Abstract
An extensive variety of THAP1 sequence variants have been associated with focal, segmental and generalized dystonia with age of onset ranging from 3 to over 60 years. In previous work, we screened 1,114 subjects with mainly adult-onset primary dystonia (Neurology 2010;74:229-238) and identified 6 missense mutations in THAP1. For this report, we screened 750 additional subjects for mutations in coding regions of THAP1 and interrogated all published descriptions of THAP1 phenotypes (gender, age of onset, anatomical distribution of dystonia, family history and site of onset) to explore the possibility of THAP1 genotype-phenotype correlations and facilitate a deeper understanding of THAP1 pathobiology. We identified 5 additional missense mutations in THAP1 (p.A7D, p.K16E, p.S21C, p.R29Q, and p.I80V). Three of these variants are associated with appendicular tremors, which were an isolated or presenting sign in some of the affected subjects. Abductor laryngeal dystonia and mild blepharospasm can be manifestations of THAP1 mutations in some individuals. Overall, mean age of onset for THAP1 dystonia is 16.8 years and the most common sites of onset are the arm and neck, and the most frequently affected anatomical site is the neck. In addition, over half of patients exhibit either cranial or laryngeal involvement. Protein truncating mutations and missense mutations within the THAP domain of THAP1 tend to manifest at an earlier age and exhibit more extensive anatomical distributions than mutations localized to other regions of THAP1.
Keywords: Dystonia, THAP1, DYT6, Spasmodic dysphonia, Tremor
1. Introduction
Screening studies from numerous groups covering several continents have shown that mutations in THAP1 (THAP domain containing, apoptosis associated protein 1) are an important cause of both early- and late-onset primary dystonia. THAP1 was the second gene to be associated with primary dystonia [1], the first being TOR1A [2]. A heterozygous GAG deletion in Exon 5 of TOR1A is responsible for DYT1 dystonia and an indel mutation in Exon 2 of THAP1 causes DYT6 dystonia. Although inadequately studied to date, other causal mutations in TOR1A appear to be quite rare. In contrast, mutations in THAP1 show greater diversity with missense mutations broadly distributed across its three exons [3, 4]. Moreover, frameshift, non-coding and homozygous mutations in THAP1 have also been associated with dystonia [4-6].
The phenotypic spectrum and anatomical patterns of clinical involvement differ among the two genes (TOR1A and THAP1) associated with primary dystonia. In the vast majority of cases, DYT1 dystonia begins in a limb [7]. In contrast, THAP1 dystonia is more heterogeneous with both craniocervical and limb onset described in various reports [3-5]. Herein, we present new cases of THAP1 dystonia and integrate clinical and genetic information derived from these subjects with the existing published literature to explore potential THAP1 genotype-phenotype relationships and facilitate a deeper understanding of THAP1 biology. For the sake of clarity, the terms DYT1 and DYT6 will be limited to dystonia due to the seminal TOR1A ΔGAG and THAP1 Exon 2 indel (c.135_139delinsGGGTTTA) mutations, respectively [1,2].
2. Newly identified missense mutations
2.1 Methods
2.11 Clinical subjects
All human studies were conducted in accordance with the Declaration of Helsinki with formal approval from the institutional review boards at each participating study site. All subjects gave written informed consent for genetic analyses, and use of their videos, audios and photographs. Recruitment of patients with primary dystonia and neurologically-normal controls has been described previously [4, 8]. Clinical diagnoses were made by means of history and examination by one or more neurologists and/or neurolaryngologists at each site. In previous work, we screened 1,114 subjects with mainly adult-onset primary dystonia and identified 6 missense mutations in conserved regions of THAP1 [4]. For this report, we screened 750 additional subjects for missense mutations in coding regions of THAP1.
2.12 Genetic analysis
High resolution melting (HRM) analyses were performed with the LightCycler® 480 Real-Time PCR system and High Resolution Master Mix (Roche) in accordance with manufacturer instructions and our laboratory protocol [4, 9]. Melting curves and difference plots were analyzed using Gene Scanning Software. For samples with shifted melting curves, PCR products were cleaned using ExoSAP-IT® (United States Biochemical) and sequenced in the forward and reverse directions on the Applied Biosystems 3130XL Genetic Analyzer. Follow-up neurological examinations were performed on probands and select family members. Mutations in Exon 5 of TOR1A were excluded in all subjects. To predict the pathological character of single amino acid mutations, missense variants were analyzed with PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) [10], SIFT Human Protein (http://sift.jcvi.org/www/SIFT_enst_submit.html) [11] and MutationTaster (http://www.mutationtaster.org) [12].
2.2 Genotypes and Phenotypes
As follow-up to previous work [4], we identified 5 additional missense mutations in THAP1 (Supplementary Table 1 and Fig. 2A). Three of these variants are associated with appendicular tremors, which were an isolated or presenting sign in some of the affected family members. Three of the 5 probands have a positive family history of dystonia (Fig. 3).
Fig. 2.
THAP1 protein. (A) Functional domains of THAP1 and the location of THAP1 coding sequence variants that have been associated with dystonia. THAP, thanatos-associated protein domain. Pro, low complexity proline-rich region. NLS, nuclear localization signal. M1?, c.2delT or c.1A>G. (B) Multiple sequence alignment of THAP1 from human, mouse, rat, chimpanzee, rhesus monkey, dog, cow, opossum, chicken, Xenopus and zebrafish. Color codes: AVFPMILW-red (small+ hydrophobic including aromatic - Y), DE-blue (acidic), RK-magenta (basic), and STYHCNGQ-green (hydroxyl + amine + basic + Q). Symbols: (*) residues in that column are identical in all sequences, (:) conserved substitutions have been observed, and (.) semi-conserved substitutions are observed.
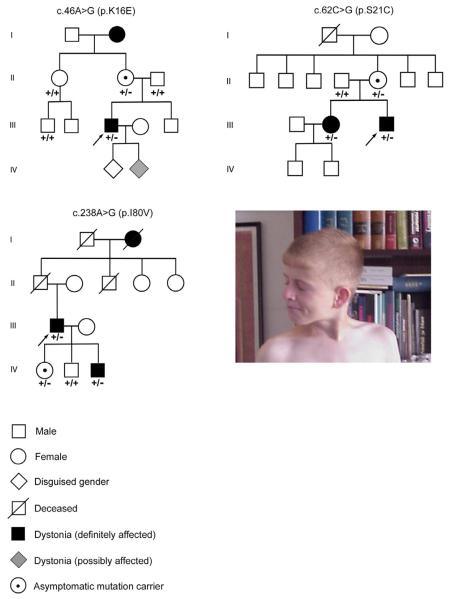
Fig. 3.
Pedigrees of three kindreds with THAP1 dystonia. All family members that provided DNA were genotyped: +/+, wild-type; and +/−, heterozygous. Probands are denoted with arrows. Photograph shows cervical dystonia with right rotational torticollis in the p.S21C proband at 12 years of age.
The p.A7D (c.20C>A) proband showed signs of subtle dysphonia at age 8 which became more overt during his teenage years. Right (dominant) hand-forearm dystonia developed during his freshman year in high school and he started to use his left hand to write. Bilateral, asymmetrical, upper-extremity tremors (dystonic) did not improve with levodopa. Trihexyphenidyl was mildly beneficial. This subject was last seen at 24 years of age. In addition to laryngeal dystonia (abductor subtype), right arm dystonia and bilateral upper extremity dystonic tremors, he also showed evidence of mild cervical, lower facial, lingual, and masticatory dystonia. His lower facial, masticatory and lingual dystonia showed moderate improvement with injections of botulinum toxin into the digastric, genioglossus, mentalis and lateral pterygoid muscles. This subject reported that none of his first or second degree relatives have obvious evidence of dystonia.
The p.K16E (c.46A>G) proband had evidence of spasmodic dysphonia (abductor subtype) and mild cervical dystonia at age 6 with significant disease progression at 14 to 16 years of age. Prior to bilateral globus pallidus interna (GPi) deep brain stimulation (DBS), generalized dystonia was manifest as spasmodic dysphonia (abductor subtype), blepharospasm, masticatory dystonia, lower facial dystonia, lingual dystonia, bilateral proximal arm dystonia (left > right), bilateral distal arm dystonia (right > left), truncal dystonia, and mild asymmetrical dystonia in the legs and feet. Gait was largely unimpaired. The laryngeal dystonia was severe, often rendering the patient’s speech unintelligible. There was no significant improvement in any aspect of the generalized dystonia with trihexyphenidyl. The cervical dystonia was painful and treatment of cervical dystonia with injections of botulinum toxin was only mildly beneficial. The patient was able to alleviate his rotational torticollis/laterocollis somewhat by touching his chin with his left hand. He had a mild positional tremor in his hands. There were no clinical signs of a cerebellar or upper motor neuron syndrome.
Although family members were not available for neurological examination, the proband’s maternal grandmother reportedly had manifest dysphonia since an early age (Fig. 3). The proband has two young children, one of whom has begun to exhibit evidence of possible lingual dystonia.
At age 43, the p.K16E proband underwent successful bilateral GPi DBS. Preoperative MRI of the brain was interpreted as normal. This patient has shown a remarkable response to DBS. Post-operative video shows improvement in cervical and appendicular dystonia (Video 1). Laryngeal dystonia has shown marked improvement within nine months after DBS operation. Now, he is able to a “shouting” voice throughout longer sentences, even though mild breathy abductor dysphonia is noted intermittently.
The p.S21C (c.62C>G) proband has shown normal psychomotor development, and was a completely healthy boy until the age of 12. The initial manifestation of dystonia was right rotational torticollis (Fig. 3). Neurological examination also revealed mild dystonic posturing of the left distal arm along with a dystonic tremor of the left hand. At age 12, the intelligence quotient (IQ) was measured as 116. At age 13, action dystonia of the left foot became manifest. He obtained modest benefit with baclofen at a maximal dosage of 65 mg/day. Clonazepam provided additional therapeutic benefit. Over a period of 6 years, several intervals of near clinical remission were documented although none lasted for more than a month. At 18 years of age, an intermittent action dystonia of the right foot was documented for the first time, but it has been inconsistently present at follow-up clinic visits. On the most recent neurological examination at 24 years of age, right hand-forearm dystonia with manifest writer’s cramp was clearly present (Video 2). Cervical dystonia has improved with injections of botulinum toxin. The proband’s mother is an asymptomatic carrier, while his sister, currently 26 years of age, had onset of hand-forearm dystonia (writer’s cramp) at 7 years of age. Over time, the task specificity of her hand-forearm dystonia faded and dystonia progressed to involve the entire right arm. In addition, she exhibits mild right leg dystonia with external rotation of the foot during prolonged walks.
The p.R29Q (c.86G>A) mutation has manifest predominantly as laryngeal dystonia (abductor spasmodic dysphonia) and bilateral dystonic arm tremors. The dystonic arm tremors began in childhood whereas the laryngeal dystonia began at age 40. Abductor spasmodic dysphonia was documented with videoendoscopy and has improved with injections of onabotulinumtoxin. Mild upper (blepharospasm) and lower facial, lingual and masticatory dystonia have been apparent on clinical examination, particularly when the patient attempts to speak. The lingual, lower facial and masticatory dystonia contributed to mild and variable dysarthria. Involvement of the cervical musculature with mild, intermittent retrocollis and subjective tightness in the posterior cervical region were also noted. The proband reported that none of his first or second degree relative had dystonia or tremors, although none were available for neurological examination.
The p.I80V (c.238A>G) proband developed right hand-forearm dystonia, mainly manifest as writer’s cramp, at 23 years of age. At the age of 45 years, right rotational torticollis appeared, and, 6 months later, a dystonic tremor of left upper limb started insidiously. Bilateral upper extremity dystonic tremors were apparent on neurological examination (Video 3). Dystonia was aggravated by movement and stress. The patient indicated that he had observed an occasional right leg tremor (action tremor precipitated by activities such as pushing down on the car pedal) for 12 years. Hypertrophy of the left sternocleidomastoid muscle was present on initial examination and the patient reported that his cervical dystonia was painful. These signs and symptoms were resistant to biperiden (6mg/day), baclofen (30 mg/day) and clonazepam (0.5mg/day) treatments. However, his cervical dystonia has responded well to injections of botulinum toxin A. Family history was remarkable for a prominent, presumably dystonic, head tremor in the paternal grandmother with onset at approximately 50 years of age and persistent until her death at the age of 80 years.
All three of the p.I80V proband’s children were examined by a neurologist, blinded to genotype. On examination, one of three children (the youngest son), who also harbors this variant, was found to have mild, asymmetrical, rest and postural hand tremors. He reported that these asymmetric upper extremity tremors first appeared at 20 years of age. On neurological examination, he had subtle dystonia of the distal right arm, a very mild rest tremor of the left thumb and mild, high-frequency postural and kinetic tremors of the upper limbs. Simultaneous accelerometric and electromyographic (EMG) short-term recordings [3 min] were used for measurement of rest, postural [with and without a load], and kinetic tremors, and exposed a rest tremor of the left thumb (9.4 Hz, root mean square [rms] amplitude = 0.041 mm/s2), along with postural (8.6 Hz, 0.34 mm/s2) and kinetic (10.1 Hz, 1.2 mm/s2) tremors of the right upper limb. Loading tests were used to exclude enhanced physiological tremors. A spiral drawing-digitizing tablet confirmed the kinetic tremor of the right upper limb (10.5 Hz).
3. THAP1
3.1 Genomic structure
THAP1 is located on Chr 8p11.21 (GRCh37/hg19 assembly: 42,691,818 - 42,698,474; 6,657 bp), reverse strand (Fig. 1A). THAP1 harbors 3 exons and is alternatively spliced into 2189 bp (Variant 1, NM_018105) and 1993 bp (Variant 2, NM_199003) transcripts. Short Interspersed Elements (SINEs) are found in Intron 1, Intron 2 and both 5′ and 3′ to the formal gene boundaries of THAP1. The proximal promoter and portions of Exon 1 and Intron 1 contain CpG islands. DNA methylation of these regions was studied in several cell lines as seen in Fig. 1 [13]. Fig. 1 also shows results of MeDIP-seq (methylated DNA immunoprecipitation and sequencing), MRE-seq (methyl-sensitive restriction enzyme digest and sequencing), and histone H3 lysine 4 trimethylation chromatin immunoprecipitation and sequencing (H3K4me3) which were generated from postmortem human frontal cortex gray matter of a 57 year-old male. MeDIP-seq enriches for methylated DNA sequences whereas MRE-seq identifies unmethylated CpG sites, and the H3K4me3 histone modification is associated with promoters.
Fig. 1.
Genomic structure of human THAP1. (A) UCSC Genome Browser on human GRCh37/hg19 assembly. From top to bottom: (1) localization of THAP1 is shown on an ideogram of Chr8, (2) UCSC and RefSeq genes on reverse strand - exon 1 is to the right of exons 2 and 3], (3) gene boundaries, (4) CpG islands, (5) DNA methylation in GM12878, H1-hESC, K562, HeLA-S3, and HepG2 cells by reduced representation bisulfite sequencing from ENCODE/Hudson Alpha, (6) H3K4me3 ChIP-seq raw signal, (7) MRE-seq CpG score, (8) MeDIP-seq CpG score, and (8) repeating elements by RepeatMasker. (B) Location of intronic and promoter sequence variants. Untranslated regions (UTRs) are shown in gray. Coding regions of Exons 1, 2 and 3, appear red, yellow and green, respectively. The major isoform (NM_018105.2) contains 3 exons and is 2189 nt in length. Aberrant transcripts containing stop codons 5′ to c.213 may be subject to nonsense mediated decay (NMD).
3.2 Functional domains of THAP1
THAP1 and related family members are defined by their THAP (Thanatos [Greek god of death]-associated protein) domain (Fig. 2). The THAP domain is a zinc-binding domain that is found in a number of proteins involved in various aspects of transcriptional regulation, apoptosis and cell-cycle control. THAP domain-containing proteins are found in mammals, zebrafish, Drosophila, C. elegans and Xenopus (Fig. 2B). However, there are no THAP proteins in plants or bacteria. The THAP domain contains a C2-CH (Cys-Xaa2-4-Cys-Xaa35-50-Cys-Xaa2-His) zinc finger that is similar to the DNA binding domain of the Drosophila P element transposase [14]. The C2-CH motif harbors 4 invariant residues (Pro26, Trp36, Phe58 and Pro78) and binds a single zinc atom [15]. The THAP domain (1-81aa) of THAP1 recognizes an 11 nucleotide target sequence (agtacgGGCAa) with GGCA forming the core motif [16]. Residues 82-90 of THAP1 are not required for DNA binding [15].
THAP1 also harbors a low complexity proline rich region and a bipartite nuclear localization signal that is located within a larger coiled-coil domain. Although THAP proteins have been shown to bind to DNA, they may also play a role in mediating protein interactions via their coiled-coiled domain. Monomeric THAP1 shows low affinity for DNA [17], and, in vivo, THAP1 probably functions as a homodimer within a larger multimeric DNA binding complex. Amino acid residues 154 to 166 are critical for dimerization of THAP1 [18].
In vitro studies suggest that THAP1 binds to the promoter region of TOR1A and regulates expression of TOR1A [19, 20]. Wild-type THAP1 was shown to reduce expression of TOR1A and mutant THAP1 decreased repression of TOR1A. However, RNAi knock-down of THAP1 in fibroblasts had no effect on TOR1A expression. Similarly, patient fibroblasts harboring THAP1 mutations showed no reduction in TOR1A expression. Overexpression of THAP1 in endothelial cells was used as an indirect means of identifying THAP1 targets [21]. TOR1A was not among the 16 genes that were significantly up-regulated or the 80 genes were down-regulated. Finally, there is no evidence that THAP1 variants act as disease modifiers in DYT1 dystonia [22].
3.3 Expression of THAP1
As seen in Supplementary Figure 1, THAP1 is widely expressed in neural and extra-neural tissues. THAP1 is expressed in whole blood. High expression levels can be found in CD34+ hematopoietic cells and cardiac myocytes. Comparable THAP1 expression is found in brain and a wide variety of extra-neural tissues such as liver, kidney, skeletal muscle, thyroid gland, and prostate. THAP1 is expressed in both the central and peripheral nervous systems. In the peripheral nervous system, THAP1 transcript has been found in the superior cervical ganglia, trigeminal ganglia, ciliary ganglia and dorsal root ganglia. Although THAP1 is widely expressed in the brain and spinal cord (http://mouse.brain-map.org), the highest levels of expression of THAP1 are seen in cerebellar Purkinje cells, the dentate gyrus and hippocampal pyramidal cells, and cerebral cortex (Supplementary Figure 2).
3.4 THAP family members in humans
THAP zinc fingers do not recognize the same DNA target sequences [15]. The human genome contains 12 THAP genes (THAP0 - THAP11). BLAST® (http://blast.ncbi.nlm.nih.gov/) query with the THAP1 protein sequence (213aa) revealed the following ordering of significant sequence alignments: THAP2 > THAP3 > THAP4 > THAP5 > THAP8 > THAP6 > THAP9 > THAP7 > THAP0> THAP11 > THAP10. THAP5, THAP6, THAP8, THAP9 and THAP10 do not have orthologues in the mouse genome [16]. THAP2 (Chr 12q21.1) and THAP3 (Chr 1p36.31) are expressed in brain (http://mouse.brain-map.org) and may be considered candidate gene for primary dystonia. Although little detail is known about THAP2, mouse Thap2 expression was down-regulated in brain following systemic hypoxia [23]. THAP3 shares in vivo expression profiles and cellular binding partners with THAP1 [24]. In particular, THAP1 and THAP3 associate with HCF-1, a cell cycle factor and transcriptional coactivator, and OCT, an enzyme that mediates O-GlcNAcylation of amino acids [24]. Furthermore, THAP3 is located within the DYT13 locus (1p36.13-36.32) [25].
4. Genotypic spectrum of THAP1 dystonia
4.1 Promoter and 5′/3′untranslated regions
A single promoter variant, 5′ to the THAP1 coding sequence, was identified in African-Americans subjects with dystonia and African-American controls [26]. Highlighting the importance of racial and ethnicity matched control groups in candidate gene studies of dystonia, this variant (g.42698477C>T/A) was not found in Caucasians (Fig. 1B and Supplementary Table 2). In this context, an initial report suggested that a nearby 5′UTR dinucleotide GA>TT variant was associated with increased risk for dystonia [27], whereas a subsequent large, well matched case-control study failed to confirm this association [8]. However, the TT variant may be pathological in the homozygous state since all three published carriers had manifest dystonia [8, 28].
Four additional UTR variants have been reported in subjects with focal dystonia (Supplementary Tables 2 and 3). Two unrelated subjects, one with blepharospasm and the other with laryngeal dystonia, were found to harbor a heterozygous c.-42C>T variant [4]. A heterozygous c.-32C>T variant was found in a male with hand-forearm dystonia, and two novel 3′UTR sequence variants were identified in a subject with generalized dystonia [29]. Although the molecular pathogenicity of these variants has not been established, they could induce cryptic effects on splicing or alter the temporal and spatial specificity of gene expression.
4.2 Intronic
Intronic variants are shown in Fig. 1B and detailed in Supplementary Table 2. Of note, the deep intronic region of Intron 1 has not been sequenced in most case-control studies of primary dystonia [26]. Initial reports suggested that the c.71+126T>C [5] and c.71+9C>A [4] variants may increase risk for primary dystonia, and follow-up studies have been underpowered to substantiate or refute the pathogenicity of these variants [22, 28, 29]. Although beyond the core splice site, the c.71+9C>A variant may possibly alter splicing efficiency and the ratio of the two THAP1 isoforms. Similarly, the c.71+126T>C variant could exert cryptic effects on splicing or reduce overall gene expression.
4.3 Coding sequence
An extensive search of the literature identified 75 coding variants in subjects with dystonia. Among this collection were 45 missense variants (Fig. 2A), 8 synonymous variants (Fig. 1B), and 22 indels or nonsense variants (Fig. 2A). More detailed information regarding individual variants is provided in Supplementary Tables 1 - 4.
4.3.1 Missense
As seen in Fig. 2, missense variants were concentrated within the THAP domain, particularly residues 6 to 32. There were no missense variants in the proline rich region or contiguous regions of THAP1 (residues 90 to 123). Several missense variants localized to the coiled-coil domain and nuclear localization signal. The closest missense variant to the C-terminus was p.D192N.
With two exceptions (p.L32H and p.N136S), all missense variants were heterozygous [5, 6]. The subject harboring p.N136S had no family history of dystonia, whereas three siblings exhibited early-onset generalized dystonia in the consanguineous kindred identified with the p.L32H variant. Heterozygous carriers of the p.L32H variant were reportedly asymptomatic.
The pathogenicity of 53 individual missense variants (45 dystonia-associated, and 8 reported in dbSNP and/or 1000 Genomes) was examined with Polyphen-2, SIFT and MutationTaster [12]. Only 16 of these missense variants are predicted to damage protein function by all three programs. Four missense variants are predicted to be benign by all three programs (p.C83R, p.M143V, p.H150P and p.D192N). These results must be interpreted with restraint given that in silico tools simply predict the pathogenicity of sequence variants and cannot substitute for functional studies. For example, MutationTaster and PolyPhen-2 are associated with accuracy rates of no better than 85.7% and 80.7%, respectively, and the false positive and negative rates for PolyPhen-2 are 21.4% and 16.8%, respectively [12]. The two novel variants (p.A7D and p.S21C) reported herein are predicted to be “probably damaging” by Polyphen-2, “damaging” by SIFT, and “disease causing” by MutationTaster.
The p.I80V variant, while predicted to be benign and tolerated by Polyphen-2 and SIFT, respectively, is classified as “disease causing” by MutationTaster. Compared to Polyphen-2, MutationTaster exhibits lower false negative and higher true positive prediction rates [12]. Although the p.I80V variant does not differ from wild-type THAP1 in its binding to the TOR1A promoter [29], there is no convincing data indicating that in vitro interactions between THAP1 and TOR1A are pathobiologically relevant [19, 20, 22]. The pathogenicity of p.I80V is suggested by its presence in two families with dystonia, co-segregation with dystonia in our family, and the 89% predicted probability of being “disease causing” by MutationTaster.
4.3.2 Synonymous
Of the 8 synonymous variants identified in an extensive literature search, 4 were also present in control subjects (Supplementary Tables 2 and 3). All of these variants were associated with segmental or focal dystonia, and onset was after 18 years of age in all but one individual. All synonymous variants were associated with sporadic dystonia. The p.L163L was found in three subjects with blepharospasm and masticatory and/or tongue involvement in China [30], but also in a control subject from Europe [28]. Although synonymous mutations have the potential to be pathogenic if they activate cryptic splice sites, leading to exon skipping or the inclusion of intronic sequences into mature transcripts, the c.489C>G (p.L163L) variant is distant from exon boundaries. In contrast, the c.267G>A (p.K89K) synonymous variant is located at the Exon 2-Intron 2 boundary and has been shown to reduce the expression of THAP1 RNA in lymphocytes [31]. Overall, however, their relatively high frequency in control subjects and apparently low penetrance in affected families suggests that synonymous variants elicit relatively small effects on the molecular biology of THAP1.
4.3.3 Indels and nonsense
The seminal THAP1 mutation identified in Amish-Mennonite families was a heterozygous 5-bp (GGGTT) insertion followed by a 3-bp deletion (AAC) in Exon 2 [1]. At the protein level, this mutation causes a frameshift and premature stop codon (p.F45Lfs*29). All reported insertion, deletion and nonsense mutations are shown in the upper half of Fig. 2. Based on the ‘50 - 54 bp rule’ from the last exon/intron junction, only those aberrant transcripts containing stop codons 5′ to c.213 - c.217 (p.71 - p.72) would be subject to nonsense-mediated decay (NMD) [32]. For instance, lymphocytes harboring a p.S21Ffs*51 variant showed normal expression levels of THAP1 RNA [33]. All reported THAP1 indels and nonsense mutations are predicted to elicit NMD or generate truncated proteins. None of the mutations shown in Fig. 2 would lead to translation of an elongated THAP1.
5. Phenotypic spectrum of THAP1 dystonia
Similar to DYT1 and non-DYT1 primary dystonia, THAP1 dystonia is not limited to one racial or ethnic group. Although initially described in Amish-Mennonites living in the Eastern United States, THAP1 dystonia has now been reported in individuals of English, German, French, Jewish, Serbian, Swedish, Italian, Chinese, Iranian, Greek, Dutch, and Mauritian/Indian ancestry [3-6, 28, 29, 34, 35]. To date, however, THAP1 dystonia has not been reported in Africans or African-Americans [26].
There have been no reports of extra-neural imaging abnormalities, congenital defects or increased extra-neural disease risk in carriers of THAP1 mutations. Despite its role in cell-cycle control, there are no convincing associations between THAP1 sequence variants and cancer. Self-reported measures of depression, psychometric test scores, motor sequence learning and visual sequence learning of manifesting and non-manifesting THAP1 mutation carriers do not differ from normal controls [36, 37].
Routine clinical imaging (MRI) is unremarkable in THAP1 dystonia. However, transcranial ultrasound shows hyperechogenicity in mutation carriers and may represent an endophenotype [38]. Microstructural abnormalities of white matter have been localized to the dorsal pons, within an area that likely includes the superior cerebellar peduncles, in both manifesting and non-manifesting carriers of THAP1 and TOR1A mutations [39]. Similarly, diffusion tensor imaging (3 Tesla) and probabilistic tractography showed reduced integrity of cerebello-thalamo-cortical fiber tracts in both manifesting and non-manifesting carriers of THAP1 and TOR1A mutations. Non-manifesting carriers were distinguished by concomitant disruption of the distal thalamo-cortical pathway [40]. One possible interpretation of these findings is that dystonia becomes manifest when the distal segment is “less disrupted,” thereby allowing aberrant cerebellar output to reach cerebral cortex [41].
With fluorodeoxyglucose positron emission tomography (FDG-PET), manifesting THAP1 and TOR1A mutation carriers showed bilateral hypermetabolism in the pre-supplementary motor area and parietal association cortices [42]. Gene specific effects were noted, however, with hypermetabolism in the putamen, anterior cingulate and cerebellar hemispheres in DYT1 dystonia, and hypometabolism in the putamen in THAP1 dystonia [42]. In another study from the same group, significant reductions in striatal D2 receptor availability (11C-raclopride binding) were identified in manifest and non-manifest THAP1 and TOR1A mutation carriers although the changes were more prominent in THAP1 than TOR1A carriers [43]. In aggregate, findings from functional imaging point out similar abnormalities of cortico-striato-pallido-thalamo-cortical and cerebello-thalamo-cortical pathways in THAP1 and DYT1 dystonia, likely neurodevelopmental in origin [41].
Microelectrode recordings obtained during DBS procedures have shown no differences in firing rates or patterns between DYT1 and THAP1 dystonia [38, 44]. In a study of 3 awake patients with THAP1 dystonia, mean GPi spike frequency was 48.4 Hz [44]. In another study of 2 patients undergoing DBS under general anesthesia with propofol and remifentanil, globus pallidus externa (GPe) and GPi mean firing rates were 15.0 Hz and 16.6 Hz, respectively. Clearly, the differences in firing rates between these two studies were due to the effects of anesthesia [44].
In terms of phenomenology, THAP1 dystonia is similar to DYT1 dystonia and non-DYT1 adult-onset primary dystonia. Sensory tricks [35], status dystonicus [45], and spontaneous remissions [34] have been reported in THAP1 dystonia. The terms “dysphonia” and “dysarthria” frequent many of the published clinical descriptions of THAP1 dystonia. However, in many reports it is not entirely clear that “dysphonia” was appropriately differentiated from “dysarthria” or that laryngeal involvement was documented by laryngoscopy or videostroboscopy. When present, dysarthria in THAP1 dystonia is often due to involvement of masticatory, lower facial and pharyngeal muscles.
Some manifestations of THAP1 dystonia may be relatively unique. THAP1 dystonia has shown us that adult-onset primary dystonia can begin in a distal lower extremity and may not be associated with foot inversion [46]. In fact, THAP1 leg dystonia may manifest with a characteristic “limping” pattern [5, 46]. Appendicular tremor may be a presenting feature of THAP1 dystonia. Clinically, this tremor is most consistent with a dystonic tremor, showing variable amplitude, significant right-left asymmetry, and, increases in amplitude with movements opposite to the direction of any associated dystonic posturing. Although “myoclonus” has been described in THAP1 dystonia, electrophysiological studies were not performed to definitively differentiate “myoclonus” from a “jerky” dystonic tremor [47].
5.1 Data analysis
The phenotypic spectrum of THAP1 dystonia associated with coding mutations is detailed in Table 1 and Supplementary Table 1. These tables and all aspects of phenotypic data analysis integrate our newly described missense variants with data assembled from a comprehensive literature search. Given the variability in reporting styles used in the different THAP1 publications, data regarding anatomical distribution was, in some cases, imputed or inferred from clinical descriptions, along with ancillary information derived from tables, figures or videos. For subject with dystonia, a “modified UDRS” score (1-14) was generated by assigning one point for each affected body area (eyes and upper face, lower face, jaw and tongue, larynx, neck, shoulder and proximal arm [right and left], distal arm and hand including elbow [right and left], pelvis and proximal leg [right and left], distal leg and foot including knee [right and left], and trunk).
Table 1.
Phenotypic spectrum of THAP1 dystonia associated with coding mutations
| Phenotypic Feature | |||||
|---|---|---|---|---|---|
| Gender | Number | Percentage | |||
| Male | 60 | 46.2% | |||
| Female | 70 | 53.8% | |||
| Family history of dystonia | Number | Percentage | |||
| Yes | 99 | 76.2% | |||
| No | 31 | 23.8% | |||
| Age of Onset (yrs) | Number | Range | Mean | Skewness | Kurtosis |
| 119 | 3 - 62 | 16.8 ± 12.6 | 1.8 | 2.7 | |
| Duration (yrs) | Number | Range | Mean | Skewness | Kurtosis |
| 113 | 1-70 | 23.8 ± 15.8 | 0.8 | 0.3 | |
| Site of onset | Number | Percentage | |||
| Upper face | 1/122 | 0.8% | |||
| Lower face | 1/122 | 0.8% | |||
| Tongue-jaw | 3/122 | 2.5% | |||
| Larynx | 11/122 | 9.0% | |||
| Neck | 30/122 | 24.6% | |||
| Hand-forearm | 8/122 | 6.6% | |||
| Leg | 14/122 | 11.5% | |||
| Cranial | 10/122 | 8.2% | |||
| Arm (unspecified) | 44/122 | 36.1% | |||
| Total arm | 52/122 | 42.6% | |||
| Distribution | Number | Percentage | |||
| Focal | 21/130 | 16.2% | |||
| Segmental | 45/130 | 34.6% | |||
| Multifocal | 7/130 | 5.4% | |||
| Generalized | 56/130 | 43.1% | |||
| Hemidystonia | 1/130 | 0.8% | |||
| Anatomical sites | Number | Percentage | |||
| Cranium | 56/111 | 50.5% | |||
| Upper face | 21/111 | 18.9% | |||
| Lower Face | 32/111 | 28.8% | |||
| Jaw-Tongue-Pharynx | 47/111 | 42.3% | |||
| Larynx | 49/111 | 44.1% | |||
| Neck | 85/111 | 76.6% | |||
| Arm | 79/111 | 71.2% | |||
| Leg | 67/130 | 51.5% | |||
| Modified UDRS | Number | Range | Mean | Skewness | Kurtosis |
| 130 | 1 - 14 | 5.9 ± 3.7 | 0.5 | −0.9 |
5.2 Results
Our analysis indicated that THAP1 dystonia was slightly more prevalent in females (Table 1). Age of onset for THAP1 dystonia ranged from 3 to over 60 years of age (Table 1). In comparison to DYT1 dystonia, THAP1 dystonia tended to manifest at older ages, with mean age of onset in the late teen years. The distribution of age of onset showed a moderate positive skew with many patients first manifesting dystonia in their 20s and 30s. Given the relatively recent identification of the causal gene, there is no true prospective data on gene carriers. Retrospective disease duration has ranged from less than a year to 70 years with a mean of over 20 years.
Some sense of anatomical spread can be surmised by comparing site of onset to most recent anatomical distribution. Common sites of onset were the arm (42.6%) and neck (24.6%). Ultimately, 43.1% of affected individuals progressed to generalized dystonia. Eventually, the cranium (upper face, lower face, and/or jaw/tongue/pharynx) was affected in approximately half of patients with THAP1 dystonia. However, blepharospasm was relatively infrequent, with many more subjects showing involvement of the jaw, tongue or pharynx. On average, about 6 body areas were affected at the time of neurological assessment. For example, a relatively common anatomical pattern yielding a modified UDRS score of 6 was segmental dystonia with involvement of the proximal and distal arm (unilateral), neck, larynx, lower face, and jaw-tongue.
6. Genotype-phenotype and phenotype-phenotype correlations
Statistical analyses of genotype-phenotype and phenotype-phenotype correlations were performed with SAS® (Cary, NC, USA). All coding mutations were assigned an amino acid number (1 - 213) corresponding to the first amino acid altered by the mutation. Variants predicted to be subjected to NMD were assigned a value of 1. Non-parametric correlations (τB) were used for analysis of ordinal variables (site of onset and distribution). Mutations near the N-terminus of THAP1 were associated with an earlier age of onset and tended to be associated with more extensive anatomical distributions (modified UDRS, p = 0.072; and distribution, p = 0.054). Similar to DYT1 dystonia, age of onset was inversely associated with both site of onset and ultimate anatomical distribution (Table 2) [48].
Table 2.
Genotype-phenotype and phenotype-phenotype correlations for coding mutations
| Amino Acid Number (1 - 213) | Age of Onset (3 - 62 yrs) | |
|---|---|---|
| Age of Onset (3 - 62 yrs) | r = 0.42, p < 0.0001 | NA |
| Duration (1 - 70 yrs) | r = −0.023, p = 0.83 | r = −0.29, p = 0.0020 |
| Modified UDRS (1 -14) | Kendall Tau b (τB) = −0.12, p = 0.072 | Kendall Tau b (τB) = −0.40, p < 0.0001 |
| Site of Onset (1 - 8) | Kendall Tau b (τB) = 0.080, p = 0.28 | Kendall Tau b (τB) = 0.32, p <0.0001 |
| Distribution (1 - 5) | Kendall Tau b (τB)= −0.14, p = 0.054 | Kendall Tau b (τB) = −0.44, p < 0.0001 |
Site of onset (leg = 1, trunk =2, arm =3, neck =4, larynx =5, jaw/tongue/pharynx or cranium =6, lower face = 7, upper face = 8). Distribution (focal = 1, segmental = 2, multifocal = 3, hemidystonia = 4, generalized = 5). NA, not applicable.
In separate analyses, genotype was dichotomized to THAP domain (1-81aa) or non-THAP domain (82-213aa) and effects on age of onset, site of onset, and anatomical distribution were determined with independent two-sample t (parametric) or Wilcoxin (nonparametric) tests. Mutations within the THAP domain were associated with an earlier age of onset (mean = 14.6 yrs) than non-THAP domain (mean = 26.5 yrs) mutations (p = 0.0047). THAP domain mutations were also associated with more extensive anatomical distributions (p < 0.020), but had no significant effect on site of onset.
Each THAP1 sequence variant probably exhibits a unique penetrance value that is modified by environment factors and overall genetic background. Proposing specific values for penetrance is also compromised by intrafamilial variability in age of onset and assigning affection status to subtle phenotypes. Based on 33 individuals from Amish-Mennonite Family M (p.F45Lfs*29) including 13 affected individuals, Kaplan-Meier risk up to age 34 years was 60% with a confidence interval of 23% to 93% [1, 49]. Combining data from Amish-Mennonite Families M and C, both of which harbor the p.F45Lfs*29 frameshift mutation, extended the analysis to 76 individuals (16 affected and 60 unaffected) ranging in age from 6 to 65 years [49]. The larger dataset generated lifetime penetrance in first-degree relatives of 57% with a confidence interval of 23% to 91% [49].
Among cases described in the literature, family history of dystonia was 76.2% (Table 1). This number is probably artificially inflated given that several published studies focused their mutation screening efforts on early-onset cases or cases with a positive family history of dystonia. In large series of mainly adult-onset primary dystonia, a family history of dystonia in first- or second-degree relatives was closer to 50% [4, 5, 28, 29]. Limiting analysis to coding variants (Supplementary Table 1), 69/85 (81%) of those localized to the THAP domain (1-81aa) were associated with a positive family history of dystonia. In comparison, a positive family history was noted for only 13/28 (46%) of those variants localized to other regions of THAP1. The difference in family history between THAP domain and non-THAP domain variants is highly significant (χ2 = 12.77, df = 1, p = 0.001). Given that family history included both first and second degree relatives in several reports and total fertility rates of approximately 2 children per woman in the United States and Europe from 1980 to the present [50, 51], penetrance is probably less than 50% for most coding variants located outside the THAP domain of THAP1.
7. Response to treatment
7.1 Oral pharmacotherapy and injections of botulinum toxin
There is no convincing evidence that THAP1 dystonia responds to levodopa [44]. Reported responses to anticholinergics such as trihexyphenidyl, baclofen and benzodiazepines are variable, and mild to moderate, at best [44]. Similar to other primary dystonias, focal manifestations such as cervical dystonia, laryngeal dystonia and hand-forearm dystonia do respond positively to injections of botulinum toxin [52]. Management of abductor laryngeal dystonia is often limited by the skill of the injector [53]. Treatment of dysarthria may be inadequate due to combined involvement of the lower face, masticatory muscles, tongue and pharynx [53].
7.2 Deep brain stimulation
The results of DBS for treatment of THAP1 dystonia are shown in Table 3. In all subjects, dystonia was treated with bilateral placement of electrodes in the GPi. As is the case for other primary dystonia, improvement in THAP1 dystonia took weeks to month after the onset of stimulation. DBS seemingly had the largest effects on appendicular and cervical dystonia. The effects of DBS on dysphonia and dysarthria appeared to be more variable and took longer to become manifest. Pre- and post-operative dystonia rating scale scores were available for 9 cases - improvement ranged from 16 - 93% with a mean of 41%.
Table 3.
Response to bilateral GPi DBS in THAP1 dystonia
| Variant | Gender | Age of Onset |
Distribution | Age at Surgery |
Response to Surgery | Reference |
|---|---|---|---|---|---|---|
| N69del | M | 7 | G (neck, arms, legs, trunk). Pre-op BFMDRS: 24 |
9 | post-op BFMDRS: 18 (25% ↓) | Groen et al. [28] |
| N69del | M | 10 | G (tongue-jaw, neck, arms, legs, trunk). Pre-op BFMDRS: 40 |
39 | post-op BFMDRS: 18 (55% ↓) | Groen et al.[28] |
| N136K | F | 9 | G (upper face, tongue-jaw, larynx, neck, arms, legs, trunk). Pre-op BFMDRS: 79.5 |
48 | post-op BFMDRS: 50 (37% ↓) | Groen et al.[28] |
| T59I | M | 6 | G (upper face, tongue-jaw, larynx, neck, arms, legs, trunk). Pre-op BFMDRS: 31 |
23 | post-op BFMDRS: 26 (16% ↓) | Groen et al.[28] |
| L71L | M | 54 | Pre-op TWSTRS: 20 | 70 | post-op TWSTRS: 16 (20% ↓) | Groen et al.[28] |
| R13H | M | 6 | S (upper face, lower face, tongue-jaw, larynx, neck, arms, trunk). Pre-op BFMDRS: 37 |
28 | mild improvement in neck and arm dystonia; but no significant improvement in laryngeal, lower facial, or tongue-jaw dystonia |
Zittel et al. [38] |
| S130Sfs*4 | M | 9 | G (lower face, tongue-jaw, larynx, neck, arms, legs, trunk). Pre-op BFMDRS: 71 |
28 | moderate improvement in appendicular dystonia; but no significant improvement in cranial, laryngeal or neck dystonia |
Zittel et al. [38] |
| S6P | F | 13 | G (tongue-jaw, neck, larynx, arms, legs) |
NA | mild to markeda | Clot et al.[47] |
| A7Efs*23 | M | 9 | S (tongue-jaw, neck, larynx, arms) |
NA | mild to markeda | Clot et al.[47] |
| N69del | M | 11 | G (upper face, neck, arms, legs) |
NA | mild to markeda | Clot et al.[47] |
| P30R | M | 8 | G (larynx, tongue-jaw, neck, trunk, arms, legs). Pre-op BFMDS: 41 |
11 | marked improvement over 2 mo. Post-op BFMDS: 3 (93% ↓) |
Jech et al.[45] |
| F45Lfs*29 | M | 3 | G (larynx, neck, arm, leg). Pre- op BFMDRS: 40 |
15 | mild improvement. Post-op BFMDRS: 27 (33% ↓) | Panov et al.[44] |
| M1?(c.2delT) | F | 25 | G (arm, tongue-jaw, leg). Pre- op BFMDRS: 20.5 |
36 | mild improvement. Post-op BFMDRS: 16.5 (20% ↓) | Panov et al.[44] |
| C54Y | M | 6 | G (jaw, neck, arm, leg). Pre-op BFMDRS: 77.5 |
23 | moderate improvement Post-op BFMDRS: 21 (73% ↓) |
Panov et al.[44] |
| K16E | M | 6 | G (upper face, lower face, tongue-jaw, larynx, neck, arms, legs, trunk) |
43 | mild improvement in laryngeal and tongue- jaw dystonia; moderate improvement in facial, neck, arm, leg and trunk dystonia |
LeDoux et al. |
BFMDRS, Burke-Fahn-Marsden Dystonia Rating Scale - motor score. TWSTRS-M, Toronto Western Spasmodic Torticollis Rating Scale - motor score.
Clot et al.[47] summarized the results in their 3 patients as mild to marked.
8. Conclusions
THAP1 dystonia often begins in an arm during the late teen years and early-adult life. Involvement of the neck, larynx and lower cranial musculature is common. Given that THAP1 has only 3 exons, genetic testing is reasonable for subjects within two standard deviations of the mean age of onset (16.8 + 12.6*2 = 42 yrs), especially if dystonia is segmental and affects the craniocervical musculature. Preimplantation genetic testing may be considered, particularly for sequence variants involving the THAP domain of THAP1.
Although no post-mortem pathological studies have been published to date, based on imaging studies and the absence of dementia, Parkinsonism, and spasticity, there is no evidence for overt neurodegeneration in THAP1 dystonia. In fact, spontaneous resolution has been reported for one patient with a THAP1 deletion mutation. This information suggests that THAP1 dystonia is a network disorder, possibly amenable to sensorimotor retraining. Patients with intractable segmental and generalized dystonia can benefit from DBS, but should be made aware that improvements in laryngeal and cranial dystonia may be marginal.
Supplementary Material
Acknowledgements
This study was supported by the Neuroscience Institute at the University of Tennessee Health Science Center (M.S.L.), Dystonia Medical Research Foundation (M.S.L. and Z.K.W.), NIH grants R01NS048458 and R01NS069936 (M.S.L.), NIH U54 Dystonia Coalition (1U54NS065701) Pilot Projects Program (M.S.L.), and the Parkinson’s & Movement Disorder Foundation (M.S.L.). At Mayo Clinic Florida, work was supported by the NIH National Institute of Neurological Disease and Stroke Morris K. Udall Center of Excellence for Parkinson Disease Research grant (P50-NS57567 and P50 NS072187-01S2) (Z.K.W. and J.A.VG.), NINDS R01 NS057567 (Z.K.W.), NINDS 1RC2NS070276 (Z.K.W.), and CR 90052030 Mayo Clinic Jacksonville Research Committee (Z.K.W. and J.A.VG). A.P. received funding from the Swedish Parkinson Academy. The Swedish patient was cared for by the Clinical Movement Disorders Team at Skåne University Hospital, Lund, Sweden, where Dr. Lars Wictor, Department of Neurology, recorded the pre-operative video, and Dr. Hjalmar Bjartmarz, Department of Neurosurgery, performed the surgical procedure and was responsible for clinical follow-up and DBS adjustments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix. Supplementary material
Supplementary data associated with this article can be found in the online version.
References
- [1].Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–8. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- [2].Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–8. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- [3].Bressman SB, Raymond D, Fuchs T, Heiman GA, Ozelius LJ, Saunders-Pullman R. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 2009;8:441–6. doi: 10.1016/S1474-4422(09)70081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xiao J, Zhao Y, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–38. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Houlden H, Schneider SA, Paudel R, Melchers A, Schwingenschuh P, Edwards M, et al. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 2010;74:846–50. doi: 10.1212/WNL.0b013e3181d5276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schneider SA, Ramirez A, Shafiee K, Kaiser FJ, Erogullari A, Bruggemann N, et al. Homozygous THAP1 mutations as cause of early-onset generalized dystonia. Mov Disord. 2011;26:858–61. doi: 10.1002/mds.23561. [DOI] [PubMed] [Google Scholar]
- [7].Bressman SB, Sabatti C, Raymond D, de Leon D, Klein C, Kramer PL, et al. The DYT1 phenotype and guidelines for diagnostic testing. Neurology. 2000;54:1746–52. doi: 10.1212/wnl.54.9.1746. [DOI] [PubMed] [Google Scholar]
- [8].Xiao J, Zhao Y, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, et al. The c.-237_236GA>TT THAP1 sequence variant does not increase risk for primary dystonia. Mov Disord. 2011;26:549–52. doi: 10.1002/mds.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiao J, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, Karimi M, et al. High-throughput mutational analysis of TOR1A in primary dystonia. BMC Med Genet. 2009;10:24. doi: 10.1186/1471-2350-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- [12].Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–6. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- [13].Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–70. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roussigne M, Cayrol C, Clouaire T, Amalric F, Girard JP. THAP1 is a nuclear proapoptotic factor that links prostate-apoptosis-response-4 (Par-4) to PML nuclear bodies. Oncogene. 2003;22:2432–42. doi: 10.1038/sj.onc.1206271. [DOI] [PubMed] [Google Scholar]
- [15].Bessiere D, Lacroix C, Campagne S, Ecochard V, Guillet V, Mourey L, et al. Structure-function analysis of the THAP zinc finger of THAP1, a large C2CH DNA-binding module linked to Rb/E2F pathways. J Biol Chem. 2008;283:4352–63. doi: 10.1074/jbc.M707537200. [DOI] [PubMed] [Google Scholar]
- [16].Clouaire T, Roussigne M, Ecochard V, Mathe C, Amalric F, Girard JP. The THAP domain of THAP1 is a large C2CH module with zinc-dependent sequence-specific DNA-binding activity. Proc Natl Acad Sci U S A. 2005;102:6907–12. doi: 10.1073/pnas.0406882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Campagne S, Saurel O, Gervais V, Milon A. Structural determinants of specific DNA-recognition by the THAP zinc finger. Nucleic Acids Res. 2010;38:3466–76. doi: 10.1093/nar/gkq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sengel C, Gavarini S, Sharma N, Ozelius LJ, Bragg DC. Dimerization of the DYT6 dystonia protein, THAP1, requires residues within the coiled-coil domain. J Neurochem. 2011;118:1087–100. doi: 10.1111/j.1471-4159.2011.07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kaiser FJ, Osmanoric A, Rakovic A, Erogullari A, Uflacker N, Braunholz D, et al. The dystonia gene DYT1 is repressed by the transcription factor THAP1 (DYT6) Ann Neurol. 2010;68:554–9. doi: 10.1002/ana.22157. [DOI] [PubMed] [Google Scholar]
- [20].Gavarini S, Cayrol C, Fuchs T, Lyons N, Ehrlich ME, Girard JP, et al. Direct interaction between causative genes of DYT1 and DYT6 primary dystonia. Ann Neurol. 2010;68:549–53. doi: 10.1002/ana.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cayrol C, Lacroix C, Mathe C, Ecochard V, Ceribelli M, Loreau E, et al. The THAP-zinc finger protein THAP1 regulates endothelial cell proliferation through modulation of pRB/E2F cell-cycle target genes. Blood. 2007;109:584–94. doi: 10.1182/blood-2006-03-012013. [DOI] [PubMed] [Google Scholar]
- [22].Kamm C, Uflacker N, Asmus F, Schrader C, Wolters A, Wittstock M, et al. No evidence for THAP1/DYT6 variants as disease modifiers in DYT1 dystonia. Mov Disord. 2011;26:2136–7. doi: 10.1002/mds.23777. [DOI] [PubMed] [Google Scholar]
- [23].Trollmann R, Rehrauer H, Schneider C, Krischke G, Huemmler N, Keller S, et al. Late-gestational systemic hypoxia leads to a similar early gene response in mouse placenta and developing brain. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1489–99. doi: 10.1152/ajpregu.00697.2009. [DOI] [PubMed] [Google Scholar]
- [24].Mazars R, Gonzalez-de-Peredo A, Cayrol C, Lavigne AC, Vogel JL, Ortega N, et al. The THAP-zinc finger protein THAP1 associates with coactivator HCF-1 and O-GlcNAc transferase: a link between DYT6 and DYT3 dystonias. J Biol Chem. 2010;285:13364–71. doi: 10.1074/jbc.M109.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Valente EM, Bentivoglio AR, Cassetta E, Dixon PH, Davis MB, Ferraris A, et al. DYT13, a novel primary torsion dystonia locus, maps to chromosome 1p36.13--36.32 in an Italian family with cranial-cervical or upper limb onset. Ann Neurol. 2001;49:362–6. [PubMed] [Google Scholar]
- [26].Puschmann A, Xiao J, Bastian RW, Searcy JA, LeDoux MS, Wszolek ZK. An African-American family with dystonia. Parkinsonism Relat Disord. 2011;17:547–50. doi: 10.1016/j.parkreldis.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Djarmati A, Schneider SA, Lohmann K, Winkler S, Pawlack H, Hagenah J, et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol. 2009;8:447–52. doi: 10.1016/S1474-4422(09)70083-3. [DOI] [PubMed] [Google Scholar]
- [28].Groen JL, Ritz K, Contarino MF, van de Warrenburg BP, Aramideh M, Foncke EM, et al. DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Mov Disord. 2010;25:2420–7. doi: 10.1002/mds.23285. [DOI] [PubMed] [Google Scholar]
- [29].Lohmann K, Uflacker N, Erogullari A, Lohnau T, Winkler S, Dendorfer A, et al. Identification and functional analysis of novel THAP1 mutations. Eur J Hum Genet. 2012;20:171–5. doi: 10.1038/ejhg.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Song W, Chen Y, Huang R, Chen K, Pan P, Yang Y, et al. Novel THAP1 gene mutations in patients with primary dystonia from Southwest China. J Neurol Sci. 2011;309:63–7. doi: 10.1016/j.jns.2011.07.023. [DOI] [PubMed] [Google Scholar]
- [31].Cheng FB, Ozelius LJ, Wan XH, Feng JC, Ma LY, Yang YM, et al. THAP1/DYT6 sequence variants in non-DYT1 early-onset primary dystonia in China and their effects on RNA expression. J Neurol. 2011 doi: 10.1007/s00415-011-6196-5. [DOI] [PubMed] [Google Scholar]
- [32].Silva AL, Romao L. The mammalian nonsense-mediated mRNA decay pathway: to decay or not to decay! Which players make the decision? FEBS Lett. 2009;583:499–505. doi: 10.1016/j.febslet.2008.12.058. [DOI] [PubMed] [Google Scholar]
- [33].Cheng FB, Wan XH, Feng JC, Wang L, Yang YM, Cui LY. Clinical and genetic evaluation of DYT1 and DYT6 primary dystonia in China. Eur J Neurol. 2011;18(3):497–503. doi: 10.1111/j.1468-1331.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- [34].Blanchard A, Roubertie A, Simonetta-Moreau M, Ea V, Coquart C, Frederic MY, et al. Singular DYT6 phenotypes in association with new THAP1 frameshift mutations. Mov Disord. 2011;26:1774–6. doi: 10.1002/mds.23641. [DOI] [PubMed] [Google Scholar]
- [35].Bonetti M, Barzaghi C, Brancati F, Ferraris A, Bellacchio E, Giovanetti A, et al. Mutation screening of the DYT6/THAP1 gene in Italy. Mov Disord. 2009;24:2424–7. doi: 10.1002/mds.22861. [DOI] [PubMed] [Google Scholar]
- [36].Carbon M, Argyelan M, Ghilardi MF, Mattis P, Dhawan V, Bressman S, et al. Impaired sequence learning in dystonia mutation carriers: a genotypic effect. Brain. 2011;134:1416–27. doi: 10.1093/brain/awr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, et al. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130:2858–67. doi: 10.1093/brain/awm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zittel S, Moll CK, Bruggemann N, Tadic V, Hamel W, Kasten M, et al. Clinical neuroimaging and electrophysiological assessment of three DYT6 dystonia families. Mov Disord. 2010;25:2405–12. doi: 10.1002/mds.23279. [DOI] [PubMed] [Google Scholar]
- [39].Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–54. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–7. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2011;42:202–9. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carbon M, Su S, Dhawan V, Raymond D, Bressman S, Eidelberg D. Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology. 2004;62:1384–90. doi: 10.1212/01.wnl.0000120541.97467.fe. [DOI] [PubMed] [Google Scholar]
- [43].Carbon M, Niethammer M, Peng S, Raymond D, Dhawan V, Chaly T, et al. Abnormal striatal and thalamic dopamine neurotransmission: Genotype-related features of dystonia. Neurology. 2009;72:2097–103. doi: 10.1212/WNL.0b013e3181aa538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Panov F, Tagliati M, Ozelius LJ, Fuchs T, Gologorsky Y, Cheung T, et al. Pallidal deep brain stimulation for DYT6 dystonia. J Neurol Neurosurg Psychiatry. 2012;83:182–7. doi: 10.1136/jnnp-2011-300979. [DOI] [PubMed] [Google Scholar]
- [45].Jech R, Bares M, Krepelova A, Urgosik D, Havrankova P, Ruzicka E. DYT 6--a novel THAP1 mutation with excellent effect on pallidal DBS. Mov Disord. 2011;26:924–5. doi: 10.1002/mds.23599. [DOI] [PubMed] [Google Scholar]
- [46].Van Gerpen JA, Ledoux MS, Wszolek ZK. Adult-onset leg dystonia due to a missense mutation in THAP1. Mov Disord. 2010;25:1306–7. doi: 10.1002/mds.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Clot F, Grabli D, Burbaud P, Aya M, Derkinderen P, Defebvre L, et al. Screening of the THAP1 gene in patients with early-onset dystonia: myoclonic jerks are part of the dystonia 6 phenotype. Neurogenetics. 2011;12:87–9. doi: 10.1007/s10048-010-0264-3. [DOI] [PubMed] [Google Scholar]
- [48].O’Riordan S, Raymond D, Lynch T, Saunders-Pullman R, Bressman SB, Daly L, et al. Age at onset as a factor in determining the phenotype of primary torsion dystonia. Neurology. 2004;63:1423–6. doi: 10.1212/01.wnl.0000142035.26034.c2. [DOI] [PubMed] [Google Scholar]
- [49].Saunders-Pullman R, Raymond D, Senthil G, Kramer P, Ohmann E, Deligtisch A, et al. Narrowing the DYT6 dystonia region and evidence for locus heterogeneity in the Amish-Mennonites. Am J Med Genet A. 2007;143A:2098–105. doi: 10.1002/ajmg.a.31887. [DOI] [PubMed] [Google Scholar]
- [50].ESHRE Capri Worshop Group Europe the continent with the lowest fertility. Hum Reprod Update. 2010;16(6):590–602. doi: 10.1093/humupd/dmq023. [DOI] [PubMed] [Google Scholar]
- [51].Hamilton BE, Ventura SJ. Fertility and abortion rates in the United States, 1960-2002. Int J Androl. 2006;29:34–45. doi: 10.1111/j.1365-2605.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- [52].Cheng FB, Wan XH, Feng JC, Wang L, Yang YM, Cui LY. Clinical and genetic evaluation of DYT1 and DYT6 primary dystonia in China. Eur J Neurol. 2011;18:497–503. doi: 10.1111/j.1468-1331.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- [53].LeDoux MS. Meige syndrome: what’s in a name? Parkinsonism Relat Disord. 2009;15:483–9. doi: 10.1016/j.parkreldis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.