Summary
To determine effect of partner involvement and couple counseling on uptake of interventions to prevent HIV-1 transmission, women attending a Nairobi antenatal clinic were encouraged to return with partners for voluntary HIV-1 counseling and testing (VCT) and offered individual or couple posttest counseling. Nevirapine was provided to HIV-1-seropositive women and condoms distributed to all participants. Among 2104 women accepting testing, 308 (15%) had partners participate in VCT, of whom 116 (38%) were couple counseled. Thirty-two (10%) of 314 HIV-1-seropositive women came with partners for VCT; these women were 3-fold more likely to return for nevirapine (P = 0.02) and to report administering nevirapine at delivery (P = 0.009). Nevirapine use was reported by 88% of HIV-infected women who were couple counseled, 67% whose partners came but were not couple counseled, and 45% whose partners did not present for VCT (P for trend = 0.006). HIV-1-seropositive women receiving couple counseling were 5-fold more likely to avoid breast-feeding (P = 0.03) compared with those counseled individually. Partner notification of HIV-1-positive results was reported by 138 women (64%) and was associated with 4-fold greater likelihood of condom use (P = 0.004). Partner participation in VCT and couple counseling increased uptake of nevirapine and formula feeding. Antenatal couple counseling may be a useful strategy to promote HIV-1 prevention interventions.
Keywords: voluntary counseling and testing, couple counseling, mother-to-child HIV-1 transmission, breastfeeding, nevirapine, condom use, partner notification
Many countries in sub-Saharan Africa are in the process of integrating antenatal voluntary HIV-1 counseling and testing (VCT), antiretroviral prophylaxis, and counseling regarding infant feeding into routine maternal and child health services.1 Even with greater availability of interventions to protect infants from HIV-1 acquisition, many women choose not to receive their HIV-1 test results, and many who learn that they are HIV infected do not implement interventions to prevent vertical or sexual transmission. In both research and non-research settings, <75% of HIV-infected pregnant women who are tested learn their HIV-1 status2,3 and <50% of these women obtain antiretrovirals to prevent mother-to-child HIV-1 transmission2,4–6 or use condoms postpartum.7,8
Not disclosing HIV-1 test results to a sexual partner may impede a woman’s access to interventions to prevent vertical and sexual HIV-1 transmission. There is evidence that lack of partner support is associated with poor uptake of antiretroviral medication and the inability to modify infant feeding practices.9,10 Sexual abstinence and condom use have also been shown to be more common among postpartum women who reveal HIV-positive results to partners.7 These associations between partner involvement and uptake of interventions underscore the importance of involving the male partner in HIV-1 prevention efforts initiated in the antenatal setting.
Antenatal VCT involves counseling and testing the woman alone, with the expectation that she will disclose results to her partner. Using this model, the majority of pregnant women in stable relationships who are tested as part of routine care do not inform their partner of positive HIV-1 results, fearing domestic violence, abandonment, or stigmatization.7,11,12 We hypothesized that conducting VCT for pregnant women together with partners could facilitate notification and increase partner participation in the decision-making process. This could in turn improve maternal access to mother-to-child HIV-1 prevention interventions. Studies among discordant couples in nonantenatal settings support this hypothesis and demonstrate that couple counseling is associated with significant behavior change, including sustainable increases in condom use.13–15 In addition, when comparing couple counseling and individual counseling, provision of counseling to couples appears to be more cost-effective in averting HIV-1 infections.16
To examine whether couple counseling in the antenatal setting could be used as a strategy to increase use of interventions to prevent perinatal and sexual HIV-1 transmission, we introduced couple VCT into a Nairobi antenatal clinic and determined the prevalence and correlates of partner participation and couple counseling. The study’s primary aim was to assess the impact of partner involvement, specifically being counseled as a couple, on perinatal intervention uptake and condom use.
SUBJECTS AND METHODS
Study Population and Clinical Procedures
Women attending a Nairobi City Council clinic were enrolled at their first antenatal visit after written informed consent. At enrollment, women were provided information as a group about heterosexual and mother-to-child HIV-1 transmission and encouraged to inform male partners of VCT availability. Each woman was asked to return within 7 days for routine antenatal laboratory studies, including optional VCT, and given the choice of presenting alone or with her partner. A baseline interview was conducted at this first visit by a trained nurse counselor or the study doctor. After questionnaire administration, the pros and cons of partner testing, couple VCT, and partner notification of HIV test results were discussed on an individual basis.
Male partners were enrolled into the study and completed a baseline questionnaire at initial presentation to the clinic. Based on their preference, women and men were pretest counseled individually or as a couple by the same person who had performed the initial pretest counseling session. In addition, women and men were counseled by a female or male HIV-infected peer counselor, respectively. Rapid assays were performed for HIV-1, syphilis, and hemoglobin and results were available within 30 minutes. Each woman and man was then asked confidentially whether she or he would prefer to receive test results and posttest counseling alone or as a couple. Individual rather than couple posttest counseling was provided if this was requested by either partner. During the posttest HIV-1 counseling session, a questionnaire was administered and condoms were offered to all individuals in conjunction with counseling on safe sex during pregnancy and breastfeeding.
HIV-1–infected and uninfected women and men were asked to return 2 weeks after receiving their results for additional posttest counseling. At this visit, HIV-1–uninfected women were counseled to breastfeed their infants and HIV-1– infected women and partners were counseled about nevirapine use at delivery, exclusive breastfeeding, and the role of alternative feeding modalities. Maternal and infant doses of nevirapine were offered to all HIV-1–infected mothers at this visit or at later follow-up if this visit was missed.
At 1 week after delivery, HIV–infected women were evaluated to determine whether the infant received nevirapine and the mode of infant feeding. Mother-infant pairs were requested to return for additional counseling and optional infant HIV-1 testing at 3 and 6 months postpartum. Women and men with HIV-associated symptoms were provided trimethoprim/sulfamethoxazole prophylaxis and referred to local clinics for further management.
Laboratory Testing
Rapid HIV testing was conducted on site using 1 of 3 commercially available tests (Capillus HIV-1/HIV-2 assay, Cambridge Diagnostics Ireland, Ltd; Serocard HIV, Trinity Biotech; Uni-Gold, Trinity Biotech, Bray, Ireland). Positive tests were confirmed using another rapid HIV assay (Determine HIV-1/HIV-2, Abbott Laboratories, Abbott Park) and indeterminate results (<1% of all tests performed in this study) were confirmed with an HIV-1/HIV-2 enzyme-linked immunosorbent assay. To determine infant HIV-1 infection status, infant blood specimens were collected on filter paper at 3 months. HIV-1 DNA gag sequences were detected in filter paper specimens using polymerase chain reaction assays.17
Statistical Methods
Baseline characteristics for women and men enrolled in the study were compared using paired t tests for continuous variables and Pearson x2 or Fisher exact tests for dichotomous variables. Correlates of couple counseling, partner presentation for VCT, and partner notification were determined with Pearson x2, Fisher exact test, and independent t tests. Covariates found to be significant in univariate analyses (P < 0.05) were examined in multivariate models using logistic regression. Nevirapine uptake and infant feeding choices were evaluated with respect to partner involvement, couple counseling, and partner notification of HIV-1 status using tests for linear associations, Pearson x2, and Fisher exact tests. Condom use before and after HIV-1 testing were compared among women and partners using the McNemar test. Associations between reported condom use after testing and partner participation in VCT, couple counseling, and partner notification were evaluated with Pearson x2 and Fisher exact tests.
RESULTS
Follow-up of Study Participants
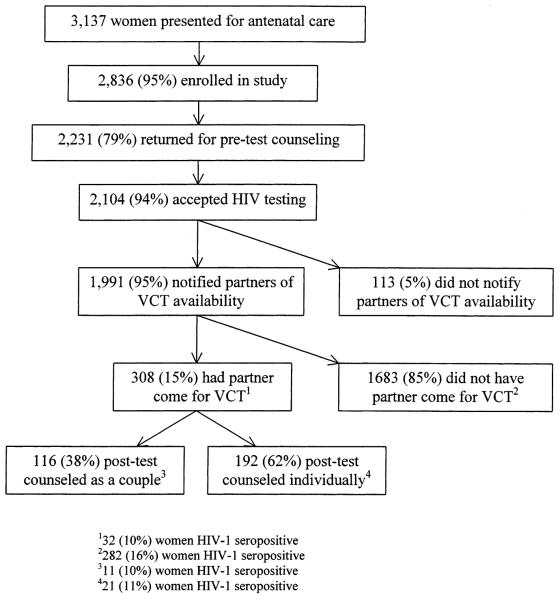
Between September 2001 and December 2002, 3137 pregnant women presented to the antenatal clinic and 2836 women enrolled in the study (Fig. 1). A total of 2231 (79%) returned to the clinic for a second study visit, of whom 2104 (94%) accepted HIV-1 counseling and testing and 1991 (89%) reported inviting their partner for HIV-1 counseling. Among 308 partners who came for testing, 116 couples (38%) elected to receive posttest counseling together and 192 (62%) to receive posttest counseling individually (Fig. 1). A total of 170 (55%) of the 308 men who presented for the first VCT visit returned for additional counseling on interventions to prevent mother-to-child transmission approximately 2 weeks after receiving HIV-1 test results.
FIGURE 1.
Enrollment and follow-up of pregnant women.
HIV-1 seroprevalence was 15% among the 2104 women who were tested and 11% among 301 men tested. Among the 314 HIV-1–seropositive women, 69% returned for follow-up 2 weeks after receiving HIV-1 test results, a lower proportion than among the 1788 HIV-1–seronegative women, of whom 79% returned (odds ratio [OR] = 0.6; 95% CI 0.5–0.8; P < 0.001). A total of 122 (56%) of these 217 HIV-1–seropositive women presented to the study clinic during the 1st week post-partum and were evaluated for nevirapine administration and infant feeding practices (Fig. 2). Among these motherinfant pairs, 67 infants (55%) were tested for HIV-1 at 3 months of age, 8 (12%) of whom were HIV-1 infected.
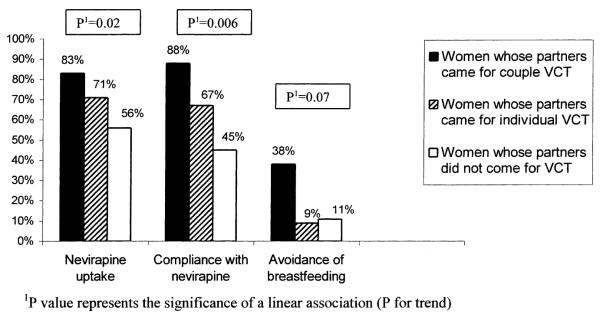
FIGURE 2.
Trends for nevirapine uptake and compliance with nevirapine regimen.
Cohort Description
Overall, 1539 (54%) of enrolled women were in formalized marriages, 2538 (90%) lived with their partners, and 816 (29%) were employed. Men were older, more educated, reported more lifetime sexual partners, and were more likely to have ever used a condom than their female partners (Table 1). A greater proportion of men also had a history of a sexually transmitted infection and prior HIV-1 testing (Table 1).
TABLE 1.
Comparison of Baseline Characteristics for Women Whose Partners Came to Clinic for VCT and Their Partners
| Characteristics | Women (n = 308) |
Men (n = 308) |
P Value* |
|---|---|---|---|
| Sociodemographic | |||
| Age [mean (SD)] (y) | 24 (4.6) | 29.5 (5.5) | <0.001 |
| Education [mean (SD)] (y) | 9 (2.7) | 10 (2.8) | <0.001 |
| Employed [n (%)] | 84 (26.9%) | 305 (98.1%) | <0.001 |
| Sexual history | |||
| Lifetime sexual partners [mean (SD)] |
2 (1.7) | 5 (6.6) | <0.001 |
| Age at sexual debut [mean (SD)] |
18.1 (2.9) | 17.6 (3.5) | 0.02 |
| Ever used condom [mean (SD)] |
69 (22%) | 149 (48%) | <0.001 |
| History of STI [mean (SD)] |
28 (9%) | 127 (40%) | <0.001 |
| Prior HIV testing [mean (SD)] |
25 (8%) | 45 (15%) | 0.006 |
P values calculated using paired t tests for continuous variables with determination of mean difference and χ2 tests for dichotomous variables.
STI, sexually transmitted infection.
Among women whose partners came to clinic, HIV-1 seroprevalence was significantly lower than among women whose partners did not present for VCT (10 vs. 16%; P = 0.02) (Fig. 1). Overall, 255 couples (85%) were concordant HIV-1 seronegative, 19 couples (6%) were concordant HIV-1 seropositive, and 26 couples (9%) were HIV-1 serodiscordant. In 13 (50%) of the serodiscordant couples, the man was HIV-1 infected and in the remaining 13 couples the woman was HIV-1 infected.
Correlates of Partner VCT, Couple Counseling, and Partner Notification
Factors associated with the partner coming to the antenatal clinic for VCT included living with the partner, being married, in a monogamous marriage, and older at sexual debut (Table 2). These same characteristics were correlated with acceptance of couple counseling (Table 2). In multivariate analysis, the 2 correlates that retained significant associations with partner participation and couple counseling were living together and older age at sexual debut (Table 2).
TABLE 2.
Comparison of Baseline Characteristics for Women Whose Partners Did Not Come to the Antenatal Clinic for VCT, Women Whose Partners Came for VCT, and Women Whose Partners Participated in VCT as a Couple
| Women Whose Partners Did Not Come for VCT* (n = 1796) |
Women Whose Partners Came for VCT* (n = 308) |
P Value†§ |
Women Counseled as a Couple* (n = 116) |
P Valueठ|
|
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age [mean (SD)] (y) | 23.7 (4.4) years | 24.1 (4.6) 6 years | 0.13 | 23.8 (4.4) years | 0.79 |
| Duration of education [mean (SD)] (y) | 8.7 (2.5) years | 9.0 (2.7) years | 0.10 | 9.1 (2.8) years | 0.09 |
| Employed [n (%)] | 541 (30.1%) | 84 (27.4%) | 0.18 | 30 (25.8%) | 0.19 |
| Live with partner [n (%)] | 1588 (88.5%) | 303 (98.4%) | <0.001∥ | 115 (99.1%) | <0.001¶ |
| Married [n (%)] | 1609 (89.6%) | 302 (98.4%) | <0.001# | 114 (98.3%) | <0.001# |
| Formalized marriage‡‡ [n (%)] | 979 (60.7%) | 197 (65.2%) | 0.08 | 69 (59.5%) | 0.523 |
| Polygamous marriage [n (%)] | 125 (7.8%) | 11 (3.6%) | 0.005# | 2 (1.7%) | 0.013# |
| Duration of relationship [mean (SD)] | 3.7 (3.8) years | 3.7 (4.0) years | 0.88 | 3.4 (3.9) years | 0.31 |
| Monthly rent in $US [mean (SD)] | 23 (12) | 23 (12) | 0.79 | 24 (12) | 0.77 |
| Number of rooms in house [mean (SD)] | 1.1 (0.3) | 1.1 (0.4) | 0.64 | 1.1 (0.3) | 0.20 |
| Reproductive/sexual | |||||
| Lifetime sexual partners [mean (SD)] | 2.3 (1.8) | 2.2 (1.7) | 0.55 | 2.0 (1.3) | 0.10 |
| Age at sexual debut [mean (SD)] | 17.6 (2.7) years | 18.2 (2.9) years | 0.04†† | 18.5 3.1) years | 0.003** |
| Number of pregnancies [mean (SD)] | 1.1 (1.2) | 1.2 (1.3) | 0.90 | 1 (1.4) | 0.25 |
| Ever used a condom [mean (SD)] | 364 (20.1%) | 66 (21.4%) | 0.35 | 26 (22.4%) | 0.32 |
| Ever used a condom with current partner [mean (SD)] | 235 (13.1%) | 36 (11.7%) | 0.28 | 14 (12.1%) | 0.44 |
| Used a condom during last sex [mean (SD)] | 19 (1.1%) | 1 (0.3%) | 0.19 | 0 (0.0%) | 0.30 |
| History of STI [mean (SD)] | 179 (10.0%) | 28 (9.1%) | 0.34 | 9 (7.8%) | 0.28 |
| Prior HIV testing [mean (SD)] | 145 (8.1%) | 25 (8.1%) | 0.55 | 11 (9.5%) | 0.34 |
Among 2104 women who returned to the study clinic for VCT.
Comparison between women whose partners came and women whose partners did not come to clinic.
Comparison between women who were counseled together with their partners and women whose partners did not come to clinic.
Variables significant in univariate analyses (P < 0.05) were included in the multivariate model.
Variable significant in multivariate analysis: OR = 5.0; 95% CI 1.3–19.7; P = 0.02.
Variable significant in multivariate analysis: OR = 20; 95% CI 1.5–257; P = 0.02.
Variable not significant in multivariate analysis: P > 0.05.
Variable significant in multivariate analysis: OR = 1.1; 95% CI 1.0–1.2; P = 0.001.
Variable significant in multivariate analysis: OR = 1.1; 95% CI 1.0–1.1; P = 0.004.
Formalized marriage included women married in church, civil, or traditional ceremonies. STI, sexually transmitted infection.
Partner notification after women received HIV test results was correlated with being unemployed, living with the partner, being formally married, reporting fewer lifetime sexual partners, older age at first sex, never having used a condom, and being HIV-1 uninfected. Overall, 64% of 217 seropositive women reported informing their partners compared with 95% of 1410 seronegative women (OR = 0.09; 95% CI 0.07–0.14; P < 0.001).
Uptake of Nevirapine and Avoidance of Breastfeeding
During follow-up visits, HIV-1–infected women were offered maternal and infant doses of nevirapine for use at the time of delivery. Overall, 200 (64%) of the 314 HIV-1–infected mothers received nevirapine. A total of 113 women presented postpartum and reported administering either the maternal or infant doses of nevirapine, with 111 (91%) taking the intrapartum dose and 108 (89%) giving nevirapine to their newborn.
We evaluated nevirapine uptake in 3 distinct groups of women accepting VCT: women who received posttest counseling and test results as a couple; women whose partners came to clinic for VCT, but who were posttest counseled individually; and women whose partners did not come to the study clinic for VCT. A higher proportion of women who were counseled as a couple returned to receive nevirapine (83%), compared with women whose partners came to clinic and were counseled separately (71%) and compared with women whose partners did not come to clinic for VCT (56%) (P for trend = 0.02) (Fig. 2). Nevirapine administration at delivery was also highest among women who were couple counseled (88%), followed by women whose partners came but were not couple counseled (67%), and women who did not have partner participation in VCT (45%) (P for trend = 0.006) (Fig. 2). Women who received nevirapine and did not return for follow-up post-partum were excluded from the analysis because they may or may not have used the nevirapine that they had received antenatally. Women who never received nevirapine antenatally were considered to have not used nevirapine at the time of delivery because nevirapine was not routinely offered to women at delivery sites in Nairobi during the study period.
In a separate analysis we evaluated the strength of these associations. Women whose partners came to clinic for VCT were approximately 3 times more likely to return to receive nevirapine (OR = 3.1; 95% CI 1.2–8.4; P = 0.02). Women whose partners came to clinic for VCT were also 3-fold more likely to have returned for follow-up and to report taking the maternal or infant dose of nevirapine (OR = 3.4; 95% CI 1.3–9; P = 0.009). Couple counseling was significantly associated with follow-up postpartum and nevirapine utilization. Women who were counseled as a couple were 8-fold more likely to return for follow-up and report nevirapine use at delivery than women who did not receive couple counseling (OR = 8.0; 95% CI 1.0–66; P = 0.03). These relationships remained robust after adjusting in a multivariate model for older age at sexual debut and living with the partner.
HIV-1-seropositive women who were couple counseled were also more likely to choose not to breastfeed their infants (OR = 0.2; 95% CI 0.04–0.9; P = 0.03). Three (38%) of the 8 women who received counseling as a couple chose not to breastfeed compared with 12 (11%) of the 114 women who did not receive counseling as a couple, a finding that retained significance in multivariate analysis. HIV-1 infection status of the partner was not associated with infant feeding decisions (P = 0.9).
At 3 months postpartum, 67 (55%) of the 122 motherinfant pairs seen after delivery returned for infant HIV-1 testing. Among the infants who were tested, 8 (12%) were HIV-1 infected. There was a trend for partner notification to be associated with lower infant HIV-1 infection prevalence (OR = 0.2; 95% CI 0.04–1.1; P = 0.07). The study was underpowered to evaluate associations between either partner involvement or couple counseling and infant HIV-1 infection. Infant infection risk when the partner came for VCT was 11% (1/9) compared with 12% (7/58) when he did not come, and for infants whose mothers did vs. did not receive couple counseling, risk was 0% (0/2) and 12.5% (8/65), respectively (P > 0.05 for both comparisons).
Condom Use Among Women Receiving HIV-1 Test Results
At baseline, reported condom use before VCT was significantly higher among women who subsequently tested HIV-1 seropositive than among women who tested HIV-1 seronegative (26 vs. 19%; P = 0.007). Among the 871 women who were sexually active between baseline evaluation and the return visit, reported condom use increased from 14 to 38% (P < 0.001). No increase in reported condom use was observed among men who were sexually active during the period between VCT and the 2-week visit (16% before testing vs. 19% after testing; P = 0.6).
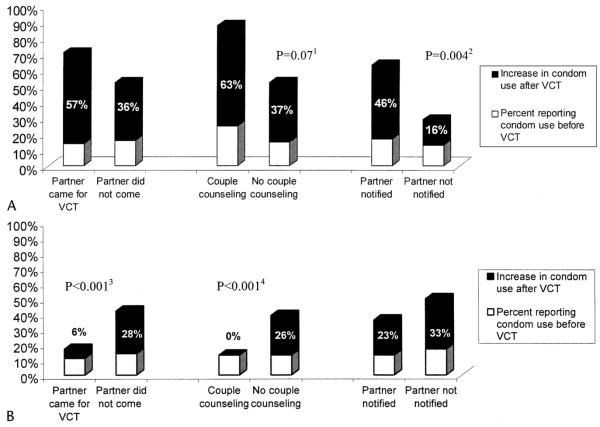
Among 95 HIV-seropositive women who reported sexual activity after receiving HIV test results, partner notification was associated with a 4-fold increased likelihood of reporting condom use in the interval since their last visit (OR = 4.2; 95% CI 1.5–11.5; P = 0.004). Couple counseling was associated with a trend for increased condom use (OR = 6.5; 95% CI 0.77–55; P = 0.07) (Fig. 3). The opposite relationship between couple counseling and condom use was found for 766 HIV-uninfected women who reported sexual activity at the 2-week posttest visit. Women who were counseled about HIV-negative test results as a couple were significantly less likely to use a condom posttest than women counseled individually (OR = 0.2; 95% CI 0.1–0.43; P < 0.001) (Fig. 3). The majority (97%) of these women and their partners were concordant HIV-1 seronegative. A similar effect on condom uptake was observed among women whose partners participated in VCT but who received individual posttest counseling (OR = 0.3; 95% CI 0.2–0.4; P < 0.001) (Fig. 3).
FIGURE 3.
Percent of women reporting condom use at baseline and after VCT. A, HIV-1–seropositive women. 1Significant difference between women who were couple counseled and those not couple counseled (P = 0.07). 2Significant difference between women who informed partners of HIV status and those who did not (P = 0.004). B, HIV-1-seronegative women. 3Significant difference between women whose partners came for VCT vs. did not come (P < 0.001). 4Significant difference between women who were couple counseled and those not couple counseled (P < 0.001). Percentages indicate the increase in the proportion of women reporting condom use during follow-up over baseline.
DISCUSSION
Among HIV-1-infected women presenting for routine antenatal care in this cohort, partner participation in VCT was associated with increased uptake of interventions to prevent vertical and sexual HIV-1 transmission. Women whose partners came to the antenatal clinic for counseling were more likely to receive nevirapine during follow-up, avoid breastfeeding their infant, and report condom use. The association between partner participation and uptake of these interventions was strongest when partners who came to the clinic agreed to be counseled as a couple. These data show that partner participation in VCT can improve acceptance and utilization of preventive strategies and suggest that couple counseling in the antenatal setting may have additional benefits to individual VCT.
We hypothesize that partner participation in the antenatal VCT program and couple counseling influenced intervention uptake by promoting dialogue between women and partners about methods to prevent mother-to-child and sexual HIV-1 transmission. This may not have been the case when women simply notified partners that they were HIV-1 seropositive in the absence of partner VCT. In the partner notification scenario, women relay test results but do not necessarily provide additional information regarding specific interventions to prevent HIV-1 transmission. Conversely, men coming for individual or couple VCT were informed of the pros and cons of antiretrovirals, alternative infant feeding, and condom use. When a couple presents for posttest counseling together, the counselor tailors discussions to meet the couple’s needs and facilitates interactions that are difficult to initiate. The benefits of couple counseling are supported by the stepwise increase that we observed in intervention uptake from partner notification of a positive test result, to partner participation in individual counseling, and finally to couple counseling.
In addition to increasing intervention uptake, offering couple counseling and encouraging women to bring partners for VCT appears to have increased partner notification rates in this cohort. Among HIV-1-infected women in the cohort, 64% informed their partners of HIV-1–positive results vs. <40% in other African studies.7,11,12 The fact that women were encouraged to discuss VCT before testing and were able to provide partners with a reason for HIV-1 testing and a site for free VCT may account for higher notification rates. It is also possible that the clinic’s emphasis on including partners in the counseling process made women more aware of the important role the partner can play in preventing HIV/AIDS transmission. Correlates of partner notification included being unemployed and less sexually experienced (fewer lifetime sexual partners, older age at first sex, no prior condom use). These findings have not been reported in all studies of HIV-1 status disclosure during pregnancy7,11; however, they are consistent with reports from 2 earlier perinatal studies conducted in East Africa.9,12 One interpretation is that women who work outside the home or have more sexual experience believe that they are more likely to be blamed for infidelity and do not disclose as a result of this fear.
Another interesting finding in this study was that among HIV-1-seronegative women, partner participation was associated with lower rather than higher condom use posttest. Partner testing may have provided these men and women with the assurance that they were in a concordant HIV-seronegative relationship. We observed that >95% of the partners of seronegative women who came for testing were also HIV uninfected. However, these HIV-1-seronegative men were not different from HIV-1–seropositive men when comparing the number of lifetime sexual partners (P = 0.7), suggesting that they are at similar risk for acquiring and transmitting HIV-1. Lower condom use in this setting may place women at risk for acute HIV-1 infection, especially in relationships that are not monogamous, a potentially more common scenario during late pregnancy and the early postpartum period. We conclude that after establishing that both members are seronegative, it is important to consistently promote condoms, even if this is difficult because it suggests infidelity within the relationship. Continued emphasis on condom use and monogamy in concordant HIV-seronegative relationships is essential to protect against future transmission.
Many mother-to-child HIV-1 transmission prevention programs are already receiving support from governmental and nongovernmental organizations in sub-Saharan Africa. Thus, the idea of working within antenatal clinics to promote VCT has great potential. By offering couple counseling within the antenatal setting, couple VCT may be integrated into existing HIV/AIDS prevention programs and fulfill an important counseling service for both men and women. To effectively implement such a program, it will be necessary to explore ways to increase partner participation. In our study, relatively high rates (64%) of reported partner notification were not matched by high rates of VCT acceptance by partners (15%). Fifteen percent of women who underwent testing had partners who came for VCT and fewer than half of these women received posttest counseling as a couple. A lack of community awareness about the importance of partner VCT and cultural beliefs that men should not participate in antenatal activities may explain low partner involvement. National and local VCT campaigns promoting couple counseling can be used to address barriers to testing male partners and increase awareness in the general public.18 Poor uptake of VCT and couple counseling may also have been due to women not informing partners of VCT availability or to conflicts between clinic hours and men’s work schedules. Although we used some strategies to overcome these barriers, such as providing letters of invitation to partners to excuse them from work and holding clinics on weekends to avoid workdays, more efforts to enhance male participation are required as these met with limited success. One option would be to offer a separate clinic distinct from the antenatal screening site to make men feel more comfortable; however, this would not facilitate couple counseling. Qualitative research to explore ways to increase uptake of couple counseling will be important to increase use of this counseling model in antenatal programs.
A final consideration is that this study was a prospective cohort study and not a randomized clinical trial. Women whose partners came to clinic were a select group who differed from those whose partners did not come. These underlying differences, rather than partner participation or couple counseling, may have resulted in effects on uptake of interventions. Specifically, we found that women with partners who came to clinic were more likely to be living together and older at sexual debut than women who did not have partners who came to clinic. However, after adjusting for these cofactors in our multivariate model, all associations retained statistical significance, suggesting that partner participation and couple counseling have independent effects on intervention uptake. Nevertheless, the possibility exists that a difference between the 2 groups went unrecognized and may have contributed to the effects we observed. For example, the quality of the relationship may have independently led to improved uptake of interventions rather than couple counseling itself. A randomized comparison of couple counseling vs. conventional models of testing is necessary to better determine the benefits and risk of this approach.
To our knowledge, this study was the first to evaluate couple counseling in a prevention of mother-to-child HIV-1 transmission program. While we found it challenging to achieve high rates of partner participation, we observed that significant benefits were associated with partner involvement. These findings support using couple counseling as a strategy to reduce risk of perinatal HIV-1 transmission and emphasize the need for feasible, affordable approaches to encourage men to participate in VCT and couple counseling.
Acknowledgments
Funded by the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF). C. Farquhar is supported by the National Institutes of Health (K23-HD41879). J. Kiarie and F. John were scholars in the AIDS International Training and Research Program supported by the National Institutes of Health/Fogarty International Center (T22-TW00001). G. John-Stewart is an EGPAF Scientist and D. Mbori-Ngacha has an EGPAF Leadership Award.
REFERENCES
- 1.Dabis F, Ekpini RE. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002;359:2097–2104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 2.Meda N, Leroy V, Viho I, et al. Field acceptability and effectiveness of the routine utilization of zidovudine to reduce mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:2323–2328. doi: 10.1097/00002030-200211220-00013. [DOI] [PubMed] [Google Scholar]
- 3.Cartoux M, Meda N, Van de Perre P, et al. Acceptability of voluntary HIV testing by pregnant women in developing countries: an international survey. Ghent International Working Group on Mother-to-Child Transmission of HIV. AIDS. 1998;12:2489–2493. doi: 10.1097/00002030-199818000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Msellati P, Hingst G, Kaba F, et al. Operational issues in preventing mother-to-child transmission of HIV-1 in Abidjan, Cote d’Ivoire, 1998– 99. Bull World Health Organ. 2001;79:641–647. [PMC free article] [PubMed] [Google Scholar]
- 5.Temmerman M, Quaghebeur A, Mwanyumba F, et al. Mother-to-child HIV transmission in resource poor settings: how to improve coverage? AIDS. 2003;17:1239–1242. doi: 10.1097/00002030-200305230-00016. [DOI] [PubMed] [Google Scholar]
- 6.Stringer JS, Sinkala M, Stout JP, et al. Comparison of two strategies for administering nevirapine to prevent perinatal HIV transmission in highprevalence, resource-poor settings. J Acquir Immune Defic Syndr. 2003;32:506–513. doi: 10.1097/00126334-200304150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nebie Y, Meda N, Leroy V, et al. Sexual and reproductive life of women informed of their HIV seropositivity: a prospective cohort study in Burkina Faso. J Acquir Immune Defic Syndr. 2001;28:367–372. doi: 10.1097/00126334-200112010-00010. [DOI] [PubMed] [Google Scholar]
- 8.Desgrees-Du-Lou A, Msellati P, Viho I, et al. Contraceptive use, protected sexual intercourse and incidence of pregnancies among African HIV-infected women. DITRAME ANRS 049 Project, Abidjan 1995–2000. Int J STD AIDS. 2002;13:462–468. doi: 10.1258/09564620260079617. [DOI] [PubMed] [Google Scholar]
- 9.Farquhar C, Mbori-Ngacha DA, Bosire RK, et al. Partner notification by HIV-1 seropositive pregnant women: association with infant feeding decisions. AIDS. 2001;15:815–817. doi: 10.1097/00002030-200104130-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiarie JN, Kreiss JK, Richardson BA, et al. Compliance with antiretroviral regimens to prevent perinatal HIV-1 transmission in Kenya. AIDS. 2003;17:65–71. doi: 10.1097/01.aids.0000042938.55529.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaillard P, Melis R, Mwanyumba F, et al. Vulnerability of women in an African setting: lessons for mother-to-child HIV transmission prevention programmes. AIDS. 2002;16:937–939. doi: 10.1097/00002030-200204120-00019. [DOI] [PubMed] [Google Scholar]
- 12.Antelman G, Smith-Fawzi MC, Kaaya S, et al. Predictors of HIV-1 serostatus disclosure: a prospective study among HIV-infected pregnant women in Dar es Salaam, Tanzania. AIDS. 2001;15:1865–1874. doi: 10.1097/00002030-200109280-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–740. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 14.Merson MH, Dayton JM, O’Reilly K. Effectiveness of HIV prevention interventions in developing countries. AIDS. 2000;14(Suppl 2):S68–S84. [PubMed] [Google Scholar]
- 15.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356:103. [PubMed] [Google Scholar]
- 16.Sweat M, Gregorich S, Sangiwa G, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet. 2000;356:113–121. doi: 10.1016/S0140-6736(00)02447-8. [DOI] [PubMed] [Google Scholar]
- 17.Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenna SL, Muyinda GK, Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11(Suppl 1):S103–S110. [PubMed] [Google Scholar]