrial biogenesis [1, 6, 44, 45, 46, 47]. In fact, eNOS-deficient mice are insulin resistant and hypertensive and have defects in fatty acid metabolism and fewer mitochondria [45, 46, 47]. Furthermore, exogenous NO or cGMP increases mitochondrial biogenesis [47]. Another important factor that regulates mitochondrial biogenesis is AMP-activated protein kinase (AMPK) [48, 49]. Pharmacological drugs and other factors that activate AMPK promote mitochondrial biogenesis [6]. DNA microarray studies have shown that expression of PGC-1α, with regard to mitochondrial biogenesis, may be responsible for metabolic disorders, including the CRS and diabetes [40, 50]. These data suggest that decreased mitochondrial function is mainly attributable to the reduced number of mitochondria. Others have reported that subsarcolemmal mitochondrial electron transport activity is lower in obese and diabetic persons [33, 34]. In either case, diminished mitochondrial electron transport activity is partly attributable to the reduced mitochondrial content, but the decrement in mitochondrial function is greater than can be solely explained by mitochondrial content. Alterations in mitochondrial number and/or function that lead to decreased capacity to oxidize fat may be the underlying cause of lipid accumulation in skeletal muscle, heart, and liver which are characteristic of impaired insulin metabolic signaling and other functional abnormalities of the CRS [1, 6, 22, 23, 24]. Despite the overwhelming evidence depicting altered expression and activity for the PGC-1α gene regulatory circuit under pathological conditions such as cardiac hypertrophy and ischemic insult, the jury is still out with regard to whether such changes in PGC-1α are a cause or a consequence of these pathological changes.
Role of Oxidative Stress in Impaired Mitochondrial Respiratory Functions
ROS-Induced ROS Release
Cellular oxidative stress involves both cytosolic and mitochondrial oxidative stress. Moreover, cytosolic oxidative stress also contributes to mitochondrial dysfunction, mitochondrial oxidative stress and vice versa. This phenomenon is described as ROS-induced ROS release and contributes to a vicious cycle of enhanced oxidative stress and mitochondrial dysfunction [52]. The magnitude of oxidative stress in cells or tissues is the result of a delicate balance of ROS and antioxidant capacity. ROS are generated either through mitochondrial respiration or through the action of several oxidases including NADPH oxidase, xanthine oxidase, cyclooxygenases, and lipoxygenase [53, 54, 55]. Although physiological levels of ROS are required for normal cellular function, overproduction of ROS will result in deleterious effects on cellular function. The major form of ROS is superoxide, and the accumulation of superoxide is caused by activation of NADPH oxidase and/or impaired mitochondrial electron transport. Superoxide is converted into hydrogen peroxide by superoxide dismutase (SOD) either in the cytosol (SOD1) or mitochondria (SOD2). Superoxide is also converted to another ROS peroxynitrite (reactive nitrogen species). Peroxynitrite reacts with proteins, lipids, and nucleic acids to form 3-nitrotyrosine which, in turn, is an indirect index of peroxynitrite formation and oxidative stress in tissues [53, 54, 55, 56]. In this context, eNOS may compete with SOD, thereby reducing the catalysis of superoxide and thus superoxide accumulation [57]. The toxicity of hydrogen peroxide is suppressed by its conversion to oxygen and water by catalase or glutathione peroxidase. The effectiveness of glutathione peroxidase is, in turn, dependent on the availability of reduced glutathione, and the overall function of the glutathione system is further linked to vitamin E, vitamin C, and lipoic acid interaction [58, 59]. Therefore, oxidative stress is imposed on tissue when excessive amounts of ROS surpass different antioxidant mechanisms operative in the cell.
Mitochondrial Oxidative Stress
Mitochondria are a major intracellular source of oxidative stress [60]. Mitochondria-derived ROS are generated during OXPHOS occurring across the respiratory chain in the mitochondria. Although mitochondrial complexes I (NADH dehydrogenase) and III (ubiquinone cytochrome c oxidoreductase) are the major sites contributing to the production of ROS, complex II (succinate-ubiquinone oxidoreductase) and complex IV (cytochrome c oxidase) may also contribute to increased ROS production [60, 61]. Mitochondrial ROS excess can be caused either by impaired activity of mitochondrial respiratory chain components or mitochondrial DNA mutations [62, 63]. Mitochondrial oxidative stress is also increased by compromised antioxidant function contributed by manganese (Mn)-SOD, catalase, and the glutathione system [64]. Severe dilated cardiomyopathy is seen in mice with complete deletion of mitochondrial Mn-SOD [65], whereas improved cardiac function and prolonged life span are seen in mice with mitochondrial targeted overexpression of catalase [66]. Moreover, the activity of Mn-SOD is reduced in heart failure [67].
A major contributory factor linked to this increased risk of cardiovascular disease in the metabolic CRS and diabetes is hyperglycemia-induced mitochondrial oxidative stress [68] which can induce cellular injury and dysfunction via several pathways: through the increased flux of glucose and other sugars through the polyol pathway, increased intracellular formation of advanced glycation end products (AGEs), increased expression of the receptor for AGEs and its activating ligands, activation of protein kinase C (PKC) isoforms, and overactivation of the hexosamine pathway. In the diabetic heart, overexpression of Mn-SOD or catalase protects cardiac mitochondria from oxidative damage, improves respiration, and normalizes mass in diabetic mitochondria [69]. Mn-SOD also prevents some of the morphological abnormalities that develop in diabetic hearts and normalizes contractility in diabetic cardiomyocytes [70].
Role of NADPH Oxidase: NOX2 and NOX4
In the cardiomyocyte, an important source of ROS is the one-electron reduction of O2 to superoxide by NADPH oxidase, using NADPH as the electron donor [71, 72]. The NADPH oxidase is recognized as an important enzymatic system responsible for ROS production in diseases such as hypertension, atherosclerosis, and both type 1 and type 2 diabetes. Uncoupled eNOS is the predominant source of superoxide following the initial activation of NOX, whereas NOX remains active in the cardiovascular system [73]. Importantly, eNOS uncoupling has been shown to occur in patients with diabetes, contributing to cardiovascular complications [74]. NOX may serve as an upstream signaling molecule in mediating diabetic uncoupling of eNOS in response to angiotensin II (Ang II) [73]. The heterodimeric structure of NADPH oxidase comprises a catalytic subunit, NOX, which has five known isoforms (NOX1–5). These differ in their tissue distribution and kinetics of ROS formation [74]. NOX2-containing NADPH oxidase is mainly localized to plasmalemma and produces superoxide in the extracellular space or within the cytosol. The NOX4-containing oxidase is located in endosomes, focal adhesions, and nuclei and generates superoxide in the intracellular space. Therefore, the subcellular localization of NOX permits the site-specific release of superoxide though distinct regulatory mechanisms, thus making a significant contribution to ROS production, especially in chronic pathologic states [75]. NOX2 is expressed in cardiomyocytes and is often up-regulated in states of oxidative stress. [76]. Ang II is a potent activator of NOX2/NADPH oxidase in the heart and can play an important role in mediating oxidative stress in cardiac pathology [77, 78, 79]. Recent studies have shown the localization of the NOX4 subunit of NADPH oxidase in the mitochondria and its up-regulation by Ang II and oxidative stress [80]. Increased expression of NOX4 in the heart also resulted in increased mitochondrial oxidative stress [81]. The cysteine residues in NADH dehydrogenase flavoprotein I, a component of complex I, and in adenine nucleotide transporter 1 (ANT1), a key component of the mitochondrial permeability transition pore (MPTP) complex, together with aconitase-2, an established redox-sensitive protein, are highly oxidized, and their function is inhibited in transgenic mice with cardiac-specific overexpression of NOX4. These data suggest that these factors are directly modulated by O2− generated by NOX4 [81, 82]. However, using different animal models, a protective role for NOX4 in cardiac hypertrophy has been proposed [83]. Similarly, ROS produced by NOX4 has been identified as hydrogen peroxide (H2O2) in several studies [84].
p66Shc and Mitochondrial Oxidative Stress
The role of p66Shc the 66-kDa isoform of the mammal ShcA gene, in mitochondrial oxidative stress is an area of active investigation. The significance of p66Shc comes from the finding that genetic deletion of p66Shc in mice confers resistance to oxidative damage and prolongs life span [85]. Although initial studies have implicated its role as a docking adaptor protein for mitogenic signals, recent studies have shown that p66shc acts as a redox enzyme contributing to mitochondrial ROS [86]. When the enzyme becomes phosphorylated on serine 36, it gets translocated into the mitochondrial intermembrane space. In the mitochondria, it catalyzes the production of H2O2, contributing to mitochondrial permeability transition. One of the consequences of mitochondrial permeability transition is caspase-mediated apoptotic cell death. The expression of p66Shc is increased in diabetic patients, and this increase was correlated to glycemic control and changes in the markers of oxidative stress [87]. p66Shc−/− mice are protected from the development of certain diabetes complications, such as nephropathy, endothelial dysfunction, and cardiomyopathy [88, 89, 90], thereby favoring the role of p66Shc in mitochondrial stress associated with diabetes mellitus.
Role of PPAR-α and Fatty Acid Metabolism
The normal heart derives most of its energy from fatty acid metabolism, with only about 30% of its energy coming from glucose oxidation. During insulin resistance and diabetes, the rate of glucose uptake is reduced while that of circulating free fatty acids is increased, leading the heart to utilize even more fatty acids for its energy needs [91]. This may result in an increased mitochondrial turnover and increased fatty acid metabolism which are both seen in the early stages of obesity and insulin resistance [92]. In the chronic state, accumulation of free fatty acids and the eventual decrease in fatty acid oxidation create a closed loop of ever-increasing oxidative stress causing deleterious effects on the heart [92]. The lipotoxicity of circulating fatty acids or the intracellular accumulation of lipids contributes to mitochondrial oxidative stress through the activation of PKC, to endoplasmic reticulum (ER) stress, and to increased tissue levels of ceramide [6]. In this regard, the role of PPAR-α should be elucidated, as it is an important regulator of fatty acid metabolism in mitochondria [93, 94, 95]. PPARs have three isoforms and are primarily found in metabolic tissues in the body [92]. The expression of PPAR-α is up-regulated under stress conditions to the heart, including during diabetes, and contributes to and correlates with the increased oxidation of fatty acids seen in the early stages of cardiomyopathy [92, 94]. In addition, PPAR-α induces mitochondrial uncoupling and degradation through up-regulation of UCP2 [96] which are thought to be protective mechanisms against increased ROS production from damaged mitochondria. However, PPAR-α also affects the expression of genes involved in cardiac cell fatty acid uptake [92], and thus leads to fatty acid accumulation over time. The level of PPAR-α may be regulated in a negative feedback loop by the fatty acid uptake [similar to glucose transporters (GLUT) regulation being related to glucose uptake], and in the chronic condition leads to the down-regulation of PPAR-α protein expression. As such, PPAR-α has been shown to be down-regulated in human diabetic hearts [97]. This decreased level of PPAR-α leading to reduced fatty acid oxidation and coupled with the detrimental effects of increased fatty acids on the mitochondrial biogenesis results in the toxic accumulation of fatty acids. In compensatory pathologies such as obesity and overnutrition, PPAR-α actually increases β-oxidation of fatty acids, concomitant with limited ROS formation due to the removal of excess or damaged mitochondria through uncoupling mechanisms induced by PPAR-α [97, 98]. In decompensated conditions such as diabetes and eventual heart failure, the β-oxidation of fatty acids is diminished through the reduced expression of PPAR-α, and ROS formation is increased [97, 99]. Thus, one of the antioxidant therapies to improve or ameliorate the metabolic pathology through reduction of ROS formation involves targeting of PPAR-α [98, 100]. Emerging evidence suggests impaired mitochondrial fatty acid oxidation also plays a key role in hepatic steatosis. In this regard, Ang II-induced non-alcoholic fatty liver disease in transgenic TG(mRen2)27(Ren2) rats was characterized by oxidative stress-induced mitochondrial dysfunction and impaired β-oxidation of fatty acids [101]. Mitochondrial dysfunction and oxidative stress also precede insulin resistance and hepatic steatosis in non-alcoholic fatty liver disease in an obese rodent model [102].
70S Ribosomal Kinase, Insulin Resistance, and Oxidative Stress
The immunosuppressant drug rapamycin has been shown to attenuate ROS formation through the attenuation of mammalian target of rapamycin (mTOR) kinase signaling [103]. This property of rapamycin may be related to its ability to improve insulin signaling, as mTOR/S6K1 has been well studied in mediating insulin resistance by inhibition of IRS-1 through serine phosphorylation of this docking protein. In addition, activation of S6K1 in conditions of overnutrition has been shown to cause oxidative stress and endothelial dysfunction [104]. mTOR is also involved in promoting protein synthesis and translation, and its inhibition could directly reduce S6K1-mediated oxidative stress, and could also be an indirect compensatory effort to prevent the proliferation of cells with dysfunctional mitochondria in chronic stress conditions with elevated ROS formation. As such, mTOR expression is increased in conditions of obesity, insulin resistance, and the CRS, with an even larger increase during type 2 diabetes mellitus and its complications; but it is decreased in some cases during very late-stage decompensated heart failure [104]. In conjunction with this, the endogenous ROS levels increase sequentially, and fall only during very late-stage pathology characterized by extensive necrosis and cell death. On the other hand, ROS have been found to either activate or inhibit mTORC1 and S6K1 signaling. Low doses of ROS and short-term ROS exposure stimulate mTORC1, while high concentrations of ROS or long-term ROS treatment inhibit mTORC1 activity. Importantly, protein phosphatase 2A-mediated dephosphorylation of S6K1 and AMPK-mediated phosphorylation of Raptor (S792) contribute to ROS-induced inhibition of mTORC1 signaling [105]. Therefore, the magnitude and duration of oxidative stress and mitochondrial dysfunction may contribute to cardiac hypertrophy and transition to failure in diabetic cardiomyopathy.
ER Stress, Mitochondrial Oxidative Stress, and Insulin Resistance
The ER is an additional organelle that contributes to mitochondrial oxidative stress and insulin resistance. One of the functions of the ER is protein folding [106]. The accumulation of misfolded proteins results in the initiation of the unfolded protein response, leading to an increased generation of ER-derived ROS [107, 108]. Moreover, the interorganellar communication between mitochondria and the ER may underlie increased mitochondrial ROS generation originating from ER-derived signals [109, 110]. Although the mechanism of ER stress-induced insulin resistance is not yet clearly known, activation of stress-activated MAP kinase c-Jun N-terminal kinase (JNK) has been proposed as one of the signaling pathways linking ER stress and insulin resistance. In conditions of oxidative stress, the redox-sensitive serine kinase JNK also phosphorylates IRS-1 on serine 307 which is inhibitory for insulin metabolic signal transduction [111, 112, 113, 114, 115].
Mitochondrial Fission and Oxidative Stress
Mitochondria are dynamic organelles which are able to interchange their morphology between two distinct arrangements by undergoing the processes of mitochondrial fusion and fission to generate either an elongated interconnected mitochondrial network or a fragmented discrete phenotype, respectively [116, 117]. Changes in mitochondrial morphology are orchestrated by a group of mitochondrial fusion and fission proteins [118, 119]. Recent in vitro data have suggested that hyperglycemia induces mitochondrial fragmentation [120]. In the rat heart myoblast cell line H9c2, sustained hyperglycemia-induced mitochondrial fragmentation and mitochondrial ROS production resulted in cell death by MPTP opening and apoptosis [121]. Importantly, this detrimental process could be prevented by transfecting cells with dynamin-related protein 1 (Drp1)K38A, suggesting that the hyperglycemia-induced mitochondrial fragmentation was a Drp1-dependent process [121]. Coronary endothelial cells isolated from the diabetic murine heart (type I model of diabetes) displayed more mitochondrial fragmentation when compared with those from non-diabetic mice, and this change in mitochondrial morphology was abolished by 4 weeks of pre-treatment with an antioxidant. These findings were associated with reduced levels of optic atrophy 1 (OPA1) and increased levels of Drp1 (levels of mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and human orthologue of Fis1p (hFis1) were unchanged), although antioxidant therapy did not change the levels of these mitochondrial-shaping proteins [122]. These data have suggested a role for oxidative stress as a mediator of mitochondrial fragmentation, which has been confirmed by the fact that oxidative stress induced further mitochondrial fragmentation in coronary endothelial cells [102]. A recent study has examined the mitochondrial morphology from a streptozotocin-induced diabetic adult mouse heart and has found that the mitochondria with fission were smaller, had lower mitochondrial membrane potential, and were more predisposed to MPTP opening and apoptosis [123].
Sirtuins, Oxidative Stress, and Mitochondrial Dysfunction
Recent proteomic studies have shown that acetylation of mitochondrial proteins is one of the important mechanisms for the fine control of fuel metabolism in mitochondria through acetylation/deacetylation of key pathways including β-oxidation of fatty acids, gluconeogenesis, tricarboxylic acid (TCA) cycle, and urea cycle [124, 125]. Acetylation of mitochondrial proteins is governed by the integrated action of histone acetyl transferases (HATs) and histone deacetylases (HDACs) [126, 127, 128]. Classically, HATs and HDACs have been studied in the context of histone modifications and thereby named accordingly. Recent studies have indicated that they can act as lysine acetyl transferases and lysine deacetylases, and expanded their substrate range to transcription factors as well as mitochondrial, cytosolic, and cytoskeletal proteins [126, 127, 128]. With regard to cardiac dysfunction in the CRS and diabetes mellitus, they are implicated in cardiac contractile dysfunction, energy metabolism, hypertrophy, and fibrosis [129, 130, 131, 132, 133, 134].
The emerging role of the sirtuin (SIR) class of HDACs in mitochondrial biogenesis and mitochondrial oxidative stress, and its regulation by oxidative stress underscore the importance of protein acetylation in mitochondrial oxidative stress in the CRS and diabetes mellitus. SIR proteins (collectively known as sirtuins) belong to a conserved family of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases (class III NAD+-dependent deacetylase) implicated in the regulation of life span under calorie restriction. Their key role is in regulating glucose and lipid metabolism [135, 136, 137, 138]. In mammals, there are seven homologs of SIR (SIRT1–7). Among these, SIRT1 has been most extensively studied, but the significance of other sirtuins is being increasingly recognized. Recent studies have demonstrated a pivotal role of SIRT1 in the regulation of energy metabolism and cellular survival, in addition to its known role in life span regulation. The differential distribution of sirtuins implicates their distinct roles in cytosolic, mitochondrial, and nuclear events. SIRT1 is predominantly localized to the nuclear compartment and is implicated in regulating the transcriptional and epigenetic regulation of gene expression [135, 136, 137, 138]. In contrast, SIRT2 is cytosolic, and SIRT3, SIRT4, and SIRT5 are mainly mitochondrial sirtuins [135, 136, 137, 138].
SIRT1, Oxidative Stress, and Mitochondrial Dysfunction
SIRT1 deacetylates a wide variety of substrates implicated in insulin action, including PGC-1α, UCP2, nuclear factor kappa-B (NF-κB), forkhead Box 01 (FoxO1) proteins, sterol regulatory element-binding protein-1c (SREBP-1c), and PPAR-γ [139, 140]. Therefore, it plays a critical role in adipogenesis, and glucose and lipid metabolism in liver and skeletal muscle. Decreased expression or activity of SIRT1 is emerging as one of the major determinants of insulin resistance [141]. SIRT1 has been shown to promote insulin secretion through modulation of UCP expression and deacetylation of FoxO1 [142]. SIRT1 also exerts positive effects on insulin signaling through repression of protein tyrosine phosphatase 1B (PTP1B), a negative regulator of insulin signaling, and stimulation of protein kinase B (Akt) [143]. In adipocytes, SIRT1 promotes glucose uptake and suppression of IRS-1 serine phosphorylation [144]. SIRT1 was shown to modulate the expression of the DNA repair factor growth arrest and DNA damage-inducible protein 45 (GADD45) and the mitochondrial antioxidant enzyme Mn-SOD. This occurs by the SIRT1-mediated regulation of PGC-1α [145] and FoxO transcription factors [139, 140]. On the other hand, oxidative stress decreases SIRT1 expression, and degradation of SIRT1 occurs by its oxidative modification [146]. SIRT1 also suppresses NADPH oxidase-mediated oxidative stress through PGC-1α activation, which is sufficient to down-regulate NADPH oxidase expression in endothelial cells [147]. In hamsters treated with resveratrol, nuclear SIRT1 induced mitochondrial Mn-SOD, which reduced oxidative stress and contributed to cardiomyocyte protection. Resveratrol also suppressed myoblast death induced by Ang II through the nuclear activation of SIRT1 and induction of mitochondrial Mn-SOD [148]. Additionally, SIRT1 overexpression or activation by resveratrol leads to down-regulation of Ang II type 1 receptor (AT1R) mRNA in vascular smooth muscle cells and improves insulin resistance [148]. SIRT2 has also been suggested to ameliorate oxidative stress via deacetylation of FoxOs [149].
SIRT3
The role of SIRT3 in age-induced cardiac hypertrophy and fibrosis was revealed by exaggeration of this process in aged SIRT3 knockout mice [150]. Moreover, SIRT3 knockout mice are more sensitive to cardiac stress induced by pressure overload [151]. The protection of cardiac myocyte death during cardiac failure was induced by overexpression of nicotinamide phosphoribosyltransferase (NAMPT) in a SIRT3-dependent manner [151]. The anti-apoptotic effect of NAMPT through the suppression of translocation of the apoptosis-inducing factor from mitochondria to the nucleus is dependent on SIRT3. SIRT3 has been shown to decrease ROS production in brown adipocytes [152] as well as mitochondrial biogenesis through the transcriptional co-activator PGC-1α [153, 154]. The significance of SIRT3 in relation to overnutrition has emerged from recent findings of the effects of mitochondrial SIRT3 on hepatic mitochondrial protein acetylation [155]. High-fat diet was characterized by a significant decrease in hepatic SIRT3 activity and concomitant hyperacetylation of proteins involved in gluconeogenesis, mitochondrial oxidative metabolism, and ER stress response, and these changes were further increased in SIRT3 knockout animals. These changes were accompanied by a disruption of OXPHOS complexes II, III, and IV [155]. SIRT3 also stimulates hepatic β-oxidation through deacetylation of long-chain acyl dehydrogenase (LCAD), one of the key enzymes of β-oxidation, and hepatic SIRT3 levels increase during fasting [156]. In primary cultured cardiomyocytes, SIRT3 blocked cardiac hypertrophy by activating the FoxO3a-dependent, antioxidant-encoding genes Mn-SOD and catalase, thereby decreasing cellular levels of ROS [151]. Ang II-induced cardiac hypertrophy was also suppressed in transgenic mice expressing SIRT3 in the heart [157]. In cultured murine tubular epithelial cells, Ang II down-regulates SIRT3 mRNA, and this effect is overcome by AT1R blockade [158]. These findings suggest that SIRT3 plays a significant role in mitochondrial oxidative stress and dysregulation of mitochondrial biogenesis in metabolic syndrome and diabetes mellitus.
MicroRNA-Mediated Regulation of Mitochondrial Dysfunction and Oxidative Stress
Accumulating evidence implies that the non-coding microRNAs (miRNAs or miRs) impose another layer of regulation of mitochondrial function and oxidative stress. miRNAs are small non-coding RNAs (approx. 22 nucleotides) that modulate mRNA stability and repress post-transcriptional translation by binding to target recognition sequences predominantly located within the 3′ untranslated regions of target mRNAs [159, 160, 161, 162]. The ‘seed sequence’ that spans bases 2–8 of the 5′ portion of mature miRNAs essentially dictates what target mRNAs would be regulated by a given miRNA. Since one miRNA can regulate hundreds of mRNAs and, conversely, one mRNA can be regulated by multiple miRNAs, it is becoming increasingly clear that a modest repression of the expression of a multitude of mRNAs by a given miRNA plays a major role in modulating related biological functions. Additionally, since many miRNAs may share the same seed sequence and form a miRNA family, they have the regulatory potential to redundantly regulate complex biological functions by targeting multiple genes belonging to a common pathway simultaneously. It is now increasingly recognized that deregulation of miRNAs contributes significantly to the pathophysiological etiology of many chronic diseases such as cancer, diabetes, hypertension, atherosclerosis, and neurological disorders, to name a few. The human genome encodes more than 1,000 miRNA genes; however, details of which miRNAs modulate mitochondrial functions are only beginning to emerge.
miRNA Modulation of Glutamine Metabolism
Mitochondria are highly abundant and constitute approximately 40% of the total cardiomyocyte volume in the heart [163]. Cheng et al. [164] have shown that the most abundant miRNAs in the heart are miR-1, Let-7, miR-133, miR-126-3p, miR-30c, and miR-26a. Studies on idiopathic end-stage failing human hearts have shown that the expression of miR-24, miR-125b, miR-195, miR-199a, and miR-214 is up-regulated in these tissues [162]. The same study has also shown that miR-23a, miR-23b, miR-24, miR-195, and miR-214 were increased in response to hypertrophy. It is conceivable that the increased expression of miR-23a/b in hypertrophy may actually represent a compensatory mechanism to down-regulate mitochondrial glutaminase (GLS) [165, 166, 167]. GLS converts glutamine to glutamate that is further catabolized through the TCA cycle for the production of ATP. Glutamate serves as a substrate for glutathione synthesis as well. Proliferating cells are known to utilize glutamine as a major source for energy, nitrogen for biosynthesis, and a carbon substrate for anabolic processes [165, 166]. Recent reports show that transcriptional regulation of the oncogene Myc coordinates with the expression of genes that promote cells to engage in excessive glutamine catabolism which exceeds the cellular requirement for protein and nucleotide biosynthesis [165]. Such Myc-dependent glutaminolysis results in the reprogramming of mitochondrial metabolism to depend on glutamine catabolism and to maintain cellular viability and TCA cycle anaplerosis. Concomitantly, this stimulation of mitochondrial glutamine metabolism leads to reduced glucose carbon entering the TCA cycle and a decreased contribution of glucose to the mitochondrial-dependent synthesis of phospholipids [163]. Interestingly, miR-23a/b targets mitochondrial GLS [166, 167]. Myc up-regulates glutaminase by down-regulating the expression of miR-23a/b [166, 167]. In age-related macular degeneration patients, it was found that the miR-23 expression was significantly down-regulated. miR-23 was also down-regulated in oxidant-induced injury [167]. The miR-23 mimic could rescue H2O2-induced ARPE-19 cell death and apoptosis [167]. Collectively, these observations uncover a role for miR-23 in oxidant-induced injury and mitochondrial metabolism. Moreover, they also suggest a link between Myc regulation of miRNAs, glutamine metabolism, and energy and ROS homeostasis.
miRNA Regulation of Cardiac Mitochondrial ATP Levels
In an attempt to determine which miRNAs modulate mitochondria and ATP levels in cardiomyocytes, Nishi et al. [168] evaluated the effects of 23 miRNAs that are known to be expressed in heart tissue by individually over-expressing them in neonatal rat cardiomyocytes using a lentiviral vector. The miRNAs that emerged as regulators of ATP levels from this study were miR-15b, miR-16, miR-195, and miR-424, all sharing the same seed sequence. Over-expressing these miRNAs down-regulated cellular ATP levels and affected mitochondrial integrity. The target of these miRNAs was ADP-ribosylation factor-like 2 (Arl2) mRNA [168]. The knockdown of Arl2 by siRNA also resulted in reduced ATP levels and degeneration of mitochondria [168]. Thus, conditions that induce up-regulation of miR-15b and other miRNAs that share the same seed sequence (such as miR-195, which was shown to be up-regulated in cardiac hypertrophy) reflect mitochondrial degeneration and reduction in ATP levels in cardiomyocytes.
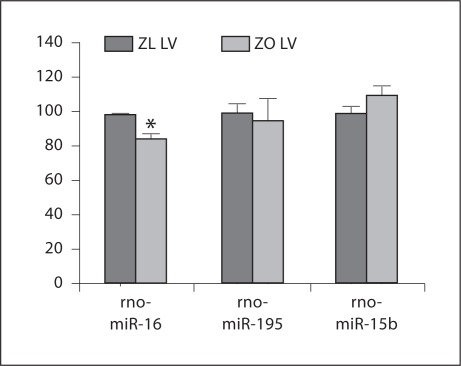
In conditions of overnutrition/obesity, however, an increase in mitochondria is observed in muscles [169, 170, 171]. For example, it is reported that maternal diet-induced obesity in mice can cause an increase in mitochondrial potential, mitochondrial DNA content, and biogenesis [169]. Concomitantly, ROS levels were raised, whereas glutathione was depleted and the redox state became more oxidized, suggestive of oxidative stress. Igosheva et al. [169] proposed that the ‘altered mitochondrial properties were associated with significant developmental impairment as shown by the increased number of obese mothers who failed to support blastocyst formation compared to lean dams’. In muscles of mice fed a high-fat diet, it has been reported that the mitochondrial content was actually increased, and it has been proposed that overconsumption, rather than mitochondrial dysfunction, is the underlying reason for the insulin resistance in these animals [170, 171]. We have demonstrated that Zucker obese (ZO) rat heart has increased mitochondria as shown in figure 2[172]. Compared to Zucker lean (ZL) controls, ZO rats exhibited a prolonged diastolic relaxation time and a reduced initial diastolic filling rate suggestive of cardiac dysfunction. They also showed increases in homeostatic model assessment of insulin resistance, NADPH oxidase activity, 3-nitrotyrosine, and NADPH oxidase-dependent superoxide [172]. Thus, the ZO rat heart tissues exhibit a scenario comparable to that seen in high-fat diet-fed mice. Our miRNA profiling experiments indicated that in fact rat (rno)-miR-16 was slightly down-regulated in ZO rat left ventricular tissue (fig. 3). Other miRNAs that shared the same seed sequence (miR-15b and miR-195) showed comparable expression levels in both ZO and ZL rat left ventricular tissues. Thus in 12-week-old ZO rat left ventricular tissue, down-regulation of Arl2, mediated by up-regulation of miRNAs that share the same seed sequence, does not seem to be occurring.
Fig. 2.
Immunostaining of mitochondrial complex IV-1 in ZO and ZL rats. Representative left ventricular sections immunostained for mitochondrial complex IV-1 of treated and untreated ZL and ZO rats. The increased level of complex IV-1 immunostaining in the ZO-C myocardium indicates an increased mitochondrial number compared with all other groups. The bar graph shows the quantification of converted signal intensities of complex IV-1 protein. ZL-C = Zucker lean-control; ZL-N = Zucker lean-nebivolol; ZO-C = Zucker obese-control; ZO-N = Zucker obese-nebivolol. * p < 0.05 vs. ZL-C; † p < 0.05 vs. ZO-C. Figure used with permission from Zhou et al. [172].
Fig. 3.
miRNA profile in the heart of ZL and ZO rats. The miRNA was isolated with an mirVana miRNA Isolation Kit (Ambion Inc.) from fresh-frozen tissues (n = 3 for each group), and was labeled with a FlashTag™ Biotin HSR RNA Labeling Kit. An Affymetrix miRNA GeneChip was used for this study (46,228 probes comprising 7,815 probe sets, including controls). The probes on this chip are derived from the Sanger miRBase miRNA database v11 (April 15, 2008, http://microrna.sanger.ac.uk) [173]. Data analysis was performed by miRNA QC tool and Significance Analysis of Microarrays (SAM) software [174]. LV = Left ventricle. * p < 0.05 for ZL versus ZO.
miRNA Regulation of Apoptosis
We have previously proposed that compensative/adaptive mechanisms are activated in ZO rat heart that may have a role in attenuating the progression to diabetic cardiomyopathy in these animals compared to Zucker diabetic fatty rats [175]. Additionally, we have recently shown that miR-200c, an miRNA only modestly expressed in the heart, is increased in ZO rat heart, and this increase in miR-200c may serve as a compensatory mechanism to down-regulate excessive activation of the nutrient sensor kinase S6K1 [176]. The miR-200 family is implicated in the epithelial-to-mesenchymal transition (EMT) that is accompanied by mitochondrial biogenesis and involved in organ fibrosis and carcinoma progression [177, 178, 179, 180, 181, 182, 183, 184]. It was reported that miR-200 is down-regulated in EMT, and that overexpression of miR-200b can attenuate EMT [179]. miR-200 regulates stem cell factors and functions as a cell growth inhibitor by targeting apoptosis inhibitor FAS-associated phosphatase-1 (FAP-1) [182]. It also regulates zinc finger E-box binding homeobox 1 (ZEB1), the EMT activator that promotes tumorigenicity [183, 184]. Expression of the miR-200c/141 cluster is down-regulated by DNA methylation in cancer, suggesting that an epigenetic silencing mechanism regulates these miRNAs. These observations have led to the idea that the members of the miR-200 family (miR-200a, b, c, miR-141, miR-429) serve as guardians of differentiation [185]. An analysis of rat (rno)-miR-200c targets predicted by miRecords [186] revealed that mitochondrial enzyme holocytochrome-c synthetase (HCCS) is also a target for miR-200c. HCCS functions as heme lyase and during apoptotic stimuli, it translocates to outside the mitochondria and suppresses the X-linked inhibitor of apoptosis protein, thus causing the activation of caspase-3 [187]. Up-regulation of miR-200c can lead to the suppression of HCCS expression that can attenuate cardiomyocyte apoptosis [188]. In this context, the increase in miR-200c observed in the ZO rat heart further supports the notion that in 12-week-old ZO rat heart compensatory/adaptive mechanisms to attenuate the progression of obesity-induced heart disease are activated via up-regulation of miR-200c.
Control of Cytochrome C Oxidase IV Expression by miR-338
miR-338 is another miRNA that modulates OXPHOS and mitochondrial functions, since it targets cytochrome c oxidase IV (COX IV) mRNA [189]. In neurons, miR-338 overexpression by transfection resulted in decreased COX IV mRNA and protein levels and mitochondrial activity, due to reduced ATP levels. In contrast, expression of anti-miRNA oligonucleotides increased COX IV levels and improved OXPHOS. Thus, it was concluded that miR-338 overexpression is detrimental to mitochondrial functions. In this context, it is noteworthy that miR-338 is one of the most abundant miRNAs seen in hepatocellular carcinoma [190].
In brief, the above examples show that we are beginning to comprehend the fundamental roles of miRNAs in the modulation of oxidative stress and mitochondrial dysfunction. While a wealth of data is available regarding the miRNA-mediated regulation of mitochondrial functions in cancer and neuronal diseases, the miRNA modulation of overnutrition-related mitochondrial dysfunction is yet to be elucidated.
Alcohol, Oxidative Stress, and Mitochondrial Dysfunction
Heavy and prolonged consumption of alcohol results in both myocardial and hepatic injuries [191, 192]. A number of theories have been put forward for the onset and development of alcoholic complications, including oxidative damage, deposition of triglycerides, altered fatty acid extraction, decreased myofilament Ca2+ sensitivity, impaired protein metabolism, and mitochondrial anomalies [193, 194, 195, 196, 197, 198, 199]. Not surprisingly, oxidative stress and mitochondrial damage have been shown to contribute to the toxic effects of alcoholic liver and myocardial injuries [200, 201, 202]. The significance of a link between alcohol and obesity/diabetes stems from the increased risk of progressive alcoholic liver and myocardial injury in obesity/diabetes [203, 204, 205]. These interrelationships may be caused by several common mechanisms of mitochondrial dysfunction and oxidative stress induced via alcohol and obesity/diabetes. The factors contributing to mitochondrial dysfunction include decreased expression of SIRT1 [206], decreased phosphorylation of AMPK [207, 208], altered mitochondrial permeability transition contributed by dysregulation of SIRT3 [209], and impaired transport of glutathione across mitochondria with decreased mitochondrial glutathione levels [210]. Long-standing alcohol consumption also leads to decreased SOD2 and catalase, thereby suppressing mitochondrial antioxidant capacity [193, 200, 204, 205, 211, 212]. In addition, chronic heavy alcohol consumption also results in insulin resistance in cardiomyocytes [213, 214, 215, 216]. The effects of alcohol are, in part, caused by ethanol metabolite acetaldehyde and increased metabolism of alcohol caused by induction of cytochrome P450 2E1 [213, 214]. The role of acetaldehyde in alcoholic-induced mitochondrial dysfunction, insulin resistance, ER stress, and activation of redox-sensitive kinases and AMPK signaling has been demonstrated in a novel transgenic mouse model with cardiac-specific overexpression of alcohol dehydrogenase, which mimics the acetaldehyde-overloaded model of alcoholic cardiomyopathy. Moreover, ethanol effects are attenuated in mitochondrial aldehyde dehydrogenase-overexpressing mice. These observations underscore the significance of oxidative metabolism of ethanol in ethanol-mediated mitochondrial toxicity. In this regard, ethanol potentiation of Ang II activation of extracellular signal-regulated kinase (ERK1/2) is noteworthy [217] since phosphorylation of NADPH oxidase by ERK1/2 contributes to increased oxidative stress [218], and oxidative stress affects SIRT1 levels [146].
Conclusion
In summary, mitochondrial dysfunction is an important player in the development of heart disease and other components of the CRS. The interplay between cytosolic and mitochondrial oxidative stress is associated with impaired mitochondrial electron transport and mitochondrial dynamics. Dysregulation of insulin and angiotensin signaling including activation of NADPH oxidase, ER stress, and activation of ROS-sensitive kinases contribute to a vicious cycle of enhanced oxidative stress and mitochondrial dysfunction. Altered acetylation of proteins in both the nuclear and mitochondrial compartment and modulation of mitochondrial function by miRNAs are also emerging as regulators of mitochondrial oxidative stress. Alcohol further augments mitochondrial oxidative stress in the CRS. Thus, in addition to being a major source of ROS generation, mitochondria are very susceptible to oxidative damage.
Acknowledgements
This research was supported by the NIH (R01 HL73101-01A1 and R01 HL107910-01 to J.R.S.), the Veterans Affairs Merit System 0018 (to J.R.S.) and the University of Missouri Mission Enhancement Fund and Forest Laboratories (to L.P.). The authors would like to thank Brenda Hunter for her assistance in editing the manuscript.
References
- 1.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med. 2010;88:993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picard M, Taivassalo T, Gouspillou G, Hepple RT. Mitochondria: isolation, structure and function. J Physiol. 2011;589:4413–4421. doi: 10.1113/jphysiol.2011.212712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal. 2011;23:1534–1545. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Rabøl R, Højberg PM, Almdal T, Boushel R, Haugaard SB, Madsbad S, Dela F. Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1372–1378. doi: 10.1210/jc.2008-1475. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Roves PM. Mitochondrial pathophysiology and type 2 diabetes mellitus. Arch Physiol Biochem. 2011;117:177–187. doi: 10.3109/13813455.2011.584538. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:101–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raju R, Jian B, Hubbard W, Chaudry I. The mitoscriptome in aging and disease. Aging Dis. 2011;2:174–180. [PMC free article] [PubMed] [Google Scholar]
- 8.Eckert A, Schmitt K, Götz J. Mitochondrial dysfunction – the beginning of the end in Alzheimer's disease? Separate and synergistic modes of tau and amyloid-β toxicity. Alzheimers Res Ther. 2011;3:15. doi: 10.1186/alzrt74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen-Bouterse SA, Zhong Q, Mohammad G, Ho YS, Kowluru RA. Oxidative damage of mitochondrial DNA in diabetes and its protection by superoxide dismutase. Free Radic Res. 2010;44:313–321. doi: 10.3109/10715760903494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou R, Yadzi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–226. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 13.Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2-emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;118:789–800. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokota T, Kinugawa S, Hirabayashi K, Matsushima S, Inoue N, Ohta Y, Hamaguchi S, Sobirin MA, Ono T, Suga T, Kuroda S, Tanaka S, Terasaki F, Okita K, Tsutsui H. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;297:H1069–H1077. doi: 10.1152/ajpheart.00267.2009. [DOI] [PubMed] [Google Scholar]
- 17.Pagel-Langenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev. 2010;31:25–51. doi: 10.1210/er.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bravard A, Bonnard C, Durand A, Chauvin MA, Favier R, Vidal H, Rieusset J. Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am J Physiol Endocrinol Metab. 2011;300:E581–E591. doi: 10.1152/ajpendo.00455.2010. [DOI] [PubMed] [Google Scholar]
- 19.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino A, Scott BT, Dillman WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologica. 2010;53:1783–1794. doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raza H, Prabu SK, John A, Avadhani NG. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int J Mol Sci. 2011;12:3133–3147. doi: 10.3390/ijms12053133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sowers JR. The heart and the kidneys: partners in disease? Cardiorenal Med. 2011;1:1–2. doi: 10.1159/000323177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulakat L, DeMarco VG, Whaley-Connell A, Sowers JR. The impact of overnutrition on insulin metabolic signaling in the heart and the kidney. Cardiorenal Med. 2011;1:102–112. doi: 10.1159/000327140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am. 2009;93:569–582. doi: 10.1016/j.mcna.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rains JL, Jain SK. Oxidative stress, insulin signaling and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian M, Shulman GI, Scheinman SJ, Lifton RP. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maassen JA, ‘T Hart LM, Van Essen E, Heine RJ, Nijpels G, Jahangir Tafrechi RS, Raap AK, Janssen GM, Lemkes HH. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53:S103–S109. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- 28.He M, Rutledge SL, Kelly DR, Palmer CA, Murdoch G, Majumder N, Nicholls RD, Pei Z, Watkins PA, Vockley J. A new genetic disorder in mitochondrial fatty acid beta-oxidation: ACAD9 deficiency. Am J Hum Genet. 2007;81:87–103. doi: 10.1086/519219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esterbauer H, Schneitler C, Oberkofler H, Ebenbichler C, Paulweber B, Sandhofer F, Ladurner G, Hell E, Strosberg AD, Patsch JR, Krempler F, Patsch W. A common polymorphism in the promoter of UCP2 is associated with decreased risk of obesity in middle-aged humans. Nat Genet. 2001;28:178–183. doi: 10.1038/88911. [DOI] [PubMed] [Google Scholar]
- 30.Sesti G, Cardellini M, Marini MA, Frontoni S, D'Adamo M, Del Guerra S, Lauro D, De Nicolais P, Sbraccia P, Del Prato S, Gambardella S, Federici M, Marchetti P, Lauro R. A common polymorphism in the promoter of UCP2 contributes to the variation in insulin secretion in glucose-tolerant subjects. Diabetes. 2003;52:1280–1283. doi: 10.2337/diabetes.52.5.1280. [DOI] [PubMed] [Google Scholar]
- 31.Muller YL, Bogardus C, Pedersen O, Baier LA. Gly482Ser missense mutation in the peroxisome proliferator-activated receptor gamma coactivator-1 is associated with altered lipid oxidation and early insulin secretion in Pima Indians. Diabetes. 2003;52:895–898. doi: 10.2337/diabetes.52.3.895. [DOI] [PubMed] [Google Scholar]
- 32.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 34.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 35.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 38.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 39.Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest. 2004;114:1518–1526. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 43.St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 44.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 45.Le Gouill E, Jimenez M, Binnert C, Pierre-Yves J, Thalmann S, Nicod P, Scherrer U, Vollenweider P. eNOS knock-out mice have defective mitochondrial beta-oxidation. Diabetes. 2007;56:2690–2696. doi: 10.2337/db06-1228. [DOI] [PubMed] [Google Scholar]
- 46.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 47.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 200;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 51.Wei Y, Clark SE, Thyfault JP, Uptergrove GM, Li W, Whaley-Connell AT, Ferrario CM, Sowers JR, Ibdah JA. Oxidative stress-mediated mitochondrial dysfunction contributes to angiotensin II-induced nonalcoholic fatty liver disease in transgenic Ren2 rats. Am J Pathol. 2009;174:1329–1337. doi: 10.2353/ajpath.2009.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol. 2011;301:H647–H653. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiomyocytes. Free Radic Biol Med. 2011;50:777–793. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Radi R, Cassina A, Hodora R. Nitric oxide and peroxynitrite interactions in mitochondria. Biol Chem. 2002;383:401–409. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- 57.Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharamcol. 2010;88:241–248. doi: 10.1139/Y10-018. [DOI] [PubMed] [Google Scholar]
- 58.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 59.Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 61.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Leloup C, Casteilla L, Carrière A, Galinier A, Benani A, Carneiro L, Pénicaud L. Balancing mitochondrial redox signaling: a key point in metabolic regulation. Antioxid Redox Signal. 2011;14:519–530. doi: 10.1089/ars.2010.3424. [DOI] [PubMed] [Google Scholar]
- 63.Bereiter-Hahn J, Jendrach M. Mitochondrial dynamics. Int Rev Cell Mol Biol. 2010;284:1–65. doi: 10.1016/S1937-6448(10)84001-8. [DOI] [PubMed] [Google Scholar]
- 64.Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol. 2010;174:182–191. doi: 10.1016/j.resp.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ungvari Z, Sonntag WE, Csiszar A. Mitochondria and aging in the vascular system. J Mol Med. 2010;88:1021–1027. doi: 10.1007/s00109-010-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 67.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 68.Sam F, Kerstetter DL, Pimental DR, Mulukutla S, Tabaee A, Bristow MR, Colucci WS, Sawyer DB. Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J Card Fail. 2005;11:473–480. doi: 10.1016/j.cardfail.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Coughlan MT, Thorburn DA, Penfold SA, Laskowski A, Harcourt BC, Sourris AC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, Renier G. Activation of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase by advanced glycation end products links oxidative stress to altered retinal vascular endothelial growth factor expression. Metabolism. 2006;55:1516–1523. doi: 10.1016/j.metabol.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 71.Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes. 2007;56:118–126. doi: 10.2337/db06-0288. [DOI] [PubMed] [Google Scholar]
- 73.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Münzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 74.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 75.Opitz N, Drummond GR, Selemidis S, Meurer S, Schmidt HH. The ’A's and ’O's of NADPH oxidase regulation: a commentary on ‘Subcellular localization and function of alternatively spliced Noxo1 isoforms’. Free Radic Biol Med. 2007;42:175–179. doi: 10.1016/j.freeradbiomed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in α1-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002;282:C926–C934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 77.Kurdi M, Booz GW. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension. 2011;57:1034–1038. doi: 10.1161/HYPERTENSIONAHA.111.172700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuroda J, Sadoshima J. NADPH oxidase and cardiac failure. J Cardiovasc Transl Res. 2010;3:314–320. doi: 10.1007/s12265-010-9184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu W, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 80.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 81.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 85.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66Shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 86.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 87.Pagnin E, Fadini G, de Toni R, Tiengo A, Calo L, Avogaro A. Diabetes induces p66Shc gene expression in human peripheral blood mononuclear cells: relationship to oxidative stress. J Clin Endocrinol Metab. 2005;90:1130–1136. doi: 10.1210/jc.2004-1283. [DOI] [PubMed] [Google Scholar]
- 88.Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55:1642–1650. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- 89.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci USA. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66Shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 91.Melenovsky V, Benes J, Skaroupkova P, Sedmera D, Strnad H, Kolar M, Vlcek C, Petrak J, Benes J, Jr, Papousek F, Oliyarnyk O, Kazdova L, Cervenka L. Metabolic characterization of volume over-load heart failure due to aorto-caval fistula in rats. Mol Cell Biochem. 2011;354:83–96. doi: 10.1007/s11010-011-0808-3. [DOI] [PubMed] [Google Scholar]
- 92.Pruimboom-Brees I, Haghpassand M, Royer R, Brees D, Aldinger C, Reagan W, Singh J, Kerlin R, Kane C, Bagley S, Hayward C, Loy J, O'Brien P, Francone OL. A critical role for peroxisomal proliferator-activated receptor-α nuclear receptors in the development of cardiomyocyte degeneration and necrosis. Am J Pathol. 2006;169:750–760. doi: 10.2353/ajpath.2006.051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdelmegeed MA, Moon K, Hardwick JP, Gonzalez FK, Song B. Role of peroxisome proliferator-activated receptor-α in fasting-mediated oxidative stress. Free Radic Biol Med. 2009;47:767–778. doi: 10.1016/j.freeradbiomed.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V, Mangelsdorf DJ, Kliewer SA, Browning JD, Burgess SC. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes. 2008;57:2012–2021. doi: 10.2337/db08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park SY, Cho YR, Finck BN, Kim HJ, Higashimori T, Hong EG, Lee MK, Danton C, Deshmukh S, Cline GW, Wu JJ, Bennett AM, Rothermel B, Kalinowski A, Russell KS, Kim YB, Kelly DP, Kim JK. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 96.Zhang X, Li L, Prabhakaran K, Zhang L, Leavesley HB, Borowitz JL, Isom GE. Uncoupling protein-2 up-regulation and enhanced cyanide toxicity are mediated by PPARα activation and oxidative stress. Toxicol Appl Pharmacol. 2007;223:10–19. doi: 10.1016/j.taap.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang H, Jin X, Wai Kei Lam C, Yan SK. Oxidative stress and diabetes mellitus. Clin Chem Lab Med. 2011;49:1773–1782. doi: 10.1515/CCLM.2011.250. [DOI] [PubMed] [Google Scholar]
- 98.Turan B. Role of antioxidants in redox regulation of diabetic cardiovascular complications. Curr Pharm Biotechnol. 2010;11:819–836. doi: 10.2174/138920110793262123. [DOI] [PubMed] [Google Scholar]
- 99.Finck BN. The role of the peroxisome proliferator-activated receptor alpha pathway in pathological remodeling of the diabetic heart. Curr Opin Clin Nutr Metab Care. 2004;7:391–396. doi: 10.1097/01.mco.0000134371.70815.32. [DOI] [PubMed] [Google Scholar]
- 100.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a ‘causal’ antioxidant therapy. Diabetes Care. 2003;26:1589–1596. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- 101.Wei Y, Clark SE, Morris EM, Thyfault JP, Uptergrove GM, Whaley-Connell AT, Ferrario CM, Sowers JR, Ibdah JA. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J Hepatol. 2008;49:417–428. doi: 10.1016/j.jhep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rector RS, Thyfault JP, Uptergrove G, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marobbio CM, Pisano I, Porcelli V, Lasorsa FM, Palmieri L. Rapamycin reduces oxidative stress in frataxin-deficient yeast cells. [DOI] [PubMed]
- 104.Rajapakse AG, Yepuri G, Carvas JM, Stein S, Matter CM, Scerri I, Ruffieux J, Montani J, Ming X, Yang Z. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: inhibition by resveratrol. PLoS One. 2011;6:e19237. doi: 10.1371/journal.pone.0019237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li M, Zhao L, Liu J, Liu A, Jia C, Ma D, Jiang Yu, Bai X. Multiple mechanisms are involved in reactive oxygen species regulation of mTOR1 signaling. Cell Signal. 2010;22:1469–1476. doi: 10.1016/j.cellsig.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 106.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 107.Gregersen N, Bross P. Protein misfolding and cellular stress: an overview. Methods Mol Biol. 2010;648:3–23. doi: 10.1007/978-1-60761-756-3_1. [DOI] [PubMed] [Google Scholar]
- 108.Ceylan-Isik AF, Sreejayan N, Ren J. Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol. 2011;50:107–116. doi: 10.1016/j.yjmcc.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 110.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 111.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lim JH, Lee HJ, Ho Jung M, Song J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal. 2009;21:169–177. doi: 10.1016/j.cellsig.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 113.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 114.Leem J, Koh EH. Interaction between mitochondria and the endoplasmic reticulum: implications for the pathogenesis of type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:242984. doi: 10.1155/2012/242984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, Kim JK, Lee AS. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 117.Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem. 2011;149:241–251. doi: 10.1093/jb/mvr002. [DOI] [PubMed] [Google Scholar]
- 118.Kuzmicic J, Del Campo A, López-Crisosto C, Morales PE, Pennanen C, Bravo-Sagua R, Hechenleitner J, Zepeda R, Castro PF, Verdejo HE, Parra V, Chiong M, Lavandero SP. Mitochondrial dynamics: a potential new therapeutic target for heart failure. Rev Esp Cardiol. 2011;64:916–923. doi: 10.1016/j.recesp.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 119.Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53:S110–S118. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- 120.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sowers JR. Role of TRIB3 in diabetic and over-nutrition induced atherosclerosis. [DOI] [PMC free article] [PubMed]
- 122.Hayden MR, Habibi J, Joginpally T, Karuparthi PR, Sowers JR. Ultrastructure study of transgenic rat aorta – part 1: endothelium and intima. Cardiorenal Med 2012. [DOI] [PMC free article] [PubMed]
- 123.Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, Hollander JM. Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol Heart Circ Physiol. 2010;298:H633–H642. doi: 10.1152/ajpheart.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: the promise of histone deacetylase inhibitors. Circ Res. 2010;106:272–284. doi: 10.1161/CIRCRESAHA.109.209338. [DOI] [PubMed] [Google Scholar]
- 127.McKinsey TA. The biology and therapeutic implications of HDACs in the heart. Handb Exp Pharmacol. 2011;206:57–78. doi: 10.1007/978-3-642-21631-2_4. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Selvi RB, Kundu TK. Reversible acetylation of chromatin: implication in regulation of gene expression, disease and therapeutics. Biotechnol J. 2009;4:375–390. doi: 10.1002/biot.200900032. [DOI] [PubMed] [Google Scholar]
- 130.Christensen DP, Dahllöf M, Lundh M, Rasmussen DN, Nielsen MD, Billestrup N, Grunnet LG, Mandrup-Poulsen T. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol Med. 2011;17:378–390. doi: 10.2119/molmed.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 2004;279:18091–18097. doi: 10.1074/jbc.M311786200. [DOI] [PubMed] [Google Scholar]
- 132.McKinsey TA. The biology and therapeutic implications of HDACs in the heart. Handb Exp Pharmacol. 2011;206:57–78. doi: 10.1007/978-3-642-21631-2_4. [DOI] [PubMed] [Google Scholar]
- 133.Bogaard HJ, Mizuno S, Hussaini AA, Toldo S, Abbate A, Kraskauskas D, Kasper M, Natarajan R, Voelkel NF. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med. 2011;183:1402–1410. doi: 10.1164/rccm.201007-1106OC. [DOI] [PubMed] [Google Scholar]
- 134.Chu CH, Lo JF, Hu WS, Lu RB, Chang MH, Tsai FJ, Tsai CH, Weng YS, Tzang BS, Huang CY. Histone acetylation is essential for ANG-II-induced IGF-IIR gene expression in H9c2 cardiomyoblast cells and pathologically hypertensive rat heart. J Cell Physiol. 2012;227:259–268. doi: 10.1002/jcp.22728. [DOI] [PubMed] [Google Scholar]
- 135.Hashimoto T, Horikawa M, Nomura T, Sakamoto K. Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology. 2010;11:31–43. doi: 10.1007/s10522-009-9225-3. [DOI] [PubMed] [Google Scholar]
- 136.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 137.Horio Y, Hayashi T, Kuno A, Kunimoto R. Cellular and molecular effects of sirtuins in health and disease. Clin Sci. 2011;121:191–203. doi: 10.1042/CS20100587. [DOI] [PubMed] [Google Scholar]
- 138.Zhong L, Mostoslavsky R. Fine tuning our cellular factories: sirtuins in mitochondrial biology. Cell Metab. 2011;13:621–626. doi: 10.1016/j.cmet.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2 (SIRT1) J Biol Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 140.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 141.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 142.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 144.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 146.Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010;24:3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10:640–647. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]
- 148.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 149.Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 150.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sack MN. Emerging characterization of the role of SIRT3-mediated mitochondrial protein deacetylation in the heart. Am J Physiol Heart Circ Physiol. 2011;301:H2191–H2197. doi: 10.1152/ajpheart.00199.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 153.Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li A, Saha A, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lombard DB, Alt FW, Cheng H-L, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. Mammalian sir2 homolog sirt3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nistala R, Hayden MR, Demarco VG, Henriksen EJ, Lackland DT, Sowers JR. Prenatal programming and epigenetics in the genesis of the cardiorenal syndrome. Cardiorenal Med. 2011;1:243–254. doi: 10.1159/000332756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285:3133–3144. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cassis P, Conti S, Remuzzi G, Benigni A. Angiotensin receptors as determinants of life span. Pflugers Arch. 2010;459:325–332. doi: 10.1007/s00424-009-0725-4. [DOI] [PubMed] [Google Scholar]
- 159.van Rooi E. The art of microRNA research. Circ Res. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- 160.Hartmann D, Thum T. MicroRNAs and vascular (dys)function. Vascul Pharmacol. 2011;55:92–105. doi: 10.1016/j.vph.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 161.Ali AS, Ali S, Ahmad A, Bao B, Philip PA, Sarkar FH. Expression of microRNAs: potential molecular link between obesity, diabetes and cancer. Obes Rev. 2011;12:1050–1062. doi: 10.1111/j.1467-789X.2011.00906.x. [DOI] [PubMed] [Google Scholar]
- 162.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barth E, Stämmler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 164.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin H, Qian J, Castillo AC, Long B, Keyes KT, Chen G, Ye Y. Effect of miR-23 on oxidant-induced injury in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:6308–6314. doi: 10.1167/iovs.10-6632. [DOI] [PubMed] [Google Scholar]
- 168.Nishi H, Ono K, Iwanaga Y, Horie T, Nagao K, Takemura G, Kinoshita M, Kuwabara Y, Mori RT, Hasegawa K, Kita T, Kimura T. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem. 2010;285:4920–4930. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA. 2008;105:7815–7820. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kraegen EW, Cooney GJ, Turner N. Muscle insulin resistance: a case of fat overconsumption, not mitochondrial dysfunction. Proc Natl Acad Sci USA. 2008;105:7627–7628. doi: 10.1073/pnas.0803901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880–888. doi: 10.1161/HYPERTENSIONAHA.109.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Griffiths-Jones S. miRBase: the microRNA sequence database. Methods Mol Biol. 2006;342:129–138. doi: 10.1385/1-59745-123-1:129. [DOI] [PubMed] [Google Scholar]
- 174.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pulakat L, Demarco VG, Ardhanari S, Chockalingam A, Gul R, Whaley-Connell AT, Sowers JR. Adaptive mechanisms to compensate for overnutrition-induced cardiovascular abnormalities. Am J Physiol Regul Integr Comp Physiol. 2011;301:R885–R895. doi: 10.1152/ajpregu.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pulakat L, Aroor A, Gul R, Sowers JR. Cardiac insulin resistance and microRNA modulators. Exp Diabetes Res. 2012;2012:654904. doi: 10.1155/2012/654904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286:25992–26002. doi: 10.1074/jbc.M111.229401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Oba S, Kumano S, Suzuki E, Nishimatsu H, Takahashi M, Takamori H, Kasuya M, Ogawa Y, Sato K, Kimura K, Homma Y, Hirata Y, Fujita T. miR-200b precursor can ameliorate renal tubulointerstitial fibrosis. PLoS One. 2010;5:e13614. doi: 10.1371/journal.pone.0013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Condorelli G, Latronico MV, Dorn GW. microRNAs in heart disease: putative novel therapeutic targets? Eur Heart J. 2010;31:649–658. doi: 10.1093/eurheartj/ehp573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Favre C, Zhdanov A, Leahy M, Papkovsky D, O'Connor R. Mitochondrial pyrimidine nucleotide carrier (PNC1) regulates mitochondrial biogenesis and the invasive phenotype of cancer cells. Oncogene. 2010;29:3964–3976. doi: 10.1038/onc.2010.146. [DOI] [PubMed] [Google Scholar]
- 182.Schickel R, Park SM, Murmann AE, Peter ME. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell. 2010;38:908–915. doi: 10.1016/j.molcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Bio. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 184.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop – a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009;37:D105–D110. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kirby DM, Thorburn DR. Approaches to finding the molecular basis of mitochondrial oxidative phosphorylation disorders. Twin Res Hum Genet. 2008;11:395–411. doi: 10.1375/twin.11.4.395. [DOI] [PubMed] [Google Scholar]
- 188.Kiryu-Seo S, Gamo K, Tachibana T, Tanaka K, Kiyama H. Unique anti-apoptotic activity of EAAC1 in injured motor neurons. EMBO J. 2006;25:3411–3421. doi: 10.1038/sj.emboj.7601225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci. 2008;28:12581–12590. doi: 10.1523/JNEUROSCI.3338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM, Tang ZY, Wang XW. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 191.George A, Figueredo VM. Alcoholic cardiomyopathy: a review. J Card Fail. 2011;17:844–849. doi: 10.1016/j.cardfail.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 192.Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 193.Awtry EH, Philippides GJ. Alcoholic and cocaine-associated cardiomyopathies. Prog Cardiovasc Dis. 2010;52:289–299. doi: 10.1016/j.pcad.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 194.Djoussé L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Curr Atheroscler Rep. 2008;10:117–120. doi: 10.1007/s11883-008-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Djoussé L, Lee IM, Buring JE, Gaziano JM. Alcohol consumption and risk of cardiovascular disease and death in women: potential mediating mechanisms. Circulation. 2009;120:237–424. doi: 10.1161/CIRCULATIONAHA.108.832360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Iacovoni A, De Maria R, Gavazzi A. Alcoholic cardiomyopathy. J Cardiovasc Med. 2010;11:884–892. doi: 10.2459/JCM.0b013e32833833a3. [DOI] [PubMed] [Google Scholar]
- 197.Jing L, Jin CM, Li SS, Zhang FM, Yuan L, Li WM, Sang Y, Li S, Zhou LJ. Chronic alcohol intake-induced oxidative stress and apoptosis: role of CYP2E1 and calpain-1 in alcoholic cardiomyopathy. Mol Cell Biochem. 2012;359:283–293. doi: 10.1007/s11010-011-1022-z. [DOI] [PubMed] [Google Scholar]
- 198.Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11:453–462. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- 199.Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2:497–506. doi: 10.1177/1753944708095137. [DOI] [PubMed] [Google Scholar]
- 200.Manzo-Avalos S, Molina SA. Cellular and mitochondrial effects of alcohol consumption. Int Environ Res Public Health. 2010;7:4281–4304. doi: 10.3390/ijerph7124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15:523–534. doi: 10.1089/ars.2010.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guo R, Ren J. Alcohol and acetaldehyde in public health: from marvel to menace. Int J Environ Res Public Health. 2010;7:1285–1301. doi: 10.3390/ijerph7041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cullmann M, Hilding A, Ostenson CG. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a Swedish population. [DOI] [PubMed]
- 204.Fan AZ, Russell M, Naimi T, Li Y, Liao Y, Jiles R, Mokdad AH. Patterns of alcohol consumption and the metabolic syndrome. J Clin Endocrinol Metab. 2008;93:3833–3838. doi: 10.1210/jc.2007-2788. [DOI] [PubMed] [Google Scholar]
- 205.Carlsson S, Hammar N, Grill V, Kaprio J. Alcohol consumption and the incidence of type 2 diabetes: a 20-year follow-up of the Finnish twin cohort study. Diabetes Care. 2003;26:2785–2790. doi: 10.2337/diacare.26.10.2785. [DOI] [PubMed] [Google Scholar]
- 206.Liang X, Hu M, Rogers CQ, Shen Z, You M. Role of SIRT1-FoxO1 signaling in dietary saturated fat-dependent upregulation of liver adiponectin receptor 2 in ethanol-administered mice. Antioxid Redox Signal. 2011;15:425–435. doi: 10.1089/ars.2010.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liangpunsakul S, Sozio MS, Shin E, Zhao Z, Xu Y, Ross RA, Zeng Y, Crabb DW. Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am J Physiol Gastrointest Liver Physiol. 2010;298:G1004–G1012. doi: 10.1152/ajpgi.00482.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ge W, Guo R, Ren J. AMP-dependent kinase and autophagic flux are involved in aldehyde dehydrogenase-2-induced protection against cardiac toxicity of ethanol. Free Radic Biol Med. 2011;51:1736–1748. doi: 10.1016/j.freeradbiomed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shulga N, Pastorino JG. Ethanol sensitizes mitochondria to the permeability transition by inhibiting deacetylation of cyclophilin-D mediated by sirtuin-3. J Cell Sci. 2010;123:4117–4127. doi: 10.1242/jcs.073502. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 210.Powell CL, Bradford BU, Craig CP, Tsuchiya M, Uehara T, O'Connell TM, Pogribny IP, Melnyk S, Koop DR, Bleyle L, Threadgill DW, Rusyn I. Mechanism for prevention of alcohol-induced liver injury by dietary methyl donors. Toxicol Sci. 2010;115:131–139. doi: 10.1093/toxsci/kfq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 213.Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008;44:992–1001. doi: 10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 214.Chen L, Wang F, Sun X, Zhou J, Gao L, Jiao Y, Hou X, Qin CY, Zhao J. Chronic ethanol feeding impairs AMPK and MEF2 expression and is associated with GLUT4 decrease in rat myocardium. Exp Mol Med. 2010;42:205–215. doi: 10.3858/emm.2010.42.3.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li SY, Gilbert SA, Li Q, Ren J. Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol. 2009;47:247–255. doi: 10.1016/j.yjmcc.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cai L. Alcoholic cardiomyopathy: acetaldehyde, insulin insensitization and ER stress. J Mol Cell Cardiol. 2008;44:979–982. doi: 10.1016/j.yjmcc.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 217.Aroor AR, Lee YJ, Shukla SD. Activation of MEK 1/2 and p42/44 MAPK by angiotensin II in hepatocyte nucleus and their potentiation by ethanol. Alcohol. 2009;43:315–322. doi: 10.1016/j.alcohol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dewas C, Fay M, Gougerot-Pocidalo MA, El-Benna J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J Immunol. 2000;165:5238–5244. doi: 10.4049/jimmunol.165.9.5238. [DOI] [PubMed] [Google Scholar]