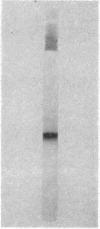
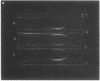
Full text
PDF
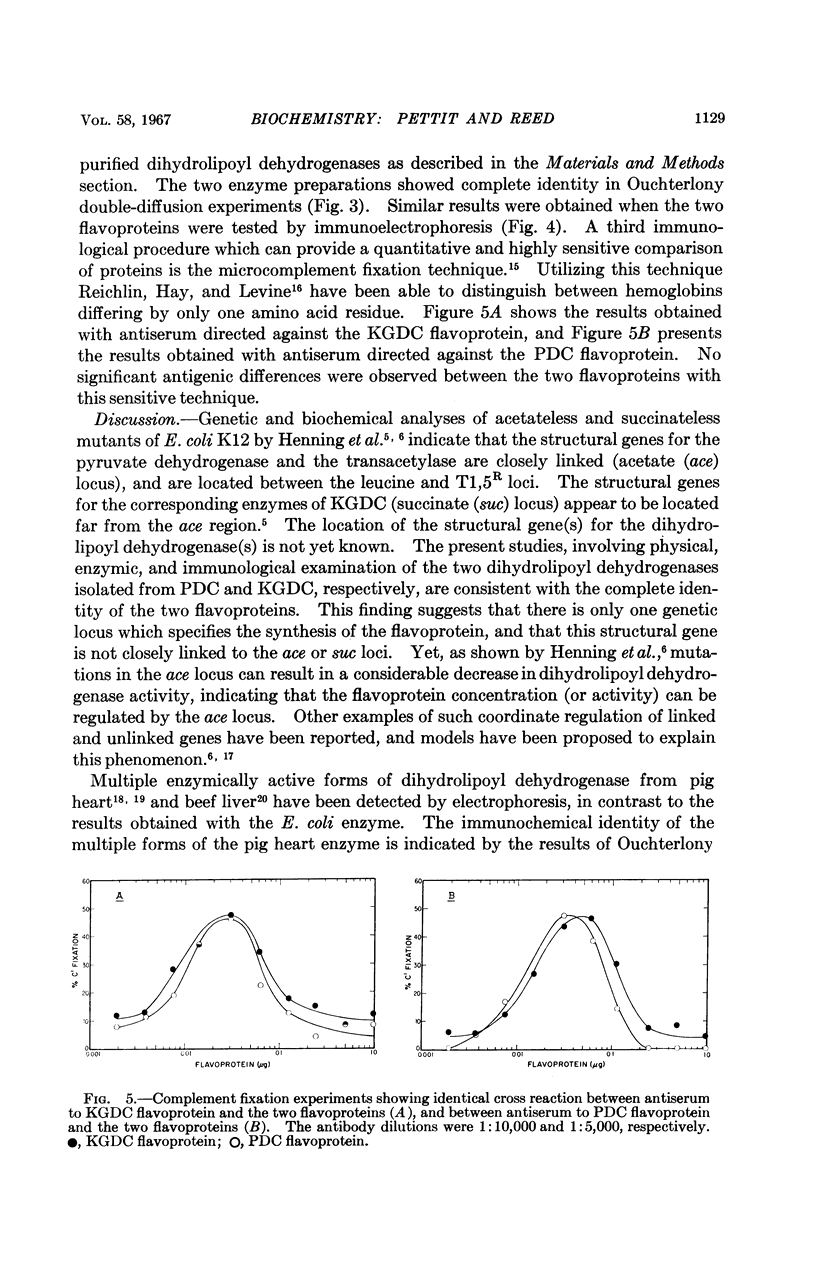

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H., PAGE E. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. V. Oxidation-reductions of the flavoproteins. J Biol Chem. 1957 Mar;225(1):479–497. [PubMed] [Google Scholar]
- Cline A. L., Bock R. M. Translational control of gene expression. Cold Spring Harb Symp Quant Biol. 1966;31:321–333. doi: 10.1101/sqb.1966.031.01.042. [DOI] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A., Jr Méthode immuno-électrophorétique d'analyse de mélanges de substances antigéniques. Biochim Biophys Acta. 1955 May;17(1):67–74. doi: 10.1016/0006-3002(55)90320-6. [DOI] [PubMed] [Google Scholar]
- HENNING U., HERZ C. EIN STRUKTURGEN-KOMPLEX FUER DEN PYRUVAT-DEHYDROGENASE-KOMPLEX VON ESCHERICHIA COLI K 12. Z Vererbungsl. 1964 Nov 11;95:260–275. [PubMed] [Google Scholar]
- Henning U., Dennert G., Hertel R., Shipp W. S. Translation of the structural genes of the E. coli pyruvate dehydrogenase complex. Cold Spring Harb Symp Quant Biol. 1966;31:227–234. doi: 10.1101/sqb.1966.031.01.031. [DOI] [PubMed] [Google Scholar]
- KAPLAN S., ENSIGN S., BONNER D. M., MILLS S. E. GENE PRODUCTS OF CRM -- MUTANTS AT THE TD LOCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:372–378. doi: 10.1073/pnas.51.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. I. Purification and properties of pyruvate and alpha-ketoglutarate dehydrogenation complexes of Escherichia coli. J Biol Chem. 1960 Jul;235:1924–1930. [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. IV. Resolution and reconstitution of the Escherichia coli pyruvate dehydrogenation complex. J Biol Chem. 1963 Jan;238:30–39. [PubMed] [Google Scholar]
- Kitto G. B., Wassarman P. M., Kaplan N. O. Enzymatically active conformers of mitochondrial malate dehydrogenase. Proc Natl Acad Sci U S A. 1966 Aug;56(2):578–585. doi: 10.1073/pnas.56.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUSTY C. J. LIPOYL DEHYDROGENASE FROM BEEF LIVER MITOCHONDRIA. J Biol Chem. 1963 Oct;238:3443–3452. [PubMed] [Google Scholar]
- MASSEY V., HOFMANN T., PALMER G. The relation of function and structure in lipoyl dehydrogenase. J Biol Chem. 1962 Dec;237:3820–3828. [PubMed] [Google Scholar]
- REICHLIN M., HAY M., LEVINE L. ANTIBODIES TO HUMAN A1 HEMOGLOBIN AND THEIR REACTION WITH A2, S, C, AND H HEMOGLOBINS. Immunochemistry. 1964 Apr;1:21–30. doi: 10.1016/0019-2791(64)90052-7. [DOI] [PubMed] [Google Scholar]
- Stein A. M., Stein J. H. Studies on the Straub diaphorase. I. Isolation of multiple forms. Biochemistry. 1965 Aug;4(8):1491–1500. doi: 10.1021/bi00884a005. [DOI] [PubMed] [Google Scholar]
- Stein A. M., Wolf B., Stein J. H. Studies on the Straub diaphorase. II. Properties of an antibody to the Straub diaphorase. Biochemistry. 1965 Aug;4(8):1500–1505. doi: 10.1021/bi00884a006. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]