Abstract
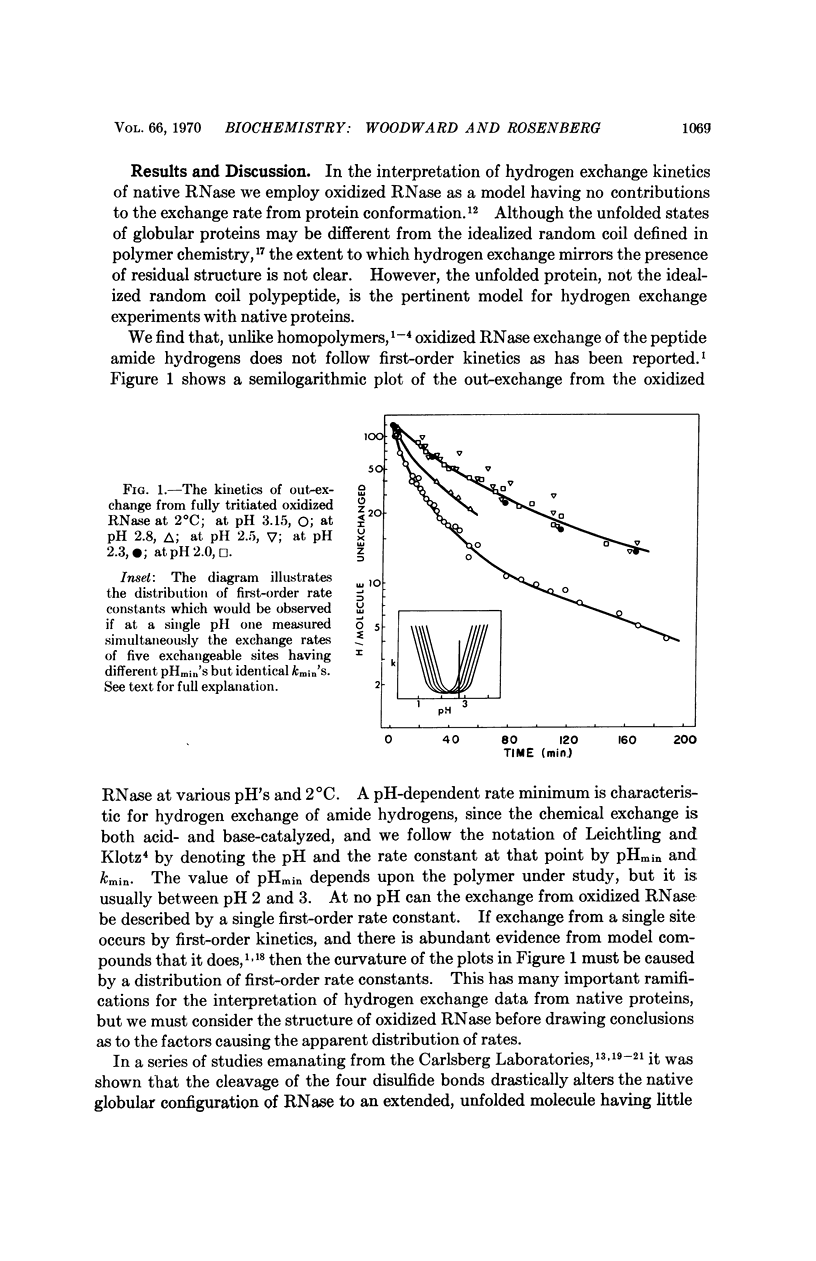
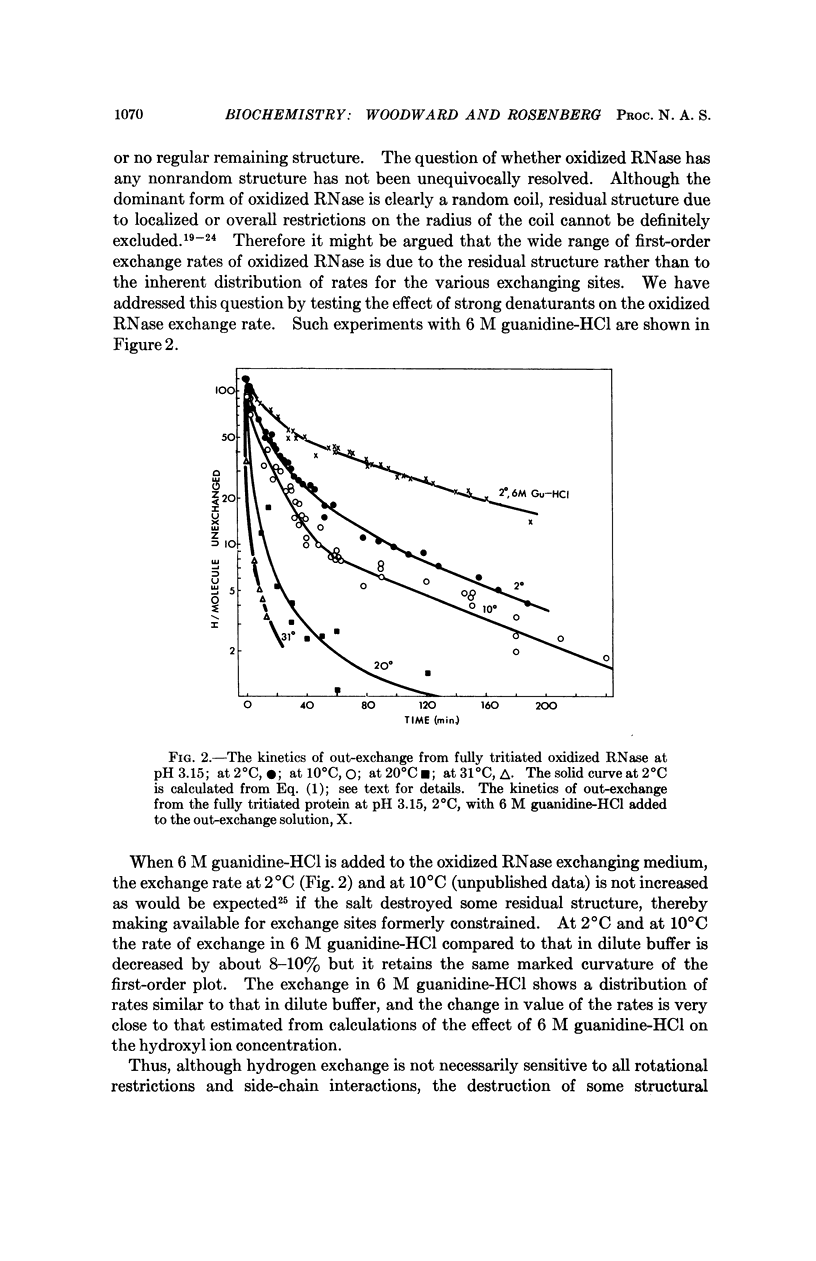
As oxidized RNase is a model for the random conformation state of RNase, the hydrogen exchange kinetics of oxidized RNase approximate the intrinsic conformation-independent chemical exchange rate of the native protein. The energy of activation, the pHmin, and the kmin of oxidized RNase exchange rates are similar to those reported for amino acid homopolymers. However, unlike the exchange from homopolymers, the exchange from oxidized RNase is characterized by a distribution of first-order rates. This distribution is important to the analysis of exchange from native proteins in terms of classes of sites which share common structural properties.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGELOW C. C. Difference spectra of ribonuclease and two ribonuclease derivatives. C R Trav Lab Carlsberg. 1960;31:305–324. [PubMed] [Google Scholar]
- BIGELOW C. C., GESCHWIND I. I. Difference specta of amino acids and proteins in aqueous media of high refractive index. C R Trav Lab Carlsberg. 1960;31:283–304. [PubMed] [Google Scholar]
- Brandts J. F., Hunt L. The thermodynamics of protein denaturation. 3. The denaturation of ribonuclease in water and in aqueous urea and aqueous ethanol mixtures. J Am Chem Soc. 1967 Sep 13;89(19):4826–4838. doi: 10.1021/ja00995a002. [DOI] [PubMed] [Google Scholar]
- ENGLANDER S. W. A HYDROGEN EXCHANGE METHOD USING TRITIUM AND SEPHADEX: ITS APPLICATION TO RIBONUCLEASE. Biochemistry. 1963 Jul-Aug;2:798–807. doi: 10.1021/bi00904a030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRINGTON W. F., SCHELLMAN J. A. Evidence for the instability of hydrogen-bonded peptide structures in water, based on studies of ribonuclease and oxidized ribonuclease. C R Trav Lab Carlsberg Chim. 1956;30(3):21–43. [PubMed] [Google Scholar]
- HARRINGTON W. F., SELA M. A comparison of the physical chemical properties of oxidized and reduced alkylated ribonuclease. Biochim Biophys Acta. 1959 Feb;31(2):427–434. doi: 10.1016/0006-3002(59)90017-4. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hade E. P., Tanford C. Isopiestic compositions as a measure of preferential interactions of macromolecules in two-component solvents. Application to proteins in concentrated aqueous cesium chloride and guanidine hydrochloride. J Am Chem Soc. 1967 Sep 13;89(19):5034–5040. doi: 10.1021/ja00995a036. [DOI] [PubMed] [Google Scholar]
- Hermans J., Jr, Lohr D., Ferro D. Unfolding and hydrogen exchange of proteins: the three-dimensional ising lattice as a model. Nature. 1969 Oct 11;224(5215):175–177. doi: 10.1038/224175a0. [DOI] [PubMed] [Google Scholar]
- Hvidt A., Nielsen S. O. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386. doi: 10.1016/s0065-3233(08)60129-1. [DOI] [PubMed] [Google Scholar]
- Jirgensons B. Circular dichroism of proteins of known and unknown conformations. Biochim Biophys Acta. 1970 Jan 20;200(1):9–17. doi: 10.1016/0005-2795(70)90037-1. [DOI] [PubMed] [Google Scholar]
- KLEE W. A., RICHARDS F. M. The reaction of O-methylisourea with bovine pancreatic ribonuclease. J Biol Chem. 1957 Nov;229(1):489–504. [PubMed] [Google Scholar]
- Miller W. G., Goebel C. V. Dimensions of protein random coils. Biochemistry. 1968 Nov;7(11):3925–3935. doi: 10.1021/bi00851a021. [DOI] [PubMed] [Google Scholar]
- NIELSEN S. O., BRYAN W. P., MIKKELSEN K. Hydrogen-deuterium exchange of small peptides in aqueous solution. Biochim Biophys Acta. 1960 Aug 26;42:550–552. doi: 10.1016/0006-3002(60)90842-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg A., Chakravarti K. Studies of hydrogen exchange in proteins. I. The exchange kinetics of bovine carbonic anhydrase. J Biol Chem. 1968 Oct 10;243(19):5193–5201. [PubMed] [Google Scholar]
- Rosenberg A., Enberg J. Studies of hydrogen exchange in proteins. II. The reversible thermal unfolding of chymotrypsinogen A as studied by exchange kinetics. J Biol Chem. 1969 Nov 25;244(22):6153–6159. [PubMed] [Google Scholar]
- Segal D. M., Harrington W. F. The tritium-hydrogen exchange of myosin and its proteolytic fragments. Biochemistry. 1967 Mar;6(3):768–787. doi: 10.1021/bi00855a018. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- WHITE F. H., Jr Regeneration of native secondary and tertiary structures by air oxidation of reduced ribonuclease. J Biol Chem. 1961 May;236:1353–1360. [PubMed] [Google Scholar]
- YOUNG D. M., POTTS J. T., Jr Structural transformations of bovine pancreatic ribonuclease in solution: a study of polarization of fluorescence. J Biol Chem. 1963 Jun;238:1995–2002. [PubMed] [Google Scholar]


