Abstract
This study was conducted in the Swedish sub-Arctic, near Abisko, in order to assess the direction and scale of possible vegetation changes in the alpine–birch forest ecotone. We have re-surveyed shrub, tree and vegetation data at 549 plots grouped into 61 clusters. The plots were originally surveyed in 1997 and re-surveyed in 2010. Our study is unique for the area as we have quantitatively estimated a 19% increase in tree biomass mainly within the existing birch forest. We also found significant increases in the cover of two vegetation types—“birch forest-heath with mosses” and “meadow with low herbs”, while the cover of snowbed vegetation decreased significantly. The vegetation changes might be caused by climate, herbivory and past human impact but irrespective of the causes, the observed transition of the vegetation will have substantial effects on the mountain ecosystems.
Keywords: Sub-Arctic, Vegetation change, Treeline, Biomass, Birch forest
Introduction
During the last 15 years, there has been an increasing focus on how climate change has and will affect the distribution and extent of ecosystems around the globe including alpine and Arctic areas (e.g., Callaghan et al. 2005). The impacts on ecosystem distribution and parameters such as plant growth and species richness are expected to be particularly substantial in Arctic and sub-Arctic areas, as the change and variation in climatic parameters such as temperature and precipitation may increase with distance from the equator (Callaghan et al. 2005; Kattsov et al. 2005; Anisimov et al. 2007). For example, there has been a mean annual temperature increase in the Arctic of about 2°C since the 1960s, exceeding the global warming trend by at least 1°C (McBean et al. 2005). Climate warming at Abisko in sub-Arctic Sweden has been slightly less pronounced, but has been sustained since 1913 and has accelerated during the last 20 years (Callaghan et al. 2010). It has been suggested that alpine plants may advance upslope or pole-ward, while they might be out-competed at their former localities and eventually displaced from local niches including mountaintops (Callaghan et al. 2005; Wilson and Nilsson 2009; Scherrer and Körner 2011). Models predict that the extent of mountain birch forest in Fennoscandia will increase with climate warming, substantially reducing the areal extent of alpine heaths (Moen et al. 2004). Indeed, field studies and remote sensing have revealed a recent increase in altitude of the treeline (e.g., Kullman 2002), and an extension and increased cover of mountain birch forest (Tømmervik et al. 2009; Rundqvist et al. 2011, this issue). Tømmervik et al. (2009) have—based on remote sensing data—estimated that tree biomass has doubled over a 43-year period, within an area of Finnmarksvidda, and Rundqvist et al. (2011) have observed an increased density and cover of mountain birch in the treeline over the last three decades, within an area near Abisko village.
Plants restricted to snowbeds are suggested to be particularly susceptible to decreased snow-fall and longer growing seasons (Callaghan et al. 2005; Björk and Molau 2007). However, it could also be suspected that heaths dominated by dwarf shrubs may be converted to heaths dominated by graminoids or larger shrubs, as experiments suggest that shrubs and graminoids may increase following warming (e.g., Walker et al. 2006). Indeed, comparison of old and new photographs has revealed an expansion of large shrubs throughout the Arctic (Sturm et al. 2001; Tape et al. 2006; Forbes et al. 2010).
A recent study in the Abisko area has, however, revealed a more complex pattern in treeline dynamics in that the treeline may be depressed at one location but increase in altitude at another nearby site that experienced the same climatic conditions (Van Boagert et al. 2011). Plant community composition and the distribution of mountain birch forest and alpine vegetation are, however, not solely determined by climate. Experiments and observations have revealed that herbivores may have a substantial impact on the distribution and abundance of plants in Arctic and sub-Arctic areas (Tenow et al. 2001; Cairns and Moen 2004; Olofsson et al. 2009; Van Bogaert et al. 2009, 2011; Babst et al. 2010). Human activities have also depressed the tree line in many areas of Scandinavia (Emanuelsson 1987; Karlsson et al. 2007).
In 1997, Dahlberg et al. (2004) conducted an extensive study in the Swedish sub-Arctic, near Abisko. They measured tree diameter on permanently marked sample plots, and estimated proportions of shrubs and vegetation cover based on the methodology of the Swedish National Forest Inventory. The rigorous sampling design used gives us a unique opportunity to resample these plots and quantitatively assess how vegetation has changed, in the tundra–birch forest ecotone between 1997 and 2010. Specifically, this study assess whether the cover of vegetation, shrub and tree cover, tree canopy cover, mean diameter of stems, number of trees, basal area and biomass have changed since 1997.
Study Area
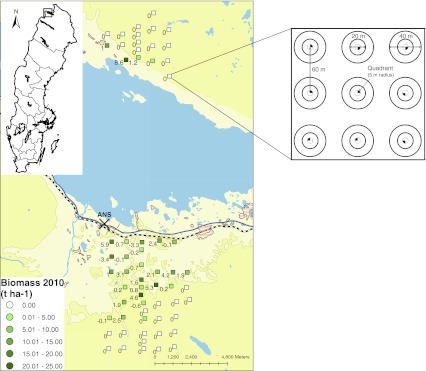
This study was conducted in two areas close to the Abisko village approximately 200 km north of the Arctic Circle, (68°20′ N, 18°50′ E; Fig. 1). One area was located on the southern side of Lake Torneträsk and the other on the northern side. Climate change is accelerating at Abisko and the mean annual temperature recorded at the beginning of the 21st century was 0.7°C (Kohler et al. 2006) which is 2.5°C greater than in the beginning of the twentieth century (Callaghan et al. 2010). The mean annual precipitation, measured at Abisko Scientific Research Station (ANS), is ~310 mm for the period 1913–2000 (Kohler et al. 2006). The mean annual precipitation on the northern side of the lake could, however, be about twice as high as recorded at ANS (Sonesson and Hoogsteger 1983). The altitudinal limit of the treeline in the area varies between 650 and 700 m above sea level (Dahlberg et al. 2004; Van Bogaert et al. 2011).
Fig. 1.
Location of the study area. The large map shows the location of the area from which biomass of the 61 clusters (squares) were re-investigated in 2010. The location of the Abisko Scientific Research Station (ANS; cross) where the meteorological station is located is also included. The biomass estimate for a cluster in 2010 is denoted by color, see legend. Numbers close to each cluster represent the change in biomass between 1997 and 2010. Each cluster consisted of nine plots and each plot consisted of two concentric circular plots and one quadrant. © Lantmäteriet, ärende nr I 2010/0345
Materials and Methods
The 1997 survey established 88 clusters with 9 permanently marked circular plots within each cluster (Dahlberg et al. 2004; Fig. 1). We re-surveyed 61 of the original 88 clusters in the summer of 2010. The clusters were organized in a systematic grid. The distance between the clusters was 1 km except in the central grid-transect where the distance was 0.5 km between the clusters. Forty-three of the re-surveyed clusters were located on the southern side of the lake, and 18 on the northern side. The altitude ranged from 390 to 1350 m above sea level. The nine plots within each cluster formed a regularly spaced grid with 3 × 3 plots with 60 m distance, and each plot consisted of two concentric circular plots of different diameter and one quadrant (Fig. 1; Dahlberg et al. 2004). We “calibrated” between the years as one of the original observers (J. Bergstedt), who designed the original sampling procedures and also did a lot of the fieldwork in 1997, introduced the field-staff in 2010.
Measurements of Stem Diameters
All trees with a DBH (diameter at breast height) ≥30 mm were measured with a caliper in each 20 m diameter plot, in both 1997 and 2010. In addition, trees <30 mm DBH with at least 1.3 m height were measured within a quadrant with a radius of 5 m (Dahlberg et al. 2004). However, willows was only measured if DBH was ≥20 mm. A fork below breast height (1.3 m height) was considered as a branch if thinner than 20 mm and as a stem if thicker than 20 mm (Dahlberg et al. 2004).
Shrub and Tree Coverage
The cover, both separately for each species and total, of all shrubs and small trees <1.3 m height, were estimated in each 20 m diameter plot. Cover of each shrub and small tree were visually estimated in 10 equal percentage classes. A specific shrub or tree taxon needed to cover at least 3 m2 to be noted. Similarly, the canopy cover of all trees, i.e., shrubs and trees ≥1.3 m height, was visually estimated in each 20 m diameter plot.
Vegetation Types
The cover of each vegetation type, in each 40 m diameter plot, was classified according to the Swedish vegetation map (Table 1; Liberkartor 1981; Rafstedt 1985). Proportional cover of each vegetation type was visually estimated in 10 equal percentage area classes. The occurrence of boulders and rocks was not considered in this estimation. A specific vegetation type needed to cover at least 3 m2 to be noted.
Table 1.
The mean cover of each vegetation class in 1997 and 2010, respectively
| Vegetation typesa | Abbrevations | Year 1997 | Year 2010 | Paired t-test | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | ||
| Grass heath | H(g) | 6.04 | 21.67 | 5.59 | 20.62 | −0.30 | 59 | 0.77 |
| Extremely dry heath | H(ex.dr) | 3.57 | 11.72 | 4.49 | 13.03 | 0.98 | 59 | 0.33 |
| Dry heath | H(dr) | 35.01 | 30.23 | 38.79 | 32.99 | 1.98 | 59 | 0.052 |
| Fresh heath | H(fr) | 9.63 | 18.41 | 9.80 | 19.73 | 0.59 | 59 | 0.56 |
| Meadow with low herbs | M(lh) | 4.06 | 12.27 | 6.47 | 14.34 | 2.08 | 59 | 0.042 |
| Meadow with tall herbs | M(th) | 1.04 | 5.27 | 1.04 | 5.83 | −0.47 | 59 | 0.64 |
| Moderate snowbed | SB(mod) | 13.86 | 26.24 | 7.38 | 18.55 | −4.43 | 59 | <0.001 |
| Extreme snowbed | SB(ex) | 0.37 | 2.87 | 0 | 0 | −1.00 | 59 | 0.32 |
| Bog and fen hummock vegetation | BoFe(hu) | 1.41 | 5.44 | 1.19 | 4.80 | −0.31 | 59 | 0.76 |
| Dry fen | Fe(dr) | 1.65 | 5.04 | 2.13 | 7.00 | 0.18 | 59 | 0.86 |
| Sloping fen | Fe(sl) | 0.67 | 2.66 | 0.67 | 2.66 | – | 59 | – |
| Wet fen | Fe(we) | 0.11 | 0.73 | 0.17 | 0.84 | 1.00 | 59 | 0.32 |
| Mosaic mire between BoFe(hu) and Fe(dr) | M(mos) | 0.17 | 1.29 | 0 | 0 | −1.00 | 59 | 0.32 |
| Willow-shrubs | W | 4.33 | 9.89 | 4.11 | 9.63 | −1.50 | 59 | 0.14 |
| Birch forest, heath type with lichens | BFo(l) | 1.06 | 4.03 | 0.96 | 4.06 | −0.58 | 59 | 0.57 |
| Birch forest, heath type with mosses | BFo(m) | 8.69 | 21.21 | 10.63 | 22.89 | 3.09 | 59 | 0.003 |
| Birch forest, meadow type with herbs | BFo(h) | 0.65 | 2.45 | 0.65 | 2.51 | −0.22 | 59 | 0.83 |
| Birch forest, sparsely grownb | BFo(sp) | 7.52 | 15.57 | 5.93 | 13.81 | −1.96 | 59 | 0.055 |
The mean values are percentages. Paired t-tests were based on arcsin-transformed values from 60 clusters. One cluster was omitted from the analyses due to missing values in 1997
Bold figures denote significant changes in cover of vegetation classes between 1997 and 2010
aVegetation types classified according to the Swedish vegetation map (Liberkartor 1981; Rafstedt 1985)
bClass 64, birch forest-sparsely grown has a canopy cover between 10 and 30%; it is in fact a mix of two birch forest types with low canopy cover, swampy birch forest and sparsely grown birches at drier soils
Data Analysis
In order to avoid spatial auto-correlation, we calculated the average of all variables for the nine plots in each cluster, and used these variables in subsequent analyses. All 61 clusters were included in all analyses except in the vegetation-type analysis. One cluster was omitted from that analysis due to missing values in 1997. All statistical analyses were performed using the R statistical package (Ihaka and Gentleman 1996). The plots were permanently marked which made it possible to conduct paired t-tests.
Analysis of the Stem Diameter Measurements
Basal areas (i.e. the cross section areas of tree trunks, at breast height, expressed as square meters per hectares) were calculated for each tree species from stem diameters. The biomass of living trees (i.e. dry weight of living tree tissues) was calculated by using allometric relationships determined in the field and summarized in the following equation (Dahlberg et al. 2004):
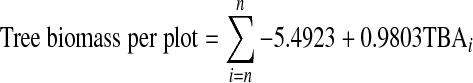 |
TBAi is the cross sectional area of an individual tree trunk at breast height (mm2), and n is the number of trees in a plot. Tree biomass per plot (kg; dry weight) was re-scaled to biomass per hectare.
We used paired t-tests to assess whether there were significant differences in mean diameter, number of trunks per ha, basal area and biomass between 1997 and 2010. Analyses were conducted for all tree species combined as well as for all focal tree species separately, i.e., mountain birch Betula pubescens ssp. czerepanovii willow Salix spp., alder Alnus incana, rowan Sorbus aucuparia, pine Pinus sylvestris and “other” tree species. We also conducted separate tests for large trees and small trees as well as for large and small trees combined. We used Spearman rank correlation tests to assess whether biomass in 1997 and 2010 and biomass change were correlated with altitude.
Shrub and Tree Coverage
The shrub cover (shrubs and trees <1.3 m in height) and tree canopy cover (cover of trees ≥1.3 m) were analyzed separately with the aid of paired t-tests. This was conducted for all shrub and tree species combined as well as for all focal shrub and tree species separately. The percentage classes were arcsin-transformed prior to analysis to meet the assumptions of normality.
Vegetation Types
We assessed whether the cover of different vegetation types had changed since 1997 until 2010 with the aid of paired t-tests on arcsin-transformed data.
Results
Mean Diameter, Number of Trunks, Basal Area and Tree Biomass
We measured the trunk diameter for a total of 6054 mountain birches, 138 alders and 207 rowans in 2010. In 1997, 154 of 549 plots had trees (≥1.3 m height) compared to 163 plots in 2010. The number of small trunks (<30 mm DBH) made up about 70% of all trunks whereas large trees (≥30 mm DBH) made up 89% of the basal area and total biomass. The mean above ground tree biomass was 4176 (SD = 6475) kg ha−1 based on all 61 clusters in 2010 (Fig. 1; Table 2). The biomass was 8785 (SD = 6923) kg ha−1 in 2010 if we instead use the mean biomass based on clusters where at least one plot had trees higher than 1.3 m (n = 29).
Table 2.
Tree characteristics of the studied clusters in 1997 and 2010, for small trees (trees <30 mm at DBH and higher than 1.3 m), large trees (trees >30 mm at DBH) and all trees (all trees higher than 1.3 m)
| Small trees | Large trees | All trees | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | 2010 | 1997 | 2010 | 1997 | 2010 | |||||||||||
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | Meana | SD | Meana | SD | ta | p | |
| Mountain birch | ||||||||||||||||
| Mean diam. (mm) | 5.4 | 7.2 | 4.6 | 6.2 | 0.23 | 27.3 | 33.8 | 29.2 | 35.7 | 0.005 | 16.5 | 24.3 | 15.7 | 26.2 | −0.45 | 0.66 |
| No. of trunks (ha−1) | 826.6 | 1695.5 | 804.3 | 1364.1 | 0.86 | 252.7 | 429.7 | 300.7 | 496.4 | 0.008 | 1079.2 | 1069.8 | 1105.0 | 1081.3 | 0.21 | 0.83 |
| Basal area (m2 ha−1) | 0.15 | 0.33 | 0.13 | 0.22 | 0.39 | 0.79 | 1.26 | 0.99 | 1.54 | <0.001 | 0.94 | 0.93 | 1.12 | 1.10 | 2.98 | 0.004 |
| TDW (kg ha−1) | 544 | 1216 | 461 | 808 | 0.39 | 2741 | 4384 | 3452 | 5330 | <0.001 | 3285 | 5216 | 3914 | 5968 | 2.92 | 0.005 |
| Willow | ||||||||||||||||
| Mean diam. (mm) | 1.7 | 5.8 | 0.3 | 2.7 | 0.038 | 11.4 | 18.0 | 11.1 | 19.4 | 0.83 | 10.3 | 16.4 | 10.9 | 19.2 | 0.35 | 0.73 |
| No. of trunks (ha−1) | 13.8 | 49.7 | 0.9 | 7.2 | 0.047 | 9.0 | 22.5 | 7.9 | 26.7 | 0.55 | 22.7 | 23.0 | 8.9 | 9.0 | −2.20 | 0.032 |
| Basal area (m2 ha−1) | <0.01 | 0.02 | <0.01 | <0.01 | 0.09 | 0.01 | 0.04 | 0.01 | 0.05 | 0.82 | 0.02 | 0.02 | 0.01 | 0.01 | −0.91 | 0.37 |
| TDW (kg ha−1) | 15 | 64 | 1 | 9 | 0.09 | 49 | 142 | 51 | 173 | 0.83 | 64 | 174 | 52 | 173 | −0.94 | 0.35 |
| Alder | ||||||||||||||||
| Mean diam. (mm) | 0.4 | 3.2 | 0.2 | 1.0 | 0.45 | 2.0 | 11.3 | 2.3 | 12.6 | 0.19 | 2.0 | 11.1 | 1.3 | 7.0 | −1.43 | 0.16 |
| No. of trunks (ha−1) | 0.5 | 3.8 | 9.31 | 65.5 | 0.27 | 9.4 | 67.4 | 11.4 | 83.4 | 0.32 | 9.9 | 10.0 | 20.7 | 21.0 | 1.09 | 0.28 |
| Basal area (m2 ha−1) | <0.01 | <0.01 | <0.01 | <0.01 | 0.30 | 0.04 | 0.32 | 0.06 | 0.45 | 0.30 | 0.04 | 0.04 | 0.06 | 0.06 | 1.03 | 0.30 |
| TDW (kg ha−1) | 0.9 | 6.8 | 1.7 | 13.2 | 0.30 | 148.7 | 1104 | 208.0 | 1553 | 0.30 | 150 | 1111 | 210 | 1566 | 1.03 | 0.30 |
| Rowan | ||||||||||||||||
| Mean diam. (mm) | 1.0 | 4.5 | 0 | – | 0.09 | 2.7 | 13.7 | 1.2 | 6.5 | 0.40 | 3.7 | 14.3 | 1.2 | 6.5 | −1.41 | 0.16 |
| No. of trunks (ha−1) | 7.9 | 53.8 | 0 | – | 0.26 | 0.2 | 0.7 | 0.2 | 1.4 | 0.68 | 8.0 | 8.1 | 0.23 | 0.24 | −1.16 | 0.25 |
| Basal area (m2 ha−1) | <0.01 | 0.01 | 0 | – | 0.22 | <0.01 | <0.01 | <0.01 | <0.01 | 0.62 | <0.01 | <0.01 | <0.01 | <0.01 | −1.56 | 0.12 |
| TDW (kg ha−1) | 7 | 43 | 0 | – | 0.22 | 2 | 10 | 1 | 6 | 0.64 | 8 | 44 | 1 | 6 | −1.54 | 0.13 |
| Pine | ||||||||||||||||
| Mean diam. (mm) | 0.4 | 2.88 | 0 | – | 0.32 | 0 | – | 0 | – | – | 0.4 | 2.8 | 0 | 0 | −1.00 | 0.32 |
| No. of trunks (ha−1) | 0.5 | 3.8 | 0 | – | 0.32 | 0 | – | 0 | – | – | 0.5 | 0.5 | 0 | 0 | −1.00 | 0.32 |
| Basal area (m2 ha−1) | <0.01 | <0.01 | 0 | – | 0.32 | 0 | – | 0 | – | – | <0.01 | <0.01 | 0 | 0 | −1.00 | 0.32 |
| TDW (kg ha−1) | <1 | 5 | 0 | – | 0.32 | 0 | – | 0 | – | – | <1 | 5 | 0 | 0 | −1.00 | 0.32 |
| Other Tree species | ||||||||||||||||
| Mean diam. (mm) | 0.1 | 1.0 | 0 | – | 0.32 | 0 | – | 0 | – | – | 0.1 | 1.0 | 0 | 0 | −1.00 | 0.32 |
| No. of trunks (ha−1) | 0.5 | 3.8 | 0 | – | 0.32 | 0 | – | 0 | – | – | 0.5 | 0.5 | 0 | 0 | −1.00 | 0.32 |
| Basal area (m2 ha−1) | <0.01 | <0.01 | 0 | – | 0.32 | 0 | – | 0 | – | – | <0.01 | <0.01 | 0 | 0 | −1.00 | 0.32 |
| TDW (kg ha−1) | <0.1 | 0.7 | 0 | – | 0.32 | 0 | – | 0 | – | – | <0.1 | 0.7 | 0 | 0 | −1.00 | 0.32 |
| All Trees | ||||||||||||||||
| Mean diam. (mm) | 5.9 | 7.4 | 4.7 | 6.2 | 0.050 | 26.6 | 32.2 | 29.0 | 33.8 | 0.006 | 14.8 | 20.5 | 15.7 | 22.6 | 0.66 | 0.51 |
| No. of trunks (ha−1) | 849.6 | 1702.9 | 814.5 | 1374.8 | 0.78 | 271.1 | 448.7 | 320.3 | 514.9 | 0.009 | 1120.8 | 1112.0 | 1134.8 | 1111.6 | 0.11 | 0.91 |
| Basal area (m2 ha−1) | 0.15 | 0.34 | 0.13 | 0.22 | 0.29 | 0.84 | 1.35 | 1.07 | 1.69 | <0.001 | 1.00 | 1.00 | 1.19 | 1.18 | 2.84 | 0.006 |
| TDW (kg ha−1) | 568 | 1235.9 | 464 | 812.4 | 0.29 | 2940 | 4701.5 | 3712 | 5862.6 | <0.001 | 3507 | 5520 | 4176 | 6476 | 2.78 | 0.007 |
Paired t-tests were based on all 61 clusters, df = 60. Bold figures denote significant changes in tree variables between 1997 and 2010
aThe mean diameter for all trees was weighted as small and large trees, respectively, were measured on different plot size
Number of trunks is the mean number of trunks based on all clusters
Basal area is the mean basal area based on all clusters
TDW is Total dry weight (kg ha−1) and corresponds to mean tree biomass per hectare
The biomass values in 1997 and 2010, respectively, were both negatively correlated with altitude (r = −0.845, p < 0.001 and r = −0.837, p < 0.001, respectively; Figs. 1, 2). The change in tree biomass documented for 1997–2010 was positively correlated with tree biomass in 1997 (r = 0.389, p = 0.002) and negatively correlated with altitude (r = −0.295, p = 0.021).
Fig. 2.
Mean biomass of trees in relation to altitude. Black circles denote clusters on the north side of the lake in 1997, and red circles denote clusters on the south-side of the lake in 1997. Arrows denote the change in biomass between 1997 and 2010
There was a 19% overall increase in basal area and tree biomass (Table 2). The increase depended mainly on the large mountain birches (≥30 mm DBH), as their mean diameter and, number of trees per hectare increased significantly (Table 2). We also found that there was a decrease (Table 2) of small willow trees (20 to <30 mm DBH), as mean diameter and number of willow trunks and basal area of willow trees decreased significantly. None of the other tree species showed any statistically significant changes.
Shrub and Tree Cover Estimates
There was an overall significant increase, from 25.0 to 32.5%, in the total cover of shrubs (i.e., all shrubs and trees <1.3 m) between 1997 and 2010. There was also a significant cover increase of dwarf birch Betula nana, juniper Juniperus communis and willows Salix spp. (Table 3). The cover of mountain birch <1.3 m and other shrub species did not differ significantly between the years. The change in shrub cover between 1997 and 2010 was positively correlated with cover of shrubs in 1997 (r = 0.417, p < 0.001). There was also a significant overall increase of tree canopy cover (trees ≥1.3 m height; Table 4), although mountain birch was the only tree species with a statistically significant increase.
Table 3.
The mean of percentages of cover, of shrubs, i.e. shrubs and trees <1.3 m height
| Tree species | 1997 | 2010 | Paired t-test | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Mountain birch | 1.5 | 2.5 | 1.2 | 2.2 | 0.63 | 60 | 0.53 |
| Willow | 7.8 | 8.1 | 8.9 | 9.9 | 2.45 | 60 | 0.017 |
| Dwarf birch | 14.0 | 11.5 | 20.1 | 16.0 | 5.97 | 60 | <0.001 |
| Juniper | 1.4 | 2.9 | 2.1 | 4.3 | 3.49 | 60 | <0.001 |
| Other shrub species | 0.2 | 1.0 | 0.3 | 1.3 | 0.17 | 60 | 0.86 |
| All species | 25.0 | 17.0 | 32.5 | 22.7 | 6.13 | 60 | <0.001 |
Paired t-tests were based on arcsin-transformed values from all 61 clusters. Bold figures denote significant changes in shrub and tree cover between 1997 and 2010
Table 4.
The mean of percentage tree canopy cover for trees ≥1.3 m high
| Tree species | 1997 | 2010 | Paired t-test | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Mountain birch | 6.9 | 11.6 | 12.6 | 19.0 | 5.10 | 60 | <0.001 |
| Willow | 0.3 | 0.7 | 0.3 | 0.9 | −1.18 | 60 | 0.24 |
| Other tree species | 0.2 | 1.3 | 0.4 | 3.3 | −0.01 | 60 | 0.99 |
| All species | 7.5 | 12.0 | 13.4 | 20.1 | 4.91 | 60 | <0.001 |
Paired t-tests were based on arcsin-transformed values from all 61 clusters. Bold figures denote significant changes in tree canopy cover between 1997 and 2010
Vegetation Types
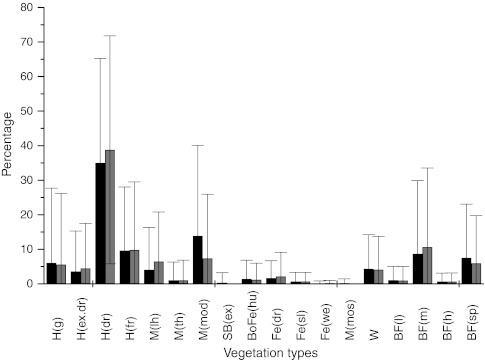
Analyses of vegetation classes revealed that the cover of two vegetation types, “Meadow with low herbs M(lh)” and “Birch forest of heath type with mosses BFo(m)”, increased significantly, while the cover of the vegetation type “Moderate snowbed vegetation SB(mod)” decreased significantly (Fig. 3; Table 1). The cover of the vegetation type “Dry heath H(dr)” increased also, while the cover of the vegetation type “Sparsely grown birch forest BFo(sp)” decreased although neither of the changes were statistically significant.
Fig. 3.
Mean cover (mean percentage ± SD) of specific vegetation classes. Black bars denote 1997 and grey bars denote 2010 data, respectively. For abbreviations of the vegetations types and statistics describing vegetation change see Table 1
Discussion
Tree basal area and biomass increased by 19% between 1997 and 2010 with the main increase occurring in the established birch forest. There were significant transitions in the cover of vegetation types; the cover of “Meadow with low herbs M(lh)” and “Birch forest of heath type with mosses BFo(m)” increased significantly, while the cover of “Moderate snowbed vegetation SB(mod)” decreased significantly. Our study concurs with the results of other studies which suggest that there has been a general increase in cover and biomass of trees and shrubs in sub-Arctic and Arctic areas (e.g., Sturm et al. 2001; Tape et al. 2006; Danby and Hik 2007; Tømmervik et al. 2009; Forbes et al. 2010; Hallinger et al. 2010; Van Bogaert et al. 2011; Rundqvist et al. 2011, this issue).
Tree biomass increased on average 1.5% per year from 3.5 t ha−1 in 1997 to 4.2 t ha−1 in 2010. We have found no other study that has estimated the tree biomass changes in the alpine–birch forest ecotone by re-surveying systematically sampled field plots. Karlsson et al. (2005) estimated—based on tree-ring series—the relative growth rate of above-ground biomass of individual trees to be 10, 5 and 2% per year in 25–30-, 50- and 100-year old trees, respectively. These results are, however, only representative for individual trees, and do not include turnover of trees in the forest. Though, it is apparent that individual mountain birch trees may have a relatively high growth potential despite the constraints of climatic conditions and browsing. Tømmervik et al. (2009)—using remote sensing techniques—estimated the annual increase in biomass to be ~2.7% in Finnmarksvidda, northern Norway over a 43-year period. Birches may, however, expand slower in steeper than in flat areas, as they are limited by the lower temperature and shorter growing season upslope (Chapin 1983; Karlsson et al. 2005). Our study was located in an area that is steeper than Finnmarksvidda, and we could thus not expect a similar expansion rate as Tømmervik et al. (2009) observed. We observed only a minor expansion of the mountain birch forest, and the biomass increase was mainly due to the growth of birches higher than 1.3 m that where already present in 1997. We also noted that the very open birch forest type decreased while the denser birch forest of heath type increased, that together with increased canopy cover suggest a densification of the mountain forest near Abisko.
It has been suggested that increased nutrient availability associated with higher soil temperatures, and a longer growing season could underpin increased tree and shrub abundance and biomass in the Arctic (e.g., Chapin 1983; Weih and Karlsson 1997; Hartley et al. 1999; Tape et al. 2006). Kammer et al. (2007) on the other hand, suggest that the observed increase may merely be a delayed re-expansion of shrubs and trees following the “Little Ice-age” that ended in the early twentieth century (Grubb 2008; see discussion in Rundqvist et al. 2011, this issue).
The observed increase in shrub and tree cover and tree biomass may, however, be related to several factors other than climate, such as reduction in herbivory pressure (e.g., Olofsson et al. 2009) or changed land management (Emanuelsson 1987; Karlsson et al. 2007). It is well known that shrubs and trees increase in abundance and biomass with decreased herbivory (Cairns and Moen 2004; Olofsson et al. 2009; Van Boagaert et al. 2011). However, the browsing pressure has probably increased rather than decreased over the last 13 years as the population of the major herbivore, reindeer, has increased in the study area since 1995 (Van Boagaert et al. 2011). Similarly, our study area has a history of outbursts of geometrid moths (mainly autumnal moth, Epirrita autumnata; Tenow et al. 2001; Karlsson and Weih 2003). The latest in 2004 severely defoliated birches in our study area, both at the north and south side of the lake (Babst et al. 2010). Thus, the biomass of shrubs and trees could potentially have been even higher without reindeers or geometrid moths (Cairns and Moen 2004; Karlsson et al. 2005; Olofsson et al. 2009; Babst et al. 2010; Van Bogaert et al. 2011). Further, we cannot exclude the possibility that the observed increased biomass and cover of shrubs and trees was caused by past anthropogenic activities associated with railway construction. Gathering of fuelwood and cutting of trees during the construction led to an unnaturally low tree line and tree cover in the Abisko area (Emanuelsson 1987). Thus, the observed increased biomass and cover of shrubs and trees may be caused by a recovery of shrubs and trees since the railroad was finished in 1903 (Emanuelsson 1987; cf. Karlsson et al. 2007).
In addition to forest changes, we observed a particularly dramatic decrease of snowbed vegetation confirming the prediction that snowbed plant communities are particularly vulnerable to climate change (Björk and Molau 2007). The decrease of snowbed vegetation reflects a shift from a sparse vegetation cover to denser vegetation characterized by species found in the surrounding vegetation (Heegard 2002; Heegaard and Vandvik 2004; Björk and Molau 2007). It is suggested that snowbed plants are restricted by growing season length and the availability of phosphorus (Björk and Molau 2007). Earlier studies indicate, however, that the immediate response of snowbed species to changed snow-depth and growing season length is highly species-specific (Galen and Stanton 1995; Sandvik et al. 2004). An alternative explanation is that graminoids have increased due to decreased lemming grazing (Virtanen 2000; Hentton and Wallgren 2001).
Further, we also found that the cover of juniper increased in agreement with findings by Hallinger et al. (2010) in the same general location. Rundqvist et al. (2011) described, a more complex pattern in which juniper increased in plots with low cover of mountain birch while it decreased in a plot with much higher cover of mountain birch. Our study revealed a different pattern with a positive correlation between the cover of juniper and basal area and cover of mountain birch. This demonstrates the complexity of vegetation change over time and the importance of fine-scaled processes (e.g., Rundqvist et al. 2011).
An increased biomass of trees, and cover of shrubs and trees, will have substantial effects on Arctic and sub-Arctic ecosystems (Callaghan et al. 2005), and species composition and diversity of vascular plants and cryptogams may change (cf., warming experiments; Walker et al. 2006). In particular, boreal species may extend their altitudinal distribution (Sundqvist et al. 2008), while alpine plant species with lower ability to compete may prevail only if they may be able to migrate upward or northward (cf. Grabherr et al. 1994; Callaghan et al. 2005; Wilson and Nilsson 2009) or survive in small niches with favorable micro-climates (Scherrer and Körner 2011). Further, reindeer might be negatively affected as the availability of palatable, nutritionally rich plants that used to occur in snowbeds decrease (Edenius et al. 2003).
There are also implications for regulatory ecosystem services (Chapin et al. 2005). The mountain birch forest is a major sink for atmospheric CO2 in the Torneträsk area (Christensen et al. 2007). Following an outbreak of the autumnal moth, such as that in 2004 (Babst et al. 2010), the defoliated forest becomes a source of CO2 to the atmosphere (Heliasz et al. 2011). However, our study has shown that despite the 2004 defoliation, there has been a net increase in biomass—and carbon drawdown—of 19%.
Acknowledgments
We sincerely thank Mats Jonasson, Patrik Åström, and Anders Petterson for conducting the field work, and the staff of the Swedish National Forest Inventory and Abisko Arctic Scientific Research Station for their support. We also thank Craig E. Tweedie and an anonymous reviewer for their valuable comments. The project was mainly funded by Swedish Environmental Protection Agency. Funding was also obtained from IPY project No 512 “Back to the Future”, Formas grant 214-2008-188.
Biographies
Henrik Hedenås
has a PhD in Ecology from the department of Ecology and Environmental Science (EMG), Umeå University. He has worked at different positions at EMG, and is currently a researcher at Abisko Scientific Research Station and temporary Forest Habitat Advisor at Species Information Centre, Swedish University of Agricultural Science.
Håkan Olsson
is since 1994 professor in forest remote sensing at the Swedish university of Agricultural Sciences. In 1997 he established the permanent field plots at Abisko as part of the Climate Impact Research Centre (CIRC) research program at the Abisko Research Station. He then initiated the re-survey of the plots in 2010.
Christer Jonasson
has a PhD in Physical Geography from Uppsala University and is Associate professor at Stockholm University. He is Station Manager for the Abisko Scientific Research Station.
Johan Bergstedt
has a PhD in Ecology from the department of IFM—Physics, Chemistry and Biology, Linköping University. He has worked as teacher and study counselor at the IFM. He has also a worked with the Swedish National Forest Inventory (NFI, performed by the department of Forest Resource Management at the Swedish University of Agricultural Sciences) for a number of years and is currently working for the NFI as well as IFM at Linköping University.
Ulrika Dahlberg
has licentiate exam from the department of Forest Resource Management, Swedish university of Agricultural sciences. She is currently working with geographical information products at Lantmäteriet.
Terry V. Callaghan
has a PhD from the University of Birmingham, UK, honorary PhDs from the Universities of Lund, Sweden, and Oulu, Finland, and a DSc from the University of Manchester, UK. He is a Royal Swedish Academy of Sciences’ Distinguished Research Professor and Member and also Professor of Arctic Ecology at the University of Sheffield, UK.
Contributor Information
Henrik Hedenås, Email: henrik.hedenas@slu.se, Email: henrik.hedenas@gmail.com.
Håkan Olsson, Email: hakan.olsson@slu.se.
Christer Jonasson, Email: christer.jonasson@ans.polar.se.
Johan Bergstedt, Email: johan.bergstedt@liu.se, Email: johan.bergstedt@gmail.com.
Ulrika Dahlberg, Email: ulrika.dahlberg@lm.se.
Terry V. Callaghan, Email: terry_callaghan@btinternet.com
References
- Anisimov OA, Vaughan DG, Callaghan TV, Furgal C, Marchant H, Prowse TD, Vilhjálmsson H, Walsh JE. Polar regions (Arctic and Antarctic) In: Parry ML, Canziani OF, Palutikof JP, Hanson CE, Linden PJ, editors. Climate change 2007: Impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Babst F, Esper J, Parlow E. Landsat TM/ETM plus and tree-ring based assessment of spatiotemporal patterns of the autumnal moth (Epirrita autumnata) in northernmost Fennoscandia. Remote Sensing of Environment. 2010;114:637–646. doi: 10.1016/j.rse.2009.11.005. [DOI] [Google Scholar]
- Björk RG, Molau U. Ecology of alpine snowbeds and the impact of global change. Arctic, Antarctic, and Alpine Research. 2007;39:34–43. doi: 10.1657/1523-0430(2007)39[34:EOASAT]2.0.CO;2. [DOI] [Google Scholar]
- Cairns DM, Moen J. Herbivory influences tree lines. Journal of Ecology. 2004;92:1019–1024. doi: 10.1111/j.1365-2745.2004.00945.x. [DOI] [Google Scholar]
- Callaghan, T.V., F. Bergholm, T.R. Christensen, C. Jonasson, U. Kokfelt, and M. Johansson. 2010. A new climate era in the sub-Arctic: Accelerating climate changes and multiple impacts. Geophysical Research Letters 37. doi:10.1029/2009GL042064.
- Callaghan TV, Björn LO, Chapin T, Chernov Y, Christensen TR, Huntley B, Ims RA, Johansson M, et al. Arctic tundra and polar desert ecosystem. In: IA AC, et al., editors. Arctic climate impact assessment (ACIA) Cambridge: Cambridge University Press; 2005. pp. 243–352. [Google Scholar]
- Chapin FS., III Direct and indirect effects of temperature on Arctic plants. Polar Biology. 1983;2:47–52. doi: 10.1007/BF00258285. [DOI] [Google Scholar]
- Chapin FS, III, Berman M, Callaghan TV, Convey P, Crepin A-S, Danell K, Ducklow H, Forbes B, et al. Polar Systems. In: Hassan R, Scholes R, Ash N, et al., editors. Ecosystems and human well-being: Current state and trends. Washington: Island Press; 2005. pp. 717–743. [Google Scholar]
- Christensen TR, Johansson T, Olsrud M, Ström L, Lindroth A, Mastepanov M, Malmer N, Friborg T, et al. A catchment-scale carbon and green house gas budget of a subarctic landscape. Philosophical Transactions of the Royal Society A. 2007;365:1643–1656. doi: 10.1098/rsta.2007.2035. [DOI] [PubMed] [Google Scholar]
- Dahlberg U, Berge TW, Petersson H, Vencatasawmy CP. Modelling biomass and leaf area index in a sub-arctic Scandinavian mountain area. Scandinavian Journal of Forest Research. 2004;19:60–71. doi: 10.1080/02827580310019266. [DOI] [Google Scholar]
- Danby RK, Hik DS. Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. Journal of Ecology. 2007;95:352–363. doi: 10.1111/j.1365-2745.2006.01200.x. [DOI] [Google Scholar]
- Edenius L, Vencatasawmy CP, Sandström P, Dahlberg U. Combining satellite imagery and ancillary data to map snowbed vegetation important to reindeer Rangifer tarandus. Arctic, Antarctic, and Alpine Research. 2003;35:150–157. doi: 10.1657/1523-0430(2003)035[0150:CSIAAD]2.0.CO;2. [DOI] [Google Scholar]
- Emanuelsson U. Human influence on vegetation in the Torneträsk area during the last three centuries. Ecological Bulletins. 1987;30:95–111. [Google Scholar]
- Forbes BC, Fauria MM, Zetterberg P. Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Global Change Biology. 2010;16:1542–1554. doi: 10.1111/j.1365-2486.2009.02047.x. [DOI] [Google Scholar]
- Galen C, Stanton ML. Response of snowbed plant species to changes in growing-season length. Ecology. 1995;76:1546–1557. doi: 10.2307/1938156. [DOI] [Google Scholar]
- Grabherr G, Gottfried M, Pauli H. Climate effects on mountain plants. Nature. 1994;369:448. doi: 10.1038/369448a0. [DOI] [PubMed] [Google Scholar]
- Grubb H. Torneträsk tree-ring width and density AD 500–2004: A test of climatic sensitivity and a new 1500-year reconstruction of north Fennoscandian summers. Climate Dynamics. 2008;31:843–857. doi: 10.1007/s00382-007-0358-2. [DOI] [Google Scholar]
- Hallinger M, Manthey M, Wilmking M. Establishing a missing link: warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist. 2010;186:890–899. doi: 10.1111/j.1469-8137.2010.03223.x. [DOI] [PubMed] [Google Scholar]
- Hartley AE, Neil C, Melillo JM, Crabtree R, Bowles FP. Plant performance and soil nitrogen mineralization in response to simulated climate change in subarctic dwarf shrub heath. Oikos. 1999;86:331–343. doi: 10.2307/3546450. [DOI] [Google Scholar]
- Heegaard H, Vandvik V. Climate change affects the outcome of competitive interactions: An application of principal response curves. Oecologia. 2004;139:459–466. doi: 10.1007/s00442-004-1523-5. [DOI] [PubMed] [Google Scholar]
- Heegard E. A model of alpine species distribution in relation to snowmelt time and altitude. Journal of Vegetation Science. 2002;13:493–504. doi: 10.1111/j.1654-1103.2002.tb02076.x. [DOI] [Google Scholar]
- Heliasz M, Johansson T, Lindroth A, Mölder M, Mastepanov M, Friborg T, Callaghan TV, Christensen TR. Quantification of C uptake in subarctic birch forest after setback by an extreme insect outbreak. Geophysical Research Letters. 2011;38:L01704. doi: 10.1029/2010GL044733. [DOI] [Google Scholar]
- Hentton, H., and H. Wallgren. 2001. Rodent dynamics and communities in the birch forest zone of northern Fennoscandia. In Nordic Mountain birch ecosystems. Man and the biosphere series, ed. F.E. Wielgolaski, Vol 27, 261–278. New York: The Parthenon Publishing Group.
- Ihaka R, Gentleman R. R: A statistical language for data analysis and graphics. Journal of Computational and Graphical Statistics. 1996;5:299–314. doi: 10.2307/1390807. [DOI] [Google Scholar]
- Kammer PM, Schöb C, Choler P. Increasing species richness on mountain summits: upward migration due to anthropogenic climate change or re-colonization? Journal of Vegetation Science. 2007;18:301–306. doi: 10.1111/j.1654-1103.2007.tb02541.x. [DOI] [Google Scholar]
- Karlsson H, Hörnberg G, Hannon G, Nordström E-M. Long-term vegetation changes in the northern Scandinavian forest limit: A human impact-climate synergy? The Holocene. 2007;17:37–49. doi: 10.1177/0959683607073277. [DOI] [Google Scholar]
- Karlsson PS, Weih M. Long-term patterns of leaf, shoot and wood production after insect herbivory in the Mountain Birch. Functional Ecology. 2003;17:841–850. doi: 10.1111/j.1365-2435.2003.00792.x. [DOI] [Google Scholar]
- Karlsson PS, Weih M, Borg C. Mountain birch growth in relation to climate and herbivores. In: Wielgolaski FE, editor. Plant Ecology, Herbivory, and Human Impact in Nordic Mountain Birch Forests. Berlin: Springer-Verlag; 2005. pp. 71–86. [Google Scholar]
- Kattsov VM, Källén E, Cattle H, Christensen J, Hanssen-Bauer I, Jóhannesen T, Karol I, Räisänen J, et al. Future climate change: Modeling and scenarios for the arctic. In: IA AC, et al., editors. Arctic climate impact assessment (ACIA) Cambridge: Cambridge University Press; 2005. pp. 243–352. [Google Scholar]
- Kohler J, Brandt O, Johansson M, Callaghan T. A long-term Arctic snow depth record from Abisko, northern Sweden, 1913–2004. Polar Research. 2006;25:91–113. doi: 10.1111/j.1751-8369.2006.tb00026.x. [DOI] [Google Scholar]
- Kullman L. Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. Journal of Ecology. 2002;90:68–77. doi: 10.1046/j.0022-0477.2001.00630.x. [DOI] [Google Scholar]
- Liberkartor. 1981. Vegetationskarta över de Svenska fjällen. Kartblad nr2, Abisko. Stockholm: LiberKartor. [Vegetation map over the Swedish mountains; in Swedish, vegetation class names in English].
- McBean G, Alekseev G, Chen D, Førland E, Fyfe J, Groisman P, King R, Melling H, et al. Arctic climate—past and present. In: IA AC, et al., editors. Arctic climate impact assessment (ACIA) Cambridge: Cambridge University Press; 2005. pp. 21–60. [Google Scholar]
- Moen J, Aune K, Edenius L, Angerbjörn A. Potential effects of climate change on treeline position in the Swedish mountains. Ecology and Society. 2004;9:16. [Google Scholar]
- Olofsson J, Oksanen L, Callaghan T, Hulme EP, Oksanen T, Suominen O. Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change Biology. 2009;15:2681–2693. doi: 10.1111/j.1365-2486.2009.01935.x. [DOI] [Google Scholar]
- Rafstedt, T. (ed.) 1985. Vegetation of the Swedish mountain area, Norrbottens county. A survey on the basis of vegetation mapping and assessment of natural values. Solna: Statens Naturvårdsverk.
- Rundqvist, S., H. Hedenås, A. Sandström, U. Emanuelsson, H. Eriksson, C. Jonasson, and T.V. Callaghan. 2011. Tree and shrub expansion over the past 34 years at the tree-line near Abisko, Sweden. Ambio. doi:10.1007/s13280-011-0174-0. [DOI] [PMC free article] [PubMed]
- Sandvik SM, Heegaard E, Elven R, Vandvik V. Response of alpine snowbed vegetation to long-term experimental warming. Ecoscience. 2004;11:150–159. [Google Scholar]
- Scherrer D, Körner C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. Journal of Biogeography. 2011;38:406–416. doi: 10.1111/j.1365-2699.2010.02407.x. [DOI] [Google Scholar]
- Sonesson M, Hoogsteger J. Recent treeline dynamics (Betula pubescens Ehrh. ssp. tortuosa (Ledeb.) Nyman) in northern Sweden. Nordicana. 1983;47:47–54. [Google Scholar]
- Sturm M, Racine C, Tape K. Climate change—increasing shrub abundance in the Arctic. Nature. 2001;411:546–547. doi: 10.1038/35079180. [DOI] [PubMed] [Google Scholar]
- Sundqvist MK, Björk RG, Molau U. Establishment of boreal forest species in alpine dwarf-shrub heath in subarctic Sweden Mountain birch advance into alpine tundra. Plant Ecology & Diversity. 2008;1:67–75. doi: 10.1080/17550870802273395. [DOI] [Google Scholar]
- Tape K, Sturm M, Racine C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biology. 2006;12:686–702. doi: 10.1111/j.1365-2486.2006.01128.x. [DOI] [Google Scholar]
- Tenow, O., H. Bylund, and B. Holmgren. 2001. Impact on mountain birch forests in the past and the future of outbreaks of two geometrid insects. In Nordic Mountain birch ecosystems. Man and the biosphere series, ed. F.E. Wielgolaski, Vol. 27, 223–239. New York: The Parthenon Publishing Group.
- Tømmervik H, Johansen B, Riseth JÅ, Karlsen SR, Solberg B, Høgda KA. Above ground biomass changes in the mountain birch forests and mountain heaths of Finnmarksvidda, northern Norway, in the period 1957–2006. Forest Ecology and Management. 2009;257:244–257. doi: 10.1016/j.foreco.2008.08.038. [DOI] [Google Scholar]
- Van Bogaert, R., K. Haneca, J. Hoogesteger, C. Jonasson, M. De Dapper, and T.V. Callaghan. 2011. A century of tree line changes in sub-Arctic Sweden show local and regional variability and only a minor role of 20th century climate warming. Journal of Biogeography. doi:10.1111/j.1365-2699.2010.02453.x.
- Bogaert R, Jonasson C, Dapper M, Callaghan TV. Competitive interaction between aspen and birch moderated by invertebrate and vertebrate herbivores and climate warming. Plant Ecology and Diversity. 2009;2:221–232. doi: 10.1080/17550870903487456. [DOI] [Google Scholar]
- Virtanen R. Effects of grazing on above-ground biomass on a mountain snowbed, NW Finland. Oikos. 2000;90:295–300. doi: 10.1034/j.1600-0706.2000.900209.x. [DOI] [Google Scholar]
- Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, Bret-Harte MS, Calef MP, et al. Plant community response to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences. 2006;103:1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih M, Karlsson PS. Growth and nitrogen utilization in seedlings of mountain birch (Betula pubescens ssp. tortuosa) as related to plant nitrogen status and temperature: A two-year study. Écoscience. 1997;4:365–373. [Google Scholar]
- Wilson SD, Nilsson C. Arctic and alpine vegetation change over years. Global Change Biology. 2009;15:1676–1684. doi: 10.1111/j.1365-2486.2009.01896.x. [DOI] [Google Scholar]