Abstract
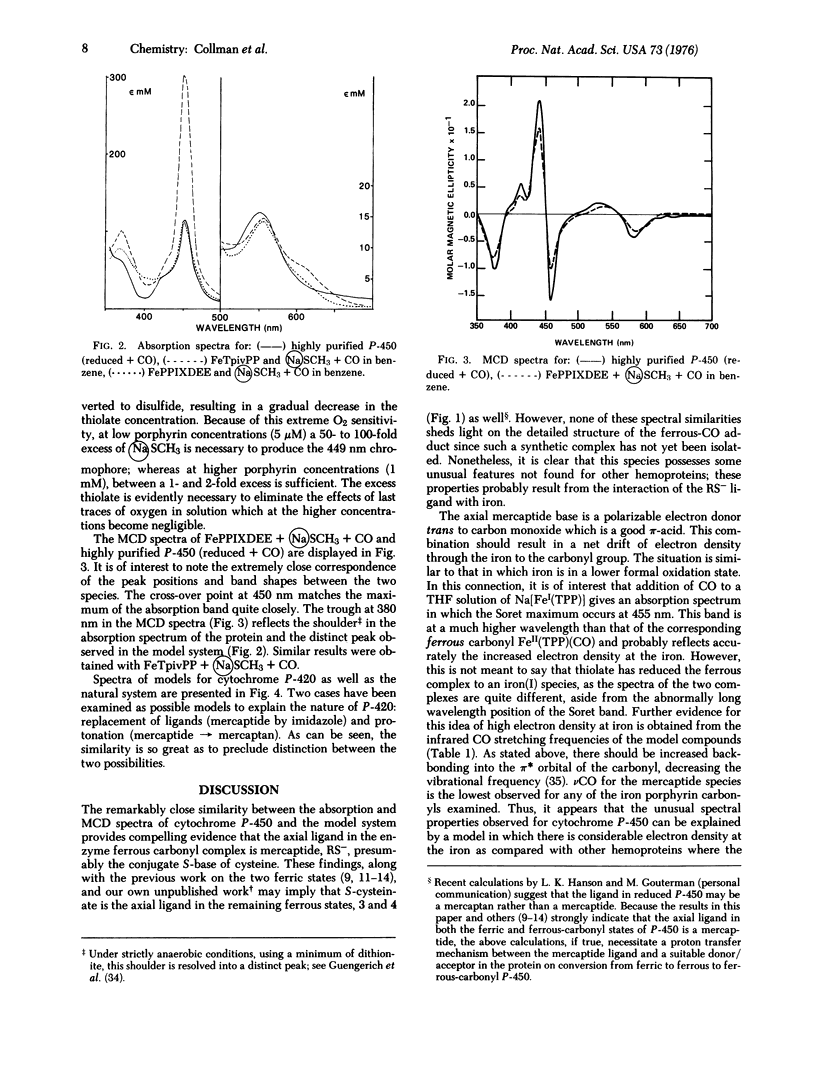
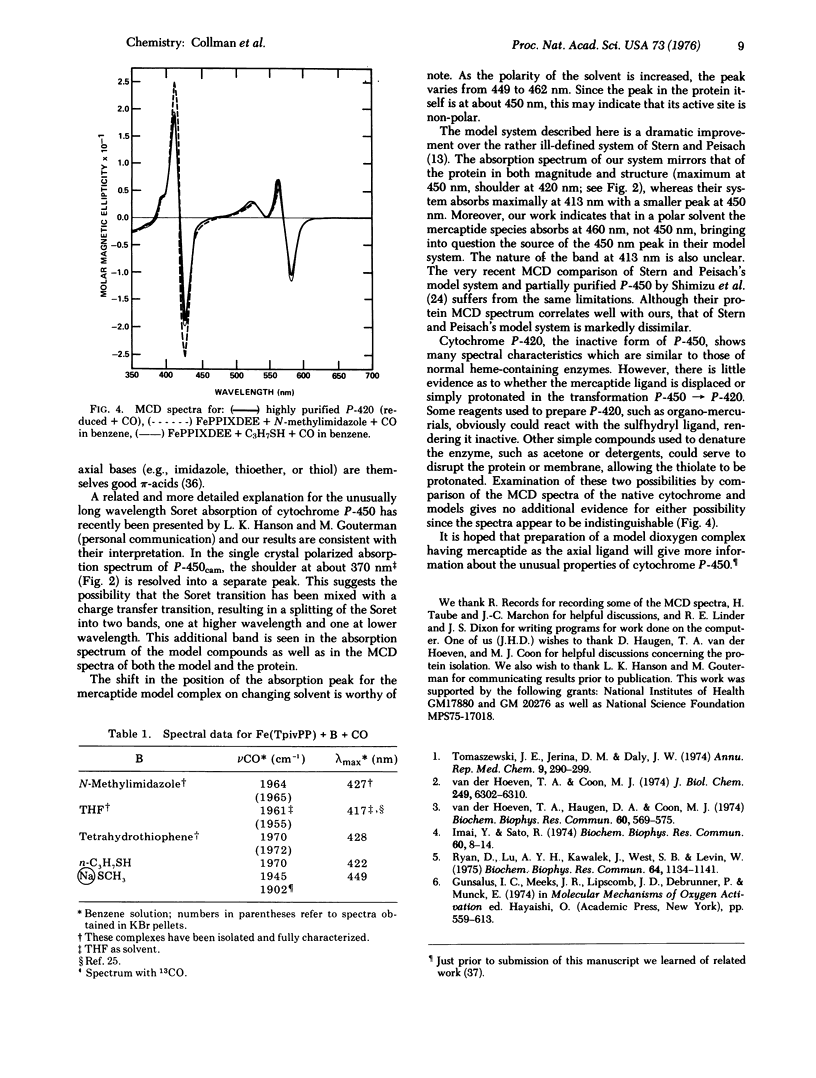
Absorption and magnetic circular dichroism (MCD) spectra have been obtained for the ferrous carbonyl adducts of cytochromes P-450 and P-420 as well as synthetic model systems. Ferrous porphyrins with sodium methyl mercaptide and CO in benzene give MCD and absorption spectra which are almost identical to those of the natural enzyme, indicating that in P-450 a mercaptide serves as the fifth ligand in the ferrous carbonyl adduct. MCD spectra of models with either propyl mercaptan or N-methylimidazole as the axial ligand are identical with that of P-420. Thus, no unambiguous assignment of the axial ligand can be made in this case. The infrared stretching frequencies of ferrous porphyrin carbonyl complexes and the absorption spectrum of the CO adduct of Na[Fe1(meso-tetraphenylporphyrin dianion)] are consistent with the concept that in P-450 considerable electron density is transferred to the iron by the mercaptide ligand.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth G., Dawson H. J., Dolinger P. M., Linder R. E., Bunnenberg E., Djerassi C. Magnetic circular dichroism studies XXXIV. Improved instrumentation for MCD measurements. Anal Biochem. 1975 May 12;65(1-2):100–108. doi: 10.1016/0003-2697(75)90496-0. [DOI] [PubMed] [Google Scholar]
- Brook D. F., Large P. J. Inhibition by carbon monoxide of the secondary-amine mono-oxygenase of Pseudomonas aminovorans and the photochemical action spectrum for its reversal. Eur J Biochem. 1975 Jul 15;55(3):601–609. doi: 10.1111/j.1432-1033.1975.tb02197.x. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Dolphin D. Letter: Ferrous porphyrin-mercaptide complexes. Models for reduced cytochrome P-450. J Am Chem Soc. 1975 Oct 1;97(20):5948–5950. doi: 10.1021/ja00853a069. [DOI] [PubMed] [Google Scholar]
- Cohen I. A., Ostfeld D., Lichtenstein B. Characterization of a d iron system. Tetraphenylporphineiron (I) anion. J Am Chem Soc. 1972 Jun 28;94(13):4522–4525. doi: 10.1021/ja00768a018. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Gagne R. R., Reed C. A., Halbert T. R., Lang G., Robinson W. T. "Picket fence porphyrins." Synthetic models for oxygen binding hemoproteins. J Am Chem Soc. 1975 Mar 19;97(6):1427–1439. doi: 10.1021/ja00839a026. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Hoard J. L., Kim N., Lang G., Reed C. A. Synthesis, stereochemistry, and structure-related properties of alpha, beta, gamma, delta-tetraphenylporphinatoiron(II). J Am Chem Soc. 1975 May 14;97(10):2676–2681. doi: 10.1021/ja00843a015. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Sorrell T. N., Hoffman B. M. Letter: Models for cytochrome P-450. J Am Chem Soc. 1975 Feb 19;97(4):913–914. doi: 10.1021/ja00837a050. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Sorrell T. N. Letter: A model for the carbonyl adduct of ferrous cytochrome P450. J Am Chem Soc. 1975 Jul 9;97(14):4133–4134. doi: 10.1021/ja00847a046. [DOI] [PubMed] [Google Scholar]
- Dawson J. H., Dolinger P. M., Trudell J. R., Barth G., Linder R. E., Bunnenberg E., Djerassi C. Magnetic circular dichroism studies XXXV. A comparison of cytochromes P-450 and P-448. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4594–4597. doi: 10.1073/pnas.71.11.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinger P. M., Kielczewski M., Trudell J. R., Barth G., Linder R. E., Bunnenberg E., Djerassi C. Magnetic circular dichroism studies. XXV. A preliminary investigation of microsomal cytochromes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):399–403. doi: 10.1073/pnas.71.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P., Ballou D. P., Coon M. J. Purified liver microsomal cytochrome P-450. Electron-accepting properties and oxidation-reduction potential. J Biol Chem. 1975 Sep 25;250(18):7405–7414. [PubMed] [Google Scholar]
- Imai Y., Sato R. A gel-electrophoretically homogeneous preparation of cytochrome P-450 from liver microsomes of phenobarbital-pretreated rabbits. Biochem Biophys Res Commun. 1974 Sep 9;60(1):8–14. doi: 10.1016/0006-291x(74)90164-8. [DOI] [PubMed] [Google Scholar]
- Imai Y., Sato R. Conversion of P-450 to P-420 by neutral salts and some other reagents. Eur J Biochem. 1967 Jun;1(4):419–426. doi: 10.1007/978-3-662-25813-2_57. [DOI] [PubMed] [Google Scholar]
- Koch S., Tang S. C., Holm R. H., Frankel R. H., Ibers J. A. Letter: Ferric porphyrin thiolates. Possible relationship to cytochrome P-450 enzymes and the structure of (p-nitrobenzenethiolato)iron(III) protoporphyrin IX dimethyl ester. J Am Chem Soc. 1975 Feb 19;97(4):916–918. doi: 10.1021/ja00837a052. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maines M. D., Anders M. W., Muller-Eberhard U. Studies on heme transfer from microsomal hemoproteins to heme-binding plasma proteins. Mol Pharmacol. 1974 Mar;10(2):204–213. [PubMed] [Google Scholar]
- Ryan D., Lu Y. H., Kawalek J., West S. B., Levin L. Highly purified cytochrome P-448 and P=450 from rat liver microsomes. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1134–1141. doi: 10.1016/0006-291x(75)90812-8. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Nozawa T., Hatano M., Imai Y., Sato R. Magnetic circular dichroism studies of hepatic microsomal cytochrome P-450. Biochemistry. 1975 Sep 23;14(19):4172–4178. doi: 10.1021/bi00690a004. [DOI] [PubMed] [Google Scholar]
- Singhal R. L., Merali Z., Kacew S., Sutherland D. J. Persistence of cadmium-induced metabolic changes in liver and kidney. Science. 1974 Mar 15;183(4129):1094–1096. doi: 10.1126/science.183.4129.1094. [DOI] [PubMed] [Google Scholar]
- Stern J. O., Peisach J. A model compound study of the CO-adduct of cytochrome P-450. J Biol Chem. 1974 Dec 10;249(23):7495–7498. [PubMed] [Google Scholar]
- Vickery L., Salmon A., Sauer K. Magnetic circular dichroism studies on microsomal aryl hydrocarbon hydroxylase: comparison with cytochrome b-5 and cytochrome P-450-cam. Biochim Biophys Acta. 1975 Mar 28;386(1):87–98. doi: 10.1016/0005-2795(75)90249-4. [DOI] [PubMed] [Google Scholar]
- van der Hoeven T. A., Coon M. J. Preparation and properties of partially purified cytochrome P-450 and reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase from rabbit liver microsomes. J Biol Chem. 1974 Oct 10;249(19):6302–6310. [PubMed] [Google Scholar]
- van der Hoeven T. A., Haugen D. A., Coon M. J. Cytochrome P-450 purified to apparent homogeneity from phenobarbital-induced rabbit liver microsomes: catalytic activity and other properties. Biochem Biophys Res Commun. 1974 Sep 23;60(2):569–575. doi: 10.1016/0006-291x(74)90278-2. [DOI] [PubMed] [Google Scholar]


