Abstract
The continued global spread and evolution of HIV diversity pose significant challenges to diagnostics and vaccine strategies. NIAID partnered with the FDA, WRAIR, academia, and industry to form a Viral Panel Working Group to design and prepare a panel of well-characterized current and diverse HIV isolates. Plasma samples that had screened positive for HIV infection and had evidence of recently acquired infection were donated by blood centers in North and South America, Europe, and Africa. A total of 80 plasma samples were tested by quantitative nucleic acid tests, p24 antigen, EIA, and Western blot to assign a Fiebig stage indicative of approximate time from initial infection. Evaluation of viral load using FDA-cleared assays showed excellent concordance when subtype B virus was tested, but lower correlations for subtype C. Plasma samples were cocultivated with phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) from normal donors to generate 30 viral isolates (50–80% success rate for samples with viral load >10,000 copies/ml), which were then expanded to 107–109 virus copies per ml. Analysis of env sequences showed that sequences derived from cultured PBMCs were not distinguishable from those obtained from the original plasma. The pilot collection includes 30 isolates representing subtypes B, C, B/F, CRF04_cpx, and CRF02_AG. These studies will serve as a basis for the development of a comprehensive panel of highly characterized viral isolates that reflects the current dynamic and complex HIV epidemic, and will be made available through the External Quality Assurance Program Oversight Laboratory (EQAPOL).
Introduction
Despite extensive efforts, HIV-1 remains a global health problem, with 2.6 million new infections in 2008.1,2 At present, HIV-1 is classified into four Groups (M, N, O, and P) with Group M further subdivided into nine major subtypes (A–D, F–H, J, and K), over 49 circulating recombinant forms (CRF), and numerous unique recombinant forms (URF).3–5 The extensive diversity and rapid evolution of HIV pose serious challenges for maintaining reliable serologic and nucleic acid tests6 for blood screening, epidemiological surveillance, diagnosis, and clinical management of infected persons.7 Different test manufacturers target different HIV genes in their nucleic acid tests (NAT) for screening and quantitative HIV RNA determinations, with varying degrees of success in correctly identifying or quantifying emerging isolates.8,9 Comparisons of recent HIV viral load assays demonstrated an underestimation of 10–40% of some non-B subtypes, CRF variants, or Group N or O viruses, and reports of failure of screening tests to detect the rare subtypes have been noted.3,10–12 Subtype G and CRF02_AG, for example, are frequently missed or underquantitated.13 Failure of HIV DNA PCR assays to correctly identify infants infected with non-subtype B has also been reported.14,15
The extensive viral diversity also has important implications for the pathogenesis and development of antiretroviral therapies and vaccines.5,16,17 The complex interactions of viruses with the human host, such as differences in usage of chemokine coreceptors CCR5 and CXCR4, play an important role in HIV transmission efficiency and disease progression.18–21 Subtype differences may also impact responses to antiretroviral therapies,5,22 and host genetic determinants of susceptibility and progression to AIDS may vary according to infecting HIV-1 subtype.6,23 The limited number of virus isolates currently available for assay development, pharmaceutical design, and the evaluation of intervention strategies constrains the ability to correctly target viral infections in different geographic regions and to control the spread of HIV. Virus panels that are currently available for assay development and evaluations were isolated more than a decade ago24,25 and are no longer fully representative of viruses currently in circulation. Many of these older isolates are incompletely characterized in molecular terms, making it difficult to reliably assess assay comparisons.
To meet the challenge of the continuing evolution of HIV diversity, the National Institute of Allergy and Infectious Diseases (NIAID) has established a Viral Panels Working Group, which includes representation from the National Heart Lung and Blood Institute (NHLBI), Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), US Military HIV Research Program (MHRP)/Walter Reed Army Institute of Research (WRAIR), National Institute for Biological Standards and Control (NIBSC), SeraCare Life Sciences, Inc., and other collaborators throughout the world to establish a set of fully characterized viruses from acute or early HIV infections. The collection will be designed to better represent current global HIV diversity and will be collected with careful attention to regulatory requirements and ethical standards of each source country and donor organization. The resulting panels, including data sheets containing demographics of source subjects and characterization information, will be made available to the scientific community, including regulatory agencies, researchers, epidemiologists, and commercial manufacturers of diagnostics and pharmaceuticals, to help control the worldwide HIV-1 epidemic through support of epidemiological testing, vaccine, and therapeutic efforts.
To develop a set of new HIV-1 subtype isolates, we focused on viruses from acute/early stage infection, when the transmitted/founder virus most relevant for detection and intervention studies predominates.26,27 Blood banks around the world are uniquely positioned to contribute sufficient volumes of plasma from early infection for global HIV surveillance and test evaluation programs. Through their screening programs, blood banks detect and defer from donation thousands of HIV-infected donors annually. Modern algorithms can discriminate recently infected donors based on HIV RNA, antigen, and antibody responses, thus allowing for identification of plasma from donors with recently acquired HIV infections. Such surveillance is well established in the United States, Europe, and South Africa, and collaborating blood banks from these areas provided plasma samples from recently HIV-infected donors for use in these studies. Additional samples were obtained from blood banks in Brazil, and rare HIV-1 subtypes or CRFs from Greece and Cameroon were provided by collaborators from NIBSC and the U.S. FDA, respectively.
A major focus of the initial efforts was to establish virus cultures from the original plasma samples, to serve as a source of high-titer, well-characterized virus to use in panels. Nucleic acid tests require larger volumes relative to serological tests, and culture can provide sufficient volumes in high titers for full characterization and manufacture of panels and dilution series. Generation of virus from plasma will also enable more detailed study of virus infectivity and biological activity. The plasma samples obtained for the initial panel studies were subjected to extensive characterization, including Fiebig staging, virus cultivation, and full length sequencing. In the course of this work, methods for plasma sample characterization and for virus isolation, expansion, and characterization were optimized and validated to generate virus for the assembly of highly characterized HIV subtype panels in a GLP environment. A panel of 30 HIV-1 isolates was generated, representative of viruses circulating in South Africa, the United States, Brazil, Greece, Poland, and Cameroon. These HIV-1 isolates have associated demographic information, are characterized with a comprehensive set of assays, including full length sequencing, and will be made available for distribution in the future through the NIAID External Quality Assurance Program Oversight Laboratory (EQAPOL) Program.
Materials and Methods
Sample collection
Acquisition of samples for use in this study was coordinated by the Blood Systems Research Institute (BSRI) and included plasma units collected by blood banks or plasmapheresis centers where back-up volumes ∼200 ml existed. Preference was given to samples from early infection (RNA positive, antibody negative), or evidence of very recent seroconversion including samples with indeterminate Western blot patterns. Samples with smaller volumes and from later stages in infection were used for some of the rare subtypes, where larger volumes were not available for study. All samples had been collected with ethical review and approval of the collection protocol and informed consent (IC) as previously established for the donor organization's collection activities.
Sample sources
Plasma samples from six different countries on four continents were submitted for evaluation. These included samples from the United States (American Red Cross), South Africa (South African National Blood Service), Poland (Institute of Hematology and Blood Transfusion), and Brazil (Fundação Pro-Cangue/Hemocentro de São Paulo). All of these had tested as NAT yield (RNA positive, antibody negative) or recently acquired infections, based on documented seroconversion and antibody results at the collecting organizations. In addition, randomly collected plasma samples from Cameroon representing CRF02_AG subtypes were provided by the FDA, and samples from Greece representing the rare CRF04-CPX subtype were provided by the National Institute of Standards and Controls (NIBSC, South Mimms, UK).
Sample characterization
All plasma specimens received were characterized by commercially available FDA-approved nucleic acid and antibody assays, and a Research Use Only (RUO) p24 antigen assay. All samples were tested by the Roche AmpliPrep/COBAS TaqMan v2.0. Additional quantitative HIV nucleic acid testing was performed for the U.S. and South Africa samples with the Roche Amplicor Monitor v1.5, the Roche AmpliPrep/COBAS TaqMan v1.0, the Abbott m2000, and the Siemens bDNA v3.0 assays. Anti-HIV reactivity was tested by the Bio-Rad/Genetic Systems Anti HIV-1/HIV-2 Plus O EIA and the Bio-Rad/Genetic Systems HIV-1 Western blot methods. The PerkinElmer HIV-1 p24 Antigen Capture Assay was used to measure plasma antigen levels and to monitor HIV appearance in culture supernatants at various times after infection for both the virus isolation and expansion studies. Prior to use in this study, this kit was evaluated and shown to exhibit high sensitivity with dilution series representing the major HIV-1 subtypes, including Group O. Samples were classified based on Fiebig stage as determined by the presence of viral RNA, p24 antigen, antibody, and specific bands on Western blot (WB) according to published methods as follows:28
| Fiebig Stage | Markers |
|---|---|
| I | HIV RNA Positive |
| II | p24 Antigen Positive |
| III | HIV ELISA Positive |
| IV | Western blot Indeterminate |
| V | Western blot Positive, no p31 |
| VI | Western blot Positive, with p31 |
Virus isolation and expansion
Virus isolation methods were based on a modification of those described in the literature for efficient recovery of infectious virus by cocultivation with phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) from normal donors.29 To further improve on the virus isolation efficiency, the plasma samples were subjected to a low-speed centrifugation (450×g) to remove particulate matter and then ultracentrifuged at 24,000×g to concentrate the virus. These steps were included to remove any toxic components that may be present in the plasma. A further refinement was the use of CD8-depleted PBMCs to reduce any virus inhibitory activity that could be present during viral expansion. CD8 depletion was performed by using the CD8 kit from STEMCELL technologies on the RoboSep Instrument following the manufacturer's instructions. Pelleted virus from 1 ml of plasma was used to infect 6×106 PHA-stimulated, CD8-depleted PBMCs. Media (RPMI-10% FBS/IL-2) was replaced every 3–4 days at which time the collected supernatants were measured for p24 antigen reactivity. Fresh PHA-stimulated PBMCs were added on days 7 and 14. The cultures were continued for up to 21 days or until p24 results were off-scale at a 1:100 dilution. Samples that showed positive p24 in culture supernatants were further expanded up to 10 ml with PHA-stimulated PBMCs and harvested after 4 additional days in culture. The resulting culture supernatants were clarified by low-speed centrifugation and stored as 1-ml aliquots at −80°C. The pelleted infected PBMCs were resuspended in fresh RPMI-20% FBS and frozen viably in 10% DMSO in liquid nitrogen. A plasma sample from an NAT yield (RNA positive, antibody negative) HIV-positive donor that had previously been shown successful in virus isolation was included as control in each virus isolation batch.
For virus expansion, 3×106 frozen infected PBMCs or 1 ml of cell culture supernatant from the initial isolation was incubated with 2 ml of 3-day PHA-stimulated PBMCs at 30×106 cells for 2 h, and diluted to 10 ml in T25 flasks. Cultures were then incubated at 37°C for 5 days and were then further expanded into 100 ml of 3-day PHA-stimulated PBMCs at 2–3×106 cells/ml and incubated for an additional 7 days. Virus was harvested from the supernatant, clarified by centrifugation at 450×g, filtered through a 0.2-μm filter, aliquoted, and stored at −80°C. Cultured virus was evaluated by the PerkinElmer p24 antigen and Roche TaqMan v2.0 assays to establish concentrations and evaluate kinetics of viral expansion.
Full length genomic sequencing
Plasma samples and expanded virus were sequenced through single genome amplification (SGA) of near full-length genomes or env genes. Viral RNA from plasma or culture supernatant was extracted by the QIAamp Viral RNA Mini Kit and served as the template for RT-PCR. Complementary DNA (cDNA) synthesis (ThermoScript RT, Invitrogen Corp., Carlsbad, CA) and nested PCR amplification were performed to amplify two half-genome sequences overlapping by 1.5 kb.30 PCR products were purified using micron YM-50 columns (Millipore Corporation, Billerica, MA) and sequenced by an Applied BioSystems 3130/3130xl Genetic Analyzer. Sequences were assembled using Sequencher, version 4.7, and sequence alignments visualized using Highlighter (www.hiv.lanl.gov). Sequences were aligned using Clustal and phylogenetic analyses were performed using the PHYLIP package. A neighbor-joining tree of sequences of interest and reference strains was constructed. Diversity metrics were obtained using DIVEIN (http://indra.mullins.microbiol.washington.edu/DIVEIN/).31 Plasma- and culture-derived env sequences were analyzed using Poisson-Fitter (http://www.hiv.lanl.gov/content/sequence/POISSON_FITTER/poisson_fitter.html).32 Recombinants were analyzed using the jumping profile hidden Markov model (jpHMM) tool provided on www.hiv.lanl.gov.
Coreceptor usage using cell culture and genotypic algorithms
Cell culture assay
Viral tropism assays (co-receptor CCR5 vs. CXCR4 usage) were performed in human osteosarcoma GHOST cell lines (NIH AIDS Research and Reference Reagent Program) that stably express either CD4 alone or in combination with one of the chemokine coreceptors CCR5 or CXCR4. Cells were cultured and seeded at 1×105 cells/well in 24-well plates and infected with the relevant virus isolates obtained from plasma specimens.33 Positive and negative control viruses for each coreceptor were included in the assay. Viral replication was monitored by measuring HIV p24 antigen levels in culture supernatants collected at days 4 and 8 using the PerkinElmer Antigen ELISA kit (cat: NEN 050B001). Increases in p24 levels in the CCR5 expressing cells over time were indicative of CCR5 tropism. In the present study, only our CXCR4 control isolate showed any increases in p24 production in the CXCR4 expressing line indicating that none of these isolates was CXCR4.
Prediction method
The genotype prediction of coreceptor usage of the HIV isolates based on V3 sequence information was evaluated using a variety of Web-based genotypic algorithms.
The Geno2pheno prediction is based on the bioinformatic prediction tool that uses the V3 sequence plus additional host-specific features to select false-positive rates (FPR) at 1%, 2.5%, 5%, 10%, 15%, and 20% (http://coreceptor.bioinf.mpi-sb.mpg.de/cgi-bin/coreceptor.pl).
The position-specific scoring matrix (PSSM) assigns a score based on the comparison with a sequence of known CXCR4 viruses (http://indra.mullins.microbiol.washington.edu/webpssm/) (PSSMX4R5 and PSSMsinsi).
The 11/25 rule predicts CXCR4 coreceptor usage in isolates based on the presence of positively charged amino acids at positions 11 and/or 25 of the third hypervariable (V3) loop of the envelope glycoprotein gp120 or cross-validation decision tree based on c4.5 p8/p12 (http://genomiac2.ucsd.edu:8080/wetcat/tropism.html).
Results
Characterization of plasma samples
Nucleic acid and serological characterizations of the 80 plasma samples collected for the pilot project are summarized in Table 1. The samples were assigned a Fiebig designation based on the results of the RNA, antigen, EIA, and WB tests as described in the Materials and Methods section, and show a good distribution among Fiebig stages I–VI. The majority of samples were collected at very early stages of infection (Fiebig stages I–V) as intended, including 27 Fiebig stage I NAT yield samples (RNA positive only) and 10 Fiebig stage II samples (RNA and p24 antigen positive, antibody negative). Most of the samples at Fiebig stage VI, indicative of older infection, are from Brazil, Cameroon, or Greece. The samples from Brazil were selected because they tested “recent” using an incidence assay and had incomplete Western blot patterns, while the samples from Cameroon and Greece were chosen for their rare subtype designation.
Table 1.
Characterization of HIV Plasma Samples
| Fiebig stage | Specimen ID | Collection date | Country of origin | Viral load (copies/ml) | p24 pg/ml | EIA | WB | Virus culture |
|---|---|---|---|---|---|---|---|---|
| I | BP00022 | 8/28/08 | South Africa | <20 | <2 | Neg | Neg | − |
| BP00024 | 11/16/07 | South Africa | <20 | <2 | Neg | Neg | − | |
| BP00017 | 5/12/08 | South Africa | <20 | <2 | Neg | Neg | − | |
| BP00026 | 5/27/08 | South Africa | <20 | <2 | Neg | Neg | − | |
| BP00003 | 7/29/08 | South Africa | 49 | <2 | Neg | Neg | − | |
| BP00030 | 9/15/08 | South Africa | 57 | <2 | Neg | Neg | − | |
| BP00020 | 12/14/07 | South Africa | 91 | <2 | Neg | Neg | − | |
| BP00007 | 7/7/08 | South Africa | 125 | <2 | Neg | Neg | − | |
| BP00056 | 11/30/06 | United States | 197 | <2 | Neg | Neg | − | |
| BP00027 | 1/15/08 | South Africa | 373 | <2 | Neg | Neg | − | |
| BP00004 | 10/15/07 | South Africa | 493 | <2 | Neg | Neg | − | |
| BP00021 | 7/10/08 | South Africa | 511 | <2 | Neg | Neg | − | |
| BP00006 | 4/21/08 | South Africa | 568 | <2 | Neg | Neg | − | |
| BP00076 | 2/6/09 | Poland | 705 | <2 | Neg | Neg | − | |
| BP00059 | 6/20/07 | United States | 1,230 | <2 | Neg | Neg | − | |
| BP00060 | 6/4/07 | United States | 4,230 | <2 | Neg | Neg | − | |
| BP00019 | 4/3/08 | South Africa | 5,380 | <2 | Neg | Ind | − | |
| BP00013 | 9/3/08 | South Africa | 6,120 | <2 | Neg | Neg | − | |
| BP00015 | 1/18/08 | South Africa | 12,840 | <2 | Neg | Neg | − | |
| BP00075 | 6/4/08 | Poland | 16,300 | <2 | Neg | Neg | + | |
| BP00077 | 1/16/06 | Poland | 17,000 | <2 | Neg | Neg | − | |
| BP00036 | 2/18/08 | South Africa | 18,950 | <2 | Neg | Neg | − | |
| BP00054 | 1/10/06 | United States | 24,600 | <2 | Neg | Neg | − | |
| BP00034 | 5/21/08 | South Africa | 25,500 | <2 | Neg | Neg | − | |
| BP00014 | 8/4/08 | South Africa | 51,600 | <2 | Neg | Neg | + | |
| BP00061 | 9/18/08 | United States | 67,000 | <2 | Neg | Neg | − | |
| BP00057 | 7/19/06 | United States | 625,000 | <2 | Neg | Neg | − | |
| II | BP00032 | 8/11/08 | South Africa | 2,320 | 2.1 | Neg | Neg | − |
| BP00074 | 4/12/08 | Poland | 56,100 | 3.9 | Neg | Neg | + | |
| BP00033 | 8/28/08 | South Africa | 92,000 | 2.2 | Neg | Neg | − | |
| BP00028 | 8/23/08 | South Africa | 162,000 | 4.8 | Neg | Neg | + | |
| BP00016 | 2/11/08 | South Africa | 183,000 | 50.9 | Neg | Neg | + | |
| BP00012 | 7/1/08 | South Africa | 302,500 | 11.1 | Neg | Neg | + | |
| BP00002 | 10/16/07 | South Africa | 553,000 | 37.7 | Neg | Neg | + | |
| BP00018 | 8/5/08 | South Africa | 1,190,000 | 104.3 | Neg | Neg | + | |
| BP00025 | 8/21/08 | South Africa | 1,480,000 | 125.0 | Neg | Neg | + | |
| BP00055 | 2/21/06 | United States | 13,200,000 | >300 | Neg | Neg | + | |
| III | BP00023 | 4/21/08 | South Africa | 1,360,000 | 203.4 | Pos | Neg | − |
| IV | BP00001 | 8/10/07 | South Africa | 497 | <2 | Pos | Ind | − |
| BP00031 | 10/25/06 | South Africa | 29,500 | <2 | Pos | Ind | − | |
| BP00037 | 6/12/07 | South Africa | 91,800 | <2 | Pos | Ind | − | |
| BP00011 | 1/12/07 | South Africa | 134,000 | <2 | Pos | Ind | − | |
| BP00069 | 5/28/08 | United States | 265,000 | 12.3 | Pos | Ind | + | |
| BP00035 | 1/24/07 | South Africa | 320,000 | 2.1 | Pos | Ind | − | |
| BP00058 | 8/13/07 | United States | 785,000 | 15.9 | Pos | Ind | + | |
| V | BP00066 | 11/7/07 | United States | 297 | <2 | Pos | Pos | − |
| BP00062 | 7/6/07 | United States | 2,440 | <2 | Pos | Pos | − | |
| BP00068 | 11/6/07 | United States | 9,420 | <2 | Pos | Pos | − | |
| BP00070 | 7/22/08 | United States | 32,300 | <2 | Pos | Pos | + | |
| BP00073 | 12/9/08 | United States | 34,000 | <2 | Pos | Pos | − | |
| BP00065 | 1/29/08 | United States | 52,200 | <2 | Pos | Pos | − | |
| BP00063 | 8/17/07 | United States | 74,900 | <2 | Pos | Pos | + | |
| BP00039 | 1/20/07 | Brazil | 106,000 | <2 | Pos | Pos | − | |
| BP00010 | 5/30/07 | South Africa | 112,000 | <2 | Pos | Pos | + | |
| BP00005 | 4/3/07 | South Africa | 190,000 | <2 | Pos | Pos | + | |
| BP00067 | 11/13/07 | United States | 199,000 | <2 | Pos | Pos | + | |
| BP00047 | 12/3/07 | Brazil | 222,000 | <2 | Pos | Pos | + | |
| BP00008 | 9/28/07 | South Africa | 471,000 | 2.8 | Pos | Pos | + | |
| BP00064 | 9/5/07 | United States | 982,000 | 48.3 | Pos | Pos | + | |
| BP00029 | 3/14/07 | South Africa | 1,510,000 | 22.6 | Pos | Pos | + | |
| VI | BP00081 | 3/13/06 | Cameroon | 113 | <2 | Pos | Pos+p31 | − |
| BP00043 | 4/26/07 | Brazil | 543 | <2 | Pos | Pos+p31 | − | |
| BP00072 | 12/15/08 | United States | 627 | <2 | Pos | Pos+p31 | − | |
| BP00038 | 1/3/07 | Brazil | 990 | <2 | Pos | Pos+p31 | − | |
| BP00053 | 8/11/04 | Greece | 1,472 | <2 | Pos | Pos+p31 | + | |
| BP00045 | 6/8/07 | Brazil | 2,400 | <2 | Pos | Pos+p31 | − | |
| BP00080 | 9/23/08 | Cameroon | 4,680 | <2 | Pos | Pos+p31 | − | |
| BP00071 | 11/14/08 | United States | 5,480 | <2 | Pos | Pos+p31 | − | |
| BP00041 | 3/3/07 | Brazil | 6,650 | <2 | Pos | Pos+p31 | − | |
| BP00051 | 9/19/00 | Greece | 7,000 | <2 | Pos | Pos+p31 | − | |
| BP00042 | 3/23/07 | Brazil | 7,480 | <2 | Pos | Pos+p31 | − | |
| BP00009 | 3/19/07 | South Africa | 14,600 | <2 | Pos | Pos+p31 | + | |
| BP00078 | unknown | Cameroon | 17,400 | <2 | Pos | Pos+p31 | + | |
| BP00049 | 9/15/00 | Greece | 24,475 | <2 | Pos | Pos+p31 | + | |
| BP00052 | 2/20/04 | Greece | 29,142 | <2 | Pos | Pos+p31 | + | |
| BP00079 | 9/23/08 | Cameroon | 44,900 | <2 | Pos | Pos+p31 | + | |
| BP00046 | 1/19/07 | Brazil | 105,000 | <2 | Pos | Pos+p31 | − | |
| BP00048 | 9/15/00 | Greece | 105,001 | <2 | Pos | Pos+p31 | + | |
| BP00040 | 2/19/07 | Brazil | 143,000 | <2 | Pos | Pos+p31 | + | |
| BP00044 | 6/1/07 | Brazil | 188,000 | <2 | Pos | Pos+p31 | + |
Plasma samples were characterized by viral load (Roche TaqMan v2.0), p24 Ag (PerkinElmer, positive cutoff 2 pg/ml), anti-HIV-1/2 Plus O EIA (Bio-Rad), and anti-HIV-1 western blot (Bio-Rad) to assign a Fiebig stage. Samples are ordered according to ascending viral load and sequential appearance of markers in each category. Positive test results and viral loads greater than 10,000 copies/ml are in bold. Also shown is success of virus isolation.
EIA, enzyme immunoassay; WB, western blot.
Isolation of virus from plasma
The initial studies for virus isolation from plasma by cocultivation with PHA-stimulated PBMCs from normal blood donors showed variable success rates, particularly with plasma samples with low viral loads. Additional optimization steps were incorporated into our standard protocol, including pelleting of virus from preclarified plasma and use of PHA-stimulated, CD8-depleted PBMCs as target cells. These refinements allowed more efficient recovery of infectious virus from the plasma without interference of inhibitors or anticoagulants, and increased the yield of virus during cultivation. Culture supernatants were collected every 3–4 days and monitored for appearance of HIV p24 antigen. A total of 30 viral isolates were successfully cultured from the 80 samples for which isolation was attempted (37.5% success rate). The success rates for virus isolation as a function of Fiebig stage or plasma viral load are shown in Table 2.
Table 2.
Virus Isolation Rates for Plasma Samples as a Function of Fiebig Stages or Viral Load
| Fiebig stage | Pos | Total | % Pos | Viral load | Pos | Total | % Pos |
|---|---|---|---|---|---|---|---|
| I | 2 | 27 | 7.4% | <1,000 | 0 | 20 | 0.0% |
| II/III | 8 | 11 | 72.7% | 1,000–10,000 | 1 | 14 | 7.1% |
| IV | 2 | 7 | 28.6% | 10,000–100,000 | 10 | 21 | 47.6% |
| V | 9 | 15 | 60.0% | 100,000–1,000,000 | 15 | 20 | 75.0% |
| VI | 9 | 20 | 45.0% | >1,000,000 | 4 | 5 | 80.0% |
| Total | 30 | 80 | 37.5% | Total | 30 | 80 | 37.5% |
Most efficient virus isolation was observed for plasma samples at Fiebig stages II and V, although virus could be isolated at each stage. Efficiency of virus isolation went up as viral load increased. Virus isolation was not achieved for samples with viral loads below 1000 copies/ml.
Virus was successfully isolated from both early (Fiebig stage II–IV) and later stage (Fiebig stage V/VI) HIV infections. Samples from Fiebig stage I yielded very few positive cultures, with only two isolates from 27 samples for which isolation was attempted. Samples with low plasma viral loads gave poor virus recovery rates, with no culture positives from samples with fewer than 1000 copies/ml, and only one of 14 (7.1%) samples in the 1–10,000 plasma virus load range was virus isolation positive (Table 2). The success rate for virus isolation increased with viral load and ranged from 47.6% to 80% for viral loads above 10,000 copies/ml. No effect of Fiebig stage on isolation rates was observed when controlling for viral load, hence there was no apparent impact on absence of presence of antibody on isolation efficiency.
Virus expansions
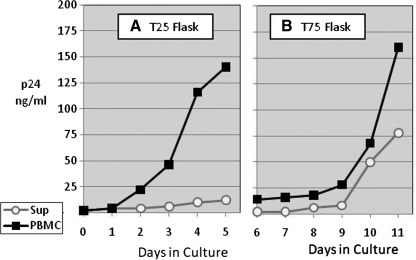
All virus isolates obtained in this study were subsequently expanded in culture, where either culture supernatant or infected PBMCs from the same initial isolation stock were used as inoculum. Figure 1 shows a typical virus growth curve where BP00002, a Fiebig stage II plasma isolate from South Africa, was used as inoculum for expansion into 10 ml (Fig. 1A) and the resulting supernatant or PBMCs was transferred to 100 ml culture on day 6 (Fig 1B). Virus grew more rapidly and to higher titers out of the infected PBMCs (open circles) than from the culture supernatant (closed squares), allowing for a smaller number of passages to minimize possible genetic drift. Similar virus expansion patterns were observed with three other isolates, including those initiated with plasma from donors in Fiebig stage V.
FIG. 1.
Culture expansion of virus. Virus from the initial HIV isolation was expanded in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) through two cycles of virus cultivation. The first cycle expanded the virus in T25 flasks (A) starting with 3×106 infected PBMCs (open circles) or 1 ml of culture supernatant (closed squares) to 10 ml culture. The second cycle expanded these to 100 ml culture in T75 flasks (B). All cultures contained 3×106 cells/ml of 3-day PHA-stimulated PBMCs from normal donors. The cultures were monitored daily for HIV p24 and harvested on day 11.
HIV-1 viral load testing
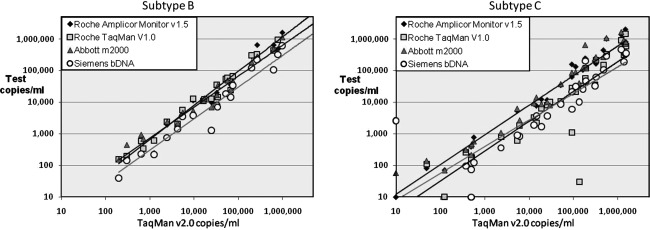
A major application of the proposed subtype panel is comparison and ultimate standardization of various quantitative HIV NAT assays. Since the current commercially available tests use different amplification technologies and are based on primers from different regions of the viral genome, we wished to compare quantitation by several assays using the plasma samples collected in different geographic regions during the pilot project. Samples from the United States and South Africa (subsequently identified as all subtype B and C, respectively) were evaluated using several FDA-licensed viral load assays according to each manufacturer's instructions, and their performance was compared. The Roche COBAS TaqMan HIV-1 v1.0 test, like the Roche Amplicor HIV-1 Monitor v1.5 test, targets the gag p24 region of HIV-1,34 while the TaqMan HIV-1 v2.0 targets both the gag and LTR regions for broader subtype quantitation, including Group O.35 The Abbott m2000 RT-PCR assay targets the pol IN region 1 (Abbott Real-Time HIV-1. Package Insert, 2007), while the Siemens Versant HIV-1 RNA 3.0 bDNA assay targets highly conserved regions of pol.36
Figure 2 shows the quantitative results of plasma viral loads for U.S. and South African samples as tested by each assay plotted relative to the value obtained by the Roche TaqMan v2.0. A wide range of HIV-1 RNA concentrations was represented in the plasma collection, with viral loads ranging from <20 copies/ml to 1.32×107 copies/ml. Four of the Fiebig stage 1 isolates from South Africa (BP00017, BP00022, BP00024, and BP00026) had screened NAT positive by the qualitative individual donation Ultrio assay (Procleix Tigris, Chiron) but were not detected on the quantitation assays. Two additional low copy (<200 copies/ml) South African samples were negative on the Siemens assay or the Roche TaqMan v1.0, but were picked up by the other assays. All assays detected and quantified HIV nucleic acid in the U.S. plasma samples. The coefficient of correlation for quantitative results between the Roche COBAS TaqMan HIV-1 v2.0 and each of the other assays is shown in Table 3.
FIG. 2.
Relationship between HIV-1 viral load measurements of plasma samples from U.S. isolates (subtype B) or South African isolates (subtype C) as tested on Roche COBAS Ampliprep/COBAS TaqMan HIV-1 Test v2.0 vs. Roche COBAS Ampliprep/COBAS TaqMan HIV-1 Test v1.0, Roche Amplicor Monitor v1.5, Siemens bDNA v3.0, and the Abbott m2000 assays.
Table 3.
Correlation of Viral Load Assays
| Roche TaqMan v2.0 vs. | Roche Amplicor Monitor v1.5 | Roche TaqMan v1.0 | Abbott m2000 | Siemens bDNA v3.0 |
|---|---|---|---|---|
| Subtype B | 0.997 | 0.948 | 0.997 | 0.952 |
| Subtype C | 0.883 | 0.872 | 0.837 | 0.891 |
The correlation coefficients for four viral load assays, each vs. the Roche TaqMan v2.0, were calculated separately for U.S. (subtype B; N=20) and South African (subtype C; N=37) samples.
The U.S. samples (Subtype B) showed a high degree of correlation (≥0.95) for the various assays over a broad linear range (102–106 copies/ml). In contrast, the South African samples (Subtype C) showed less agreement (coefficient of correlation <0.90), with some samples differing by more than a log in HIV-1 RNA concentration, and more outliers. The discrepancies observed among the various assays in quantitation of subtype C isolates underscores the importance of evaluating assays with a large number and broad range of subtyped samples. A large panel of virus isolates or plasma samples from currently circulating subtypes is crucial to guiding assay design and for standardization of assay performance to ensure that all major HIV-1 subtypes are properly detected and quantified.
Comparison of plasma- and culture-derived sequences by SGA
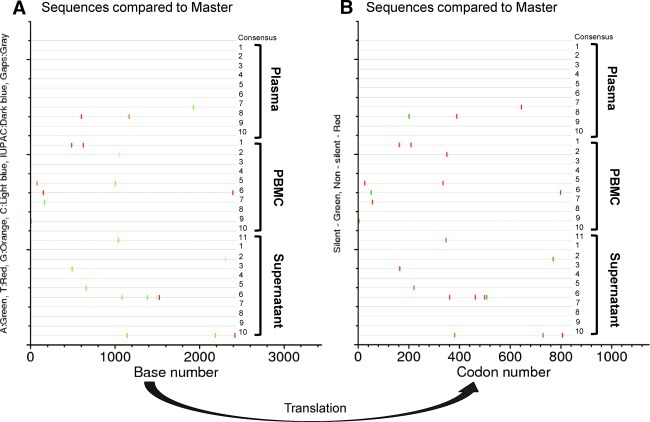
To compare plasma- and culture-derived sequences, we used the SGA approach to analyze the envelope sequences from these same three viruses. For this analysis, 10–12 sequences from viral RNA from the original plasma were compared to those of the culture supernatant virus and of proviral DNA from cultured PBMCs collected at day 8 of the second round of expansion. The sequence diversity between the original plasma (BP00002) and its corresponding expanded virus is shown by graphic representations of nucleotide (Fig. 3A) or protein (Fig. 3B) sequence alignments in which each sequence is compared to a consensus sequence and all polymorphisms are indicated by a marker (Fig. 3). A similar degree of diversity was observed in the other two samples (BP00008 and BP00067) analyzed by Highlighter (www.hiv.lanl.gov). (Supplementary Figs. S3C and D and S3E and F; Supplementary Data are available online at www.liebertonline.com/aid).
FIG. 3.
Comparison of viral sequences from plasma and derived cultured virus from a Fiebig stage II virus isolate (BP00002) from South Africa. The envelope sequence analysis of original plasma and virus cultures from supernatant or PBMCs of original isolate as described in Fig. 2 was carried out on 10–12 separate sequences of the respective virus preparations. Point mutations in each sequence are designated by a color marker to indicate specific mutation type (A). The observed mismatches mostly led to codon changes (B).
Most of the nucleotide changes were transition, not transversion. Pairwise diversity of sequences derived from original plasma and the expanded virus using the culture supernatant or infected PBMCs from the initial isolation (Table 4) showed that the viruses in each preparation have little sequence diversity between them, indicating that viral isolation and expansion for a limited time did not introduce any significant change in sequence of the viral population. These results are consistent with the interpretation that the infection most likely started with a single transmitter/founder (T/F) virus, and the degree of sequence diversity from a common origin conformed to a model of random evolution typical of acute infection.26 The variation seen in each individual followed a Poisson distribution, reflected in a star phylogeny, which allowed us to estimate the timing of infection. The estimated start of infection, relative to date of acquisition of the samples evaluated, was 38 days for BP00002, 33 days for BP00008, and 43 days for BP00067 (Table 4). These estimates were consistent with the clinical data and Fiebig characterization. These results showed that each individual was infected by a single HIV-1 variant.
Table 4.
Summary Analysis of Envelope Sequences Performed on Virus Isolated from Plasma or Viral Culture
| |
Pairwise diversity (%) |
Groups comparison |
Poisson model |
|||||
|---|---|---|---|---|---|---|---|---|
| Sample ID | Plasma (mean±SD) | PBMC (mean±SD) | SUP (mean±SD) | Plasma vs. PBMC (mean±SD) | Plasma vs. SUP (mean±SD) | PBMC vs. SUP (mean±SD) | Star phylogeny | Days (CI) |
| BP00002 | 0.024±0.034 | 0.072±0.042 | 0.079±0.072 | 0.048±0.040 | 0.051±0.059 | 0.076±0.062 | Yes | 38 (27,48) |
| BP00008 | 0.034±0.039 | 0.081±0.069 | 0.081±0.074 | 0.059±0.058 | 0.059±0.065 | 0.081±0.075 | Yes | 33 (22,43) |
| BP00067 | 0.088±0.082 | 0.083±0.057 | 0.107±0.077 | 0.087±0.078 | 0.099±0.089 | 0.093±0.071 | Yes | 43 (30,55) |
Two different models of sequence diversity comparisons were applied to the Poisson model to estimate the time from initial infection.
PBMC, peripheral blood mononuclear cells; SUP, supernatant.
Assignment of viral subtype
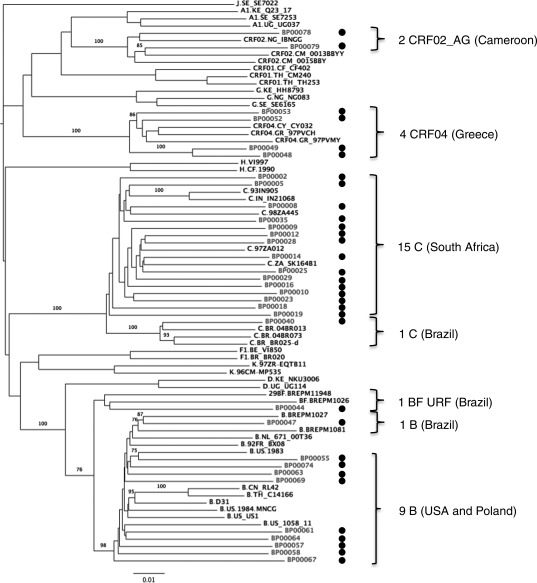
A neighbor-joining tree of 33 genome sequences from this study and 45 reference strains was constructed to designate subtype and sequence relatedness (Fig. 4). Bootstrap values of greater than 70% revealed that the eight samples from the United States and the two from Poland were subtype B. The 15 South African samples were all subtype C, the four Greek samples were CRF04_cpx, while the two Cameroonian samples were CRF02_AG. Of the three samples cultured from Brazil, one was a subtype C, one a subtype B, and one a unique recombinant between B and F. The genome structure of the B/F recombinant (nucleotide numbers corresponding to HXB2 790 to 9496) showed that the gag, vif, env, vpr, vpu, and nef genes are mostly subtype B. The pol gene is predominantly subtype F except for an insertion of subtype B just in the middle of the pol gene. We designated this B/F recombinant strain as a URF because it is not similar to any of the 10 CRF B/F recombinants identified so far (www.hiv.lanl.gov).
FIG. 4.
Subtype designation of virus isolates based on near-full-length RNA sequencing and phylogenetic analysis. The scale bar represents 1% genetic distance. Bootstrap values at relevant nodes are indicated. Reference sequences are shown alongside sequences derived from the viral panel (designated by a dot).
Characterization of expanded virus
All 30 plasma samples that had successful HIV virus isolations were expanded to approximately 80–100 ml as high-titer viral stocks, which could subsequently be used to produce virus panels. Virus yield ranged from 1 to 284 ng p24 (PerkinElmer) or from 8×106 to 4.8×109 viral RNA copies/ml (Roche TaqMan v2.0) (Table 5).
Table 5.
Characterization of Expanded Virus
| |
|
|
|
Co receptor usage |
|
|
|
|||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
Phenotype |
Genotype prediction |
Antigen and nucleic acid titer |
||||
| Sample ID | Country of origin | Fiebig stage | HIV subtype | Ghost cell assay | Geno 2 Pheno (FPR 2–10%) | Pos 8/12 | Charge rule | p24 Antigen ng/ml | RNA million copies/ml | Million copies RNA per ng p24 |
| BP00014 | South Africa | I | C | CCR5 | CXCR4 | CCR5 | CCR5 | 122 | 2,100 | 17.2 |
| BP00002 | South Africa | II | C | CCR5 | CXCR4 | CCR5 | CCR5 | 186 | 2,200 | 11.8 |
| BP00012 | South Africa | II | C | CCR5 | CXCR4 | CCR5 | CCR5 | 11 | 130 | 11.8 |
| BP00016 | South Africa | II | C | CCR5 | CXCR4 | CCR5 | CCR5 | 186 | 910 | 4.9 |
| BP00018 | South Africa | II | C | ND | ND | ND | ND | 1 | 8 | 7.9 |
| BP00025 | South Africa | II | C | ND | ND | ND | ND | 31 | 620 | 20.0 |
| BP00028 | South Africa | II | C | CCR5 | CCR5 | CCR5 | CCR5 | 284 | 1,100 | 3.9 |
| BP00010 | South Africa | IV | C | CCR5 | CCR5 | CCR5 | CCR5 | 2 | 16 | 8.0 |
| BP00005 | South Africa | V | C | CCR5 | CCR5 | CCR5 | CCR5 | 56 | 510 | 9.1 |
| BP00008 | South Africa | V | C | CCR5 | CXCR4 | CCR5 | CCR5 | 22 | 440 | 20.0 |
| BP00029 | South Africa | V | C | ND | CXCR4 | CCR5 | CCR5 | 4 | 470 | 117.5 |
| BP00009 | South Africa | VI | C | CCR5 | CXCR4 | CCR5 | CCR5 | 127 | 1,200 | 9.4 |
| BP00047 | Brazil | V | B | CCR5 | CXCR4 | CCR5 | CCR5 | 238 | 2,100 | 8.8 |
| BP00040 | Brazil | VI | C | CCR5 | CXCR4 | CXCR4 | CCR5 | 129 | 1,600 | 12.4 |
| BP00044 | Brazil | VI | BF | CCR5 | CXCR4 | CCR5 | CCR5 | 16 | 170 | 10.6 |
| BP00048 | Greece | VI | CRF04_CPX | CCR5 | CCR5 | CCR5 | CCR5 | 2 | 36 | 18.0 |
| BP00049 | Greece | VI | CRF04_CPX | CCR5 | CXCR4 | CCR5 | CCR5 | 166 | 4,800 | 28.9 |
| BP00052 | Greece | VI | CRF04_CPX | CCR5 | CXCR4 | CCR5 | CCR5 | 7 | 120 | 17.1 |
| BP00053 | Greece | VI | CRF04_CPX | CCR5 | CXCR4 | CXCR4 | CXCR4 | 61 | 2,600 | 42.6 |
| BP00055 | United States | II | B | CCR5 | CCR5 | CCR5 | CCR5 | 197 | 2,400 | 12.2 |
| BP00058 | United States | IV | B | CCR5 | CCR5 | CCR5 | CXCR4 | 50 | 270 | 5.4 |
| BP00063 | United States | V | B | CCR5 | CXCR4 | CCR5 | CCR5 | 6 | 44 | 7.3 |
| BP00064 | United States | V | B | CCR5 | CCR5 | CCR5 | CCR5 | 24 | 110 | 4.6 |
| BP00067 | United States | V | B | CCR5 | CCR5 | CCR5 | CCR5 | 3 | 34 | 11.3 |
| BP00070 | United States | V | B | CCR5 | CCR5 | CCR5 | CCR5 | 14 | 72 | 5.1 |
| BP00069 | United States | IV | B | CCR5 | CXCR4 | CXCR4 | CCR5 | 13 | 130 | 10.0 |
| BP00075 | Poland | I | Not typed | CCR5 | CCR5 | CCR5 | CCR5 | 278 | 1,500 | 5.4 |
| BP00074 | Poland | II | B | CCR5 | CXCR4 | CCR5 | CCR5 | 203 | 2,800 | 13.8 |
| BP00078 | Cameroon | VI | CRF_AG | CCR5 | CCR5 | CCR5 | CCR5 | 19 | 140 | 7.4 |
| BP00079 | Cameroon | VI | CRF_AG | CCR5 | CCR5 | CCR5 | CCR5 | 17 | 160 | 9.4 |
| Mean | 12.2 | |||||||||
| SD | 8.2 | |||||||||
ND: Not done, either culture or v3 PCR was negative.
The expanded virus was characterized in terms of viral RNA concentration (Roche TaqMan v2.0), p24 concentration (Perkin Elmer HIV Ag ELISA), and coreceptor usage. The coreceptor utilization of cultured virus was evaluated by cultivation of virus in the GHOST cell assay as described in Materials and Methods. The prediction of coreceptor usage based on sequence information was also evaluated using a variety of prediction algorithms, and results are indicated.
Coreceptor usage
The predominant coreceptor usage of each of the virus isolates was determined based on genotype and phenotype analysis (Table 5). The phenotypic analysis of coreceptor usage was performed using the GHOST cell assay.33 All samples tested exhibited a CCR5 phenotype in this assay. Several genotype software packages were also used to assign a predicted genotype based on the sequence obtained in the V3 region of the env gene of these isolates. The predictions of coreceptor usage of three of the representative assays are shown. The Geno2pheno software, assigned a CXCR4 genotype to 16 of the 28 isolates, which did not agree with the phenotype or many of the other genotype packages. The lack of concordance between the various prediction programs and the inconsistency with phenotypic results underscore the need for better standardization of the genotype prediction programs.
Discussion
The pilot studies described here were aimed at establishing systems for collection and selection of plasma samples from persons with acute or recently acquired HIV infections from which virus can be isolated, expanded, and characterized for ultimate inclusion in HIV subtype panels. Plasma samples from North America, South America, Europe, and Africa were used for initial studies. Since an important application of these panels will be for evaluation and standardization of various assays, it was essential that a rigorous, well-controlled system be used for the preparation and characterization of these materials. To ensure a reasonably homogeneous sequence, we focused the collection on early stages of infection, which are most relevant to transmission and intervention programs. Plasma samples were evaluated for Fiebig stage based on the appearance of RNA, antigen, and antibody markers. The relative timing of infection was consistent with that of star phylogeny analysis of sequence diversity within the plasma derived isolates, where tested. The well-controlled, low-passage number for virus isolation and expansion allowed for generation of large quantities of virus for use in panels with minimum change in sequence from that of the original plasma. In fact, the nucleotide differences, observed between plasma and cultured derived sequences, were less than 0.6%, which is the maximum level of intraindividual viral diversity expected within 100 days from the infection with a single T/F virus, as previously calculated using a mathematical model.26
A major challenge was successful cultivation of virus from small volumes of plasma, with particular focus on early infection, many of which had low viral loads. Our experience corroborated previous findings in the literature showing that isolation from plasma with low viral loads was difficult.37,38 The addition of high volumes of plasma from low viral load cases to in vitro cultures, intended to increase the inoculum and hence isolation success rate, resulted in clot formation or toxicity to cells. The inclusion of the ultracentrifugation step allowed us to concentrate the virus, without introducing the toxic/anticoagulant components of the plasma into the cell cultures. An additional enhancement of infectivity success was the use of CD8-depleted PBMCs from normal donors as host cells for replication.39 The use of initial infected PBMCs as starting material for expansion allowed for more efficient cell-to-cell virus transfer, and thus more rapid infection, and reduced the number of passages required for expansion of virus to high titers.40 Using these methods, efficient virus yields were obtained from plasma samples with viral loads of more than 10,000 copies/ml.
The results of these studies demonstrate the feasibility of using cultured virus derived from plasma of acute or recently infected blood donors for preparation of HIV subtype panels. Routine donor screening for HIV RNA and antibody, combined with evidence of recent seroconversion for HIV-infected repeat donors, provides information to allow for selection of large volume plasma units representing recently transmitted HIV infections for characterization and potential inclusion in panels. This approach permitted expansion to sufficient quantities of virus for the development of subtype panels and will serve as a basis for further efforts to develop more comprehensive panels of highly characterized HIV isolates and corresponding plasma, reflecting the current dynamic and complex epidemic. The optimization and implementation of virus isolation methods resulted in successful culture and expansion of 30 viral isolates from the 80 plasma samples attempted. Detailed comparisons of sequence distribution of cultured virus for three of the isolates generated in these studies indicated highly homogeneous founder virus populations, reflecting an infection established by a single HIV-1 variant about 33–43 days prior to plasma collection. The expanded virus exhibited very low sequence diversity, and was essentially undistinguishable from that of the original plasma sample.
The comparison of viral load assays on the plasma samples as shown in Fig. 2 illustrates how these panels may be useful for the evaluation of diagnostic assay performance, and sequence information may help guide new NAT assay design. The availability of sufficient identical aliquots for each panel member used in the various assays minimizes the contributions of differences in RNA integrity or introduced in sample handling, even when run by different individuals and in different laboratories, to allow direct comparison of assay performance.
The expanded virus can be used for preparation of panels, including serial dilution panels, for evaluation of NAT and Ag or Ag/Ab tests, as well as in applications in infectivity assays for examining viral pathogenesis, drug resistance, or viral neutralization studies. These panels will be useful for standardization and validation efforts, to allow for effective assessment and comparison of data on which to base sound decisions about product advancement and/or product improvement.
In addition to evaluation of HIV diagnostic assays, the viral panels described in this study will be useful in studies of coreceptor usage, an important virologic parameter for coreceptor antagonist-based treatment strategies. The diverse strains in the panel representing viruses from various Fiebig stages and geographic regions are particularly useful in comparing the performance of cell culture assays versus V3 sequence-based prediction algorithms for global use. As noted, the various coreceptor genotype prediction algorithms showed a lack of concordance with phenotype data and further studies using larger numbers of specimens and viruses are needed for a better evaluation of the performance of these in silico algorithms. The availability of well-characterized panels will allow improved standardization of algorithms for assigning coreceptor usage and facilitate their implementation for clinical use. The cultured virus from these panels will also be useful for the characterization of transmitted/founder virus in terms of virulence and infectivity of macrophage vs. CD4 T cell populations for isolates representing various subtypes, particularly those isolated from plasma at early Fiebig stage.27,41
In conclusion, the availability of subtype panels reflecting recently transmitted HIV (both in the context of the individual subject and the temporal global epidemic) such as those being developed under this Division of AIDS program and others2,42 will be of value to those developing and evaluating tests, as well as to researchers. These panels will help ensure that blood screening and diagnostic assays properly detect virus currently in circulation and that vaccine and microbicide products are properly targeted to address the extent of viral diversity.
Sequence Data
Sequences from the isolates described in this study have been submitted to GenBank (www.ncbi.nlm.nih.gov/genbank/) under accession numbers JN687677 to JN687847.
Supplementary Material
Acknowledgments
This project has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200800014C. This work was also supported by a cooperative agreement W81XWH-07-2-0067 between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. and the U.S. Department of Defense (DoD).
We wish to thank Ewa Brojer of the Institute of Hematology and Blood Transfusion for the contribution of the Polish samples and Harvey Holmes of the National Institute of Biological Standards and Technology for contributing the Greek specimens. We also thank Lequan Nguyen and Ruibin Liang at SeraCare for HIV cultivation work, Jiangqin Zhao at FDA for assistance with sequence analysis and coreceptor testing, Cathy Barrett at SeraCare and Leslie Tobler at BSRI for logistics support, Bob Coombs at the University of Washington, Seattle, WA for Abbott m2000 quantitative HIV RNA testing, Robin Dewar of SAIC-Frederick, Inc. for bDNA testing, David Montefiori at the Duke Human Vaccine Institute, Durham, NC for HIV serological testing, Anne Marie O'Sullivan, Andrea Bradfield, Adam Bates, Shana Howell, Michelle Lazzaro, William Murtaugh, and Esther Lei for HIV sequencing support, Dr. Morgane Rolland for her advice on sequencing analysis, and Francine McCutchan for helpful guidance in the design of the subtype collection.
The opinions expressed herein are those of the authors and should not be construed as representing the official views of the U.S. Department of Health and Human Services, Department of the Army, Department of Defense, or the Food and Drug Administration. The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy. The views do not necessarily reflect the official policies of the Department of Health and Human Services, nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.AIDS Epidemic Update: November 2009. www.unaids.org/globalreport/global_report.htm www.unaids.org/globalreport/global_report.htm
- 2.Los Alamos: 2011. LANL HIV Sequence Database. [Google Scholar]
- 3.Schmidt M, et al. First transmission of human immunodeficiency virus Type 1 by a cellular blood product after mandatory nucleic acid screening in Germany. Transfusion. 2009;49(9):1836–1844. doi: 10.1111/j.1537-2995.2009.02203.x. [DOI] [PubMed] [Google Scholar]
- 4.Tebit DM. Arts EJ. Tracking a century of global expansion and evolution of HIV to drive understanding and to combat disease. Lancet Infect Dis. 2011;11(1):45–56. doi: 10.1016/S1473-3099(10)70186-9. [DOI] [PubMed] [Google Scholar]
- 5.Taylor BS. Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;359(18):1965–1966. doi: 10.1056/NEJMc086373. [DOI] [PubMed] [Google Scholar]
- 6.Sezgin E, et al. Effect of host genetics on incidence of HIV neuroretinal disorder in patients with AIDS. J Acquir Immune Defic Syndr. 2010;54(4):343–351. doi: 10.1097/QAI.0b013e3181deaf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buonaguro L. Tornesello ML. Buonaguro FM. Human immunodeficiency virus type 1 subtype distribution in the worldwide epidemic: Pathogenetic and therapeutic implications. J Virol. 2007;81(19):10209–10219. doi: 10.1128/JVI.00872-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano V. de Mendoza C. Update on HIV viral-load assays: New technologies and testing in resource-limited settings. Future Virol. 2009;4(5):423–430. [Google Scholar]
- 9.Edelmann A, et al. Improvement of an ultrasensitive human immunodeficiency virus type 1 real-time reverse transcriptase-polymerase chain reaction targeting the long terminal repeat region. Transfusion. 2010;50(3):685–692. doi: 10.1111/j.1537-2995.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 10.Henquell C, et al. Difficulties in diagnosing group O human immunodeficiency virus type 1 acute primary infection. J Clin Microbiol. 2008;46(7):2453–2456. doi: 10.1128/JCM.02217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foglieni B, et al. A cluster of human immunodeficiency virus Type 1 recombinant form escaping detection by commercial genomic amplification assays. Transfusion. 2011;51(4):719–730. doi: 10.1111/j.1537-2995.2010.02942.x. [DOI] [PubMed] [Google Scholar]
- 12.Nubling CM, et al. Experience of mandatory nucleic acid test (NAT) screening across all blood organizations in Germany: NAT yield versus breakthrough transmissions. Transfusion. 2009;49(9):1850–1858. doi: 10.1111/j.1537-2995.2009.02212.x. [DOI] [PubMed] [Google Scholar]
- 13.Church D, et al. Comparison of the RealTime HIV-1, COBAS TaqMan 48 v1.0, Easy Q v1.2, and Versant v3.0 assays for determination of HIV-1 viral loads in a cohort of Canadian patients with diverse HIV subtype infections. J Clin Microbiol. 2011;49(1):118–124. doi: 10.1128/JCM.00685-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline NE. Schwarzwald H. Kline MW. False negative DNA polymerase chain reaction in an infant with subtype C human immunodeficiency virus 1 infection. Pediatr Infect Dis J. 2002;21(9):885–886. doi: 10.1097/00006454-200209000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Obaro SK, et al. Failure of serial human immunodeficiency virus type 1 DNA polymerase chain reactions to identify human immunodeficiency virus type 1 clade A/G. Pediatr Infect Dis J. 2005;24(2):183–184. doi: 10.1097/01.inf.0000151040.57772.40. [DOI] [PubMed] [Google Scholar]
- 16.Ntemgwa M, et al. Discrepancies in assignment of subtype/recombinant forms by genotyping programs for HIV type 1 drug resistance testing may falsely predict superinfection. AIDS Res Hum Retroviruses. 2008;24(7):995–1002. doi: 10.1089/aid.2008.0064. [DOI] [PubMed] [Google Scholar]
- 17.Snoeck J, et al. Discordances between interpretation algorithms for genotypic resistance to protease and reverse transcriptase inhibitors of human immunodeficiency virus are subtype dependent. Antimicrob Agents Chemother. 2006;50(2):694–701. doi: 10.1128/AAC.50.2.694-701.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuritzkes DR. HIV-1 subtype as a determinant of disease progression. J Infect Dis. 2008;197(5):638–639. doi: 10.1086/527417. [DOI] [PubMed] [Google Scholar]
- 19.Renjifo B, et al. Differences in perinatal transmission among human immunodeficiency virus type 1 genotypes. J Hum Virol. 2001;4(1):16–25. [PubMed] [Google Scholar]
- 20.Baeten JM, et al. HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Infect Dis. 2007;195(8):1177–1180. doi: 10.1086/512682. [DOI] [PubMed] [Google Scholar]
- 21.Daar ES, et al. Baseline HIV type 1 coreceptor tropism predicts disease progression. Clin Infect Dis. 2007;45(5):643–649. doi: 10.1086/520650. [DOI] [PubMed] [Google Scholar]
- 22.Weiss R, et al. Acquired immunodeficiency syndrome-related lymphoma: Simultaneous treatment with combined cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy and highly active antiretroviral therapy is safe and improves survival—results of the German Multicenter Trial. Cancer. 2006;106(7):1560–1568. doi: 10.1002/cncr.21759. [DOI] [PubMed] [Google Scholar]
- 23.Kaslow RA. Dorak T. Tang JJ. Influence of host genetic variation on susceptibility to HIV type 1 infection. J Infect Dis. 2005;191(Suppl 1):S68–S77. doi: 10.1086/425269. [DOI] [PubMed] [Google Scholar]
- 24.Huang DD. Giesler TA. Bremer JW. Sequence characterization of the protease and partial reverse transcriptase proteins of the NED panel, an international HIV type 1 subtype reference and standards panel. AIDS Res Hum Retroviruses. 2003;19(4):321–328. doi: 10.1089/088922203764969528. [DOI] [PubMed] [Google Scholar]
- 25.Brown BK, et al. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J Virol. 2005;79(10):6089–6101. doi: 10.1128/JVI.79.10.6089-6101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salazar-Gonzalez JF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206(6):1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiebig EW, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 29.Erice A, et al. Sensitive microculture method for isolation of human immunodeficiency virus type 1 from blood leukocytes. J Clin Microbiol. 1992;30(2):444–448. doi: 10.1128/jcm.30.2.444-448.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolland M, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med. 2011;17(3):366–371. doi: 10.1038/nm.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng W, et al. DIVEIN: A web server to analyze phylogenies, sequence divergence, diversity, and informative sites. Biotechniques. 2010;48(5):405–408. doi: 10.2144/000113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giorgi EE, et al. Estimating time since infection in early homogeneous HIV-1 samples using a poisson model. BMC Bioinformatics. 2010;11:532. doi: 10.1186/1471-2105-11-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vodros D. Fenyo EM. Quantitative evaluation of HIV and SIV co-receptor use with GHOST(3) cell assay. Methods Mol Biol. 2005;304:333–342. doi: 10.1385/1-59259-907-9:333. [DOI] [PubMed] [Google Scholar]
- 34.Erali M. Hillyard DR. Evaluation of the ultrasensitive Roche Amplicor HIV-1 monitor assay for quantitation of human immunodeficiency virus type 1 RNA. J Clin Microbiol. 1999;37(3):792–795. doi: 10.1128/jcm.37.3.792-795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Bel A, et al. Correction of underquantification of human immunodeficiency virus type 1 load with the second version of the Roche Cobas AmpliPrep/Cobas TaqMan assay. J Clin Microbiol. 2010;48(4):1337–1342. doi: 10.1128/JCM.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbeik T, et al. Quantitative and cost comparison of ultrasensitive human immunodeficiency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5. J Clin Microbiol. 2000;38(3):1113–1120. doi: 10.1128/jcm.38.3.1113-1120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vella S, et al. Plasma HIV-1 copy number and in vitro infectivity of plasma prior to and during combination antiretroviral treatment. Antiviral Res. 2000;47(3):189–198. doi: 10.1016/s0166-3542(00)00107-8. [DOI] [PubMed] [Google Scholar]
- 38.Piatak M, Jr, et al. Viral dynamics in primary HIV-1 infection. Lancet. 1993;341(8852):1099. doi: 10.1016/0140-6736(93)92463-4. [DOI] [PubMed] [Google Scholar]
- 39.Gorry PR, et al. Isolation of human immunodeficiency virus type 1 from peripheral blood monocytes. Methods Mol Biol. 2005;304:25–33. doi: 10.1385/1-59259-907-9:025. [DOI] [PubMed] [Google Scholar]
- 40.Sourisseau M, et al. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007;81(2):1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goonetilleke N, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206(6):1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuevas MT, et al. Biological and genetic characterization of HIV type 1 subtype B and nonsubtype B transmitted viruses: Usefulness for vaccine candidate assessment. AIDS Res Hum Retroviruses. 2010;26(9):1019–1025. doi: 10.1089/aid.2010.0018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.