ABSTRACT
BACKGROUND
Although comorbidity has been shown to affect the benefits and risks of colorectal cancer (CRC) screening, it has not been accounted for in prior cost-effectiveness analyses of CRC screening.
OBJECTIVE
To evaluate the impact of diagnosis of diabetes mellitus, a highly prevalent comorbidity in U.S. adults aged 50 and older, on health and economic outcomes of CRC screening.
DESIGN
Cost-effectiveness analysis using an integrated modeling framework.
DATA SOURCES
Derived from basic and epidemiologic studies, clinical trials, cancer registries, and a colonoscopy database.
TARGET POPULATION
U.S. 50-year-old population.
TIME HORIZON
Lifetime.
PERSPECTIVE
Costs are based on Medicare reimbursement rates.
INTERVENTIONS
Colonoscopy screening at ten-year intervals, beginning at age 50, and discontinued after age 50, 60, 70, 80 or death.
OUTCOME MEASURES
Health outcomes and cost effectiveness.
RESULTS OF BASE-CASE ANALYSIS
Diabetes diagnosis significantly affects cost-effectiveness of CRC screening. For the same CRC screening strategy, a person without diabetes at age 50 gained on average 0.07–0.13 life years more than a person diagnosed with diabetes at age 50 or younger. For a population of 1,000 patients diagnosed with diabetes at baseline, increasing stop age from 70 years to 80 years increased quality-adjusted life years (QALYs) gained by 0.3, with an incremental cost-effectiveness ratio of $206,671/QALY. The corresponding figures for 1,000 patients without diabetes are 2.3 QALYs and $46,957/QALY.
RESULTS OF SENSITIVITY ANALYSIS
Cost-effectiveness results are sensitive to cost of colonoscopy and adherence to colonoscopy screening.
LIMITATIONS
Results depend on accuracy of model assumptions.
CONCLUSION
Benefits of CRC screening differ substantially for patients with and without diabetes. Screening for CRC in patients diagnosed with diabetes at age 50 or younger is not cost-effective beyond age 70. Screening recommendations should be individualized based on the presence of comorbidities.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1972-6) contains supplementary material, which is available to authorized users.
KEY WORDS: colorectal cancer screening, cost-effectiveness analysis, health care modeling, comorbidity, optimal screening cessation, individualized guidelines
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States, with a majority of new cases diagnosed in persons aged 65 and older.1 While CRC screening has been shown to significantly reduce the risk of morbidity and mortality associated with CRC in randomized controlled trials and case-control studies,2–4 patients must have a substantial life expectancy to derive survival benefit from CRC screening.5 Diagnosis of significant comorbidity is expected to affect the benefits and risks of colorectal cancer screening, but this has not been studied in prior cost-effectiveness analyses, which have used chronological age and average longevity as a proxy for comorbidity.6 Average longevity does not take into account the heterogeneity of the elderly population which includes persons for whom screening is likely to have little impact on their life expectancy. To explore how comorbid diseases affect the cost-effectiveness of CRC screening, we chose to focus on diabetes mellitus, a highly prevalent comorbidity among older persons.7 Diabetes not only reduces a person’s life expectancy in general8,9 and overall survival after diagnosis of CRC,10 but also increases risk of developing CRC11,12 and risk of perforation during colonoscopy.13
Since there are no empirical data available at this time about how comorbid conditions, such as diabetes, affect the cost effectiveness of CRC screening, we used mathematical modeling to compare different screening strategies in different patient populations. We used the Archimedes Model, a large-scale integrated mathematical model of diseases and healthcare systems, to determine the impact of different screening cessation strategies on health and economic outcomes of colorectal cancer in patients with and without diabetes. The results of the study can be used to assist CRC screening guidelines to incorporate comorbid conditions, such as diabetes, into their recommendations, rather than to focus solely on age or assessment of health status14 to determine when to stop screening.
METHODS
The Model
We conducted the analysis by using the Archimedes Model, a large-scale integrated simulation model of human physiology, diseases, and healthcare systems.15,16 In the Archimedes Model, each individual is simulated down to the level of polyp location, adenoma histology, tumor size, fasting plasma glucose, and similar biological variables. The core of the model is a set of equations that represent the anatomy and physiology pertinent to diseases and their complications. Currently, the Archimedes Model includes diabetes, congestive heart failure, coronary artery disease, stroke, hypertension, obesity, colorectal cancer, breast cancer, and lung cancer in a single integrated model. The Archimedes Model creates virtual people, each of whom has his or her own simulated physiology and can get one or more diseases, develop symptoms, seek care, and get diagnosed and treated. To ensure that the virtual people are representative of real people, the Archimedes Model creates copies of real people using person-specific data from the National Health and Nutrition Examination Survey (NHANES). The methods for creating copies ensure that the distributions and correlations of all important variables are the same in the simulated population as in the real population.
The structure and equations of the Archimedes Model pertinent to diabetes and its complications are described elsewhere.17–19 The model predictions of diabetes-related outcomes have been validated against a large number of epidemiological and controlled clinical trials.20 The Appendix (available online) describes aspects of the diabetes sub-model that are pertinent to this analysis.
The colorectal cancer sub-model within the Archimedes Model was developed in collaboration with the American Cancer Society (Table 1 and Appendix). To build the model, we conducted systematic searches in MEDLINE, Cochrane Database of Systematic Reviews, PUBMED, Web of Science and Google Scholar, and supplemented with manual searches of references. The colorectal cancer sub-model was derived from clinical trials, retrospective analyses, population surveys, and cancer registries, including colonoscopy data from the Clinical Outcomes Research Initiative (CORI) database21 and clinical incidence data from the Surveillance Epidemiology and End Results (SEER) program.22
Table 1.
Key Parameters, Assumptions, Modeling Approaches, and Sources
| Parameter | Assumptions and approaches | References |
|---|---|---|
| 1. Natural history of CRC | ||
| Adenoma incidence | Adenoma incidence is assumed to follow a non-homogenous Poisson distribution | Rutter et al.24 |
| Villavicencio et al.48 | ||
| Johns et al.49 Cottet et al.50 | ||
| Giovannucci et al.51 | ||
| Effects of gender, family history, BMI and diabetes are represented by hazard ratios derived from author-conducted meta-analysis. | Giovannucci et al.52 | |
| Elwing et al.53 | ||
| Adenoma growth | Adenoma growth is described by a log-linear growth equation. | Wilson et al.54 |
| CORI21 | ||
| Adenoma growth rate is assumed to be a function of age, gender and race and log-normally distributed. | ||
| Size distribution of adenoma at the time of colonoscopy was matched to the CORI database. | ||
| Adenoma location | Adenomas can occur at eight different anatomical sites along the colon-rectum, namely cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, and rectum. | CORI21 |
| The distribution of anatomical sites of adenomas as a function of age and gender are extracted from the CORI database. | ||
| Adenoma histology and grade of dysplasia | Histology and grade of dysplasia are functions of adenoma size and based on author-conducted meta-analysis. | Butterly et al.55 |
| O’Brien et al.56 | ||
| Shinya et al.57 | ||
| Cancer risk and malignant transformation | The hazard rate of an adenoma developing malignancy is a function of age, gender, and adenoma location. | Scheiden et al.58 |
| Hermanek and Karrer59 | ||
| Hofstad60 | ||
| CORI21 | ||
| The form of the hazard rate is derived from author-conducted meta-analysis. The parameters are obtained by fitting the hazard rate to the CORI and SEER databases. | SEER22 | |
| Tumor growth | Tumors grow exponentially as a function of time with growth rates derived from author-conducted meta-analysis. | Bolin et al.26 |
| Umetani et al.27 | ||
| Matsui et al.28 | ||
| Welin et al.25 | ||
| Cancer diagnosis and survival | The distribution of tumor size at the time of diagnosis is derived from early SEER data. | SEER22 |
| Stein et al.61 | ||
| Survival of a patient is a function of age and tumor characteristics at the time of diagnosis and is derived from the SEER database. | ||
| Effect of diabetes on CRC-specific survival is modeled using data from a meta-analysis.61 | ||
| 2. Colonoscopy test characteristics and complications | ||
| Colonoscopy: sensitivity, specificity of adenoma detection | Adenoma size | Rex et al.62 |
| Loeve et al.63 | ||
| ○ 0-5 mm: sensitivity – 75%, specificity – 95% | ||
| ○ 5-10 mm: sensitivity – 85%, specificity – 95% | ||
| ○ >10 mm: sensitivity – 95%, specificity – 95% | ||
| Completion rate: 97% | ||
| Adverse events associated with colonoscopy | Perforation and surgical mortality are functions of age and co-morbidity and are derived from author-conducted meta-analysis. | Warren et al.64 |
| Gatto et al. 13 | ||
| Levin et al.65 | ||
| 3. Costs | ||
| Colonoscopy without polypectomy | $662 | Medicare reimbursement rate40 |
| Colonoscopy with polypectomy or biopsy | $846 | Medicare reimbursement rate40 |
| Colorectal cancer treatment | Costs of colorectal cancer treatments were estimated for initial, continuing and terminal phases and included costs of targeted therapies (e.g. bevacuzimab). See Appendix for more details. | Medicare reimbursement rate40 |
| Treatment and prevention of other diseases, including diabetes complications and cardiovascular diseases | Medication costs were obtained from Drugstore.com as of April, 2009. | Drugstore.com |
| All other costs (e.g., emergency visits, office visits and admissions, and procedures) were based on 2007 Medicare costs. | Medicare reimbursement rate | |
| 4. Health utility | ||
| Colorectal cancer | Health disutility for colorectal cancer was obtained from Ness et al.66 | Ness et al.66 |
| Other diseases | We used utility scores published by Sullivan and Ghushchyan.67,68 | Sullivan et al.67,68 |
In brief, it consists of (i) a natural history component that tracks cancer progression, including adenoma development, tumor growth, and cancer symptoms, as functions of age, gender, race/ethnicity, obesity, physical activity and family history; (ii) a screening component that allows for detection and removal of adenomas and diagnosis of preclinical CRC; and (iii) a treatment component that predicts survival following diagnosis of CRC. The model accounts for important risk factors of CRC, including age, gender, race and BMI.
We model two types of polyps, namely (i) benign polyps, which will never become cancer, and (ii) adenomatous polyps (i.e. adenomas), which have the potential for malignant transformation. The category “benign polyp” includes hyperplastic, inflammatory, and other non-neoplastic polyps and accounts for one-third of the total number of polyps.23 In the model, polyps arise in the colon and the rectum stochastically through a non-homogenous Poisson process.24 The incidence of polyps increases with age and is a function of several risk factors including gender, BMI, and family history. Polyps can occur at eight different anatomical sites: cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, and rectum. Growth of benign polyps and adenomas is modeled using a log-linear equation. As an adenoma grows in size, its histology and grade worsen. The propensity of an adenoma to become cancerous is assumed to be a function of age, adenoma size, and adenoma location. Once an adenoma becomes a malignant tumor, it will grow exponentially, with a doubling time derived from a meta-analysis of the literature.25–28 When a tumor reaches a certain size, the patient will experience CRC symptoms, and after a delay period, will be diagnosed by the healthcare system with symptomatic CRC. The distribution of tumor size at which a patient is diagnosed with symptomatic CRC is derived from early SEER data22 to minimize the effects of screening. If there is screening, malignant tumors are detectable before the symptoms surface. Readers are referred to the Appendix (available online) for a detailed description of the colorectal cancer submodel.
The colorectal cancer outcomes predicted by the Archimedes Model have been validated against several studies including the National Polyp Study,29 Minnesota FOBT Screening Trial,30 Cancer Prevention Study II Nutrition Cohort,31 Women Health Study,32 Women Health Initiative,33 the UK Flexible Sigmoidoscopy Trial34 and the Veterans Affairs Cooperative Study Group.35 Details of model validations are available in the Appendix.
Simulation setup
For the simulated trials, we used person-specific data from the 1998–2004 NHANES survey to create a simulated population of 100,000 individuals aged 50 years old, representative of the U.S. population. Of those, 12% had been diagnosed with diabetes at age 50 or younger. By using the information from real people in NHANES to create simulated individuals, we preserved the correlations of the variables pertinent to the progression of diabetes and colorectal cancer. Patients without diabetes at baseline could also develop diabetes as they aged in the simulation.
These 100,000 simulated individuals were tracked from the age of 50 to death, and were exposed to six colorectal cancer screening strategies and a control (no-screening) strategy. In the control (no-screening) strategy, simulated individuals were not screened for CRC (i.e. CRCs were diagnosed based on symptoms, and patients were treated according to current practice). In the screening strategies, simulated individuals were screened by colonoscopy at ten-year intervals with different ages beyond which screening was stopped (Table 2). Colonoscopy was selected for this study since it is considered to be the most effective CRC screening strategy. The follow-up schedule for surveillance colonoscopy is modeled after the guideline developed by the American Cancer Society and the U.S. Multi-Society Task Force on Colorectal Cancer.36 Patients are assumed to comply perfectly with recommended CRC screening schedules. During simulation, patients were provided with screening, diagnosis, treatment and monitoring for diabetes and its micro- and macro-vascular complications, according to current practice.
Table 2.
Impact of Colonoscopy Screening on CRC incidence, Life Years and Quality-Adjusted Life Years (QALYs) per 1000 Individuals, with and without Diabetes at age 50, as Compared with no Screening
| Screening strategy | Individuals with history of diabetes at age 50 | Individuals without diabetes at age 50 | ||||||
|---|---|---|---|---|---|---|---|---|
| # | Name | Description | Reduction in CRC incidence (%) | LY gained | QALY gained | Reduction in CRC incidence (%) | LY gained | QALY gained |
| 1 | No screening | Never screened for CRC | 0% | 0.0 | 0.0 | 0% | 0.0 | 0.0 |
| 2 | Screening is discontinued after age 50 | Screened once at age 50 | 43% | 128.2 | 105.5 | 45% | 198.5 | 152.4 |
| 3 | Screening is discontinued after age 60 | Screened twice, at ages 50 and 60 | 54% | 154.3 | 123.9 | 59% | 254.9 | 190.8 |
| 4 | Screening is discontinued after age 70 | Screened at ages 50, 60 and 70 | 65% | 164.1 | 129.5 | 75% | 283.2 | 206.0 |
| 5 | Screening is discontinued after age 80 | Screened at ages 50, 60, 70 and 80 | 72% | 165.9 | 129.8 | 81% | 290.5 | 208.3 |
| 6 | No stop age | Screened for CRC at 10-year intervals until death | 72% | 166.1 | 129.9 | 83% | 291.0 | 208.7 |
Cost effectiveness
We examined each screening strategy from a social perspective, based on recommendations of the Panel on Cost-Effectiveness in Health and Medicine.37 Specifically, we recorded the simulated logistic events and clinical outcomes for each person in the population, over a life-time horizon. We assigned costs to each logistic event (e.g., each test, visit, admission, and treatment), and assigned quality-of-life weights to each clinical outcome (e.g. cancer diagnosis, diabetes diagnosis, heart attack, stroke, or amputation). This allows us to calculate the time series of costs and quality-adjusted life years (QALYs) first for each person, then add them together for sub-populations of interest.
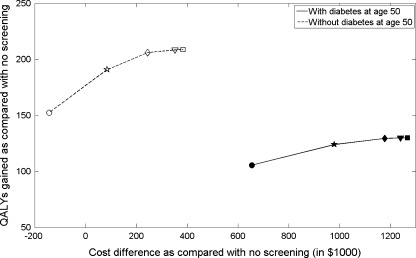
Costs of tests, visits, admissions, procedures, and treatments were primarily based on Medicare reimbursement rates (Table 1). All costs were adjusted to 2009 values using the medical care component of the Bureau of Labor Statistics Consumer Price Index. We reported costs associated with screening and treatment for CRC and total medical cost (defined as the sum of colorectal cancer costs and other medical costs captured in the Archimedes Model). Data sources used to calculate parameters for quality-adjusted life years (QALYs) are shown in Table 1. Both costs and life years are discounted at 3% annually.37 We defined the incremental cost-effectiveness ratio as cost/QALY saved for a given strategy relative to the nearest strategy on the efficient frontier (Fig. 1). We considered a strategy to be “cost-effective” as compared with another strategy if the cost/QALY was below the often-quoted benchmark of $50,000/QALY,38 although others have argued for higher thresholds.39
Figure 1.
Cost difference versus QALYs gained as compared with no-screening for different CRC screening cessation strategies per 1,000 individuals, with and without diabetes at age 50 (circle: screening cessation after age 50; pentagram: age 60; diamond: age 70; triangle: age 80; and square: no stop age).
We conducted one-way sensitivity analysis of important model parameters, including adherence to colonoscopy, costs of colonoscopy and polypectomy, costs of CRC treatment and risk of colonoscopy perforation.
Role of the Funding Source
This study was carried out by Archimedes in collaboration with Drs. Smith and Walter. Dr. Walter is supported by a grant 1R01CA134425 from the National Cancer Institute. The authors had access to all the study data, take responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication.
Results
Table 2 summarizes health outcomes of colorectal cancer screening in patients with and without a diagnosis of diabetes at age 50. Screening for CRC substantially reduced incidence of CRC (43%–83%) and added a significant number of life years as compared with no-screening. The health benefits of CRC screening increase with older stop ages. Screening for CRC without an upper stop age (strategy #6) saves 23%–32% more life years than discontinuing screening after a single screening colonoscopy at age 50 (strategy #2).
For a population representative of the general US population, the predicted life-years gained and reduction in CRC incidence from colorectal cancer screening are similar to those predicted by the MISCAN and SimCRC models,6 see Appendix for more details. Costs of CRC screening and surveillance in the general US population are also consistent with previous cost-effectiveness studies.40
Patients who have been diagnosed with diabetes at age 50 gained fewer life years from CRC screening than those without diabetes at age 50. On average, for the same screening strategy, a person without diabetes at age 50 will gain 0.07–0.13 life years more than a person with diabetes at baseline (Table 2).
Since patients without diabetes live on average 8-10 years longer than their diabetic counterparts, they have higher life time risk of developing CRC and incur more costs of colorectal cancer treatment in the no screening strategy. Screening reduces CRC treatment costs but increases total medical costs (Table 3). As screening stop age increased from 50 to 80, the difference in total medical cost compared with no screening increased from $655 to $1268 per person in individuals with diabetes at baseline. For patients without diabetes at baseline, colonoscopy screening once-only at age 50 is cost-saving, saving $143 per person as compared with no-screening.
Table 3.
Discounted Costs (in $1000) of Different CRC Screening Cessation Strategies per 1000 individuals with and Without Diabetes at Age 50
| Population | Screening strategy | Cost of CRC screening, follow-up, surveillance | Cost of CRC treatment | Total CRC cost = Cost of CRC screening + Cost of CRC treatment | Other medical costs | Total medical cost = Total CRC cost + Other medical costs | Difference in total medical cost as compared with no screening | |
|---|---|---|---|---|---|---|---|---|
| # | Name | |||||||
| With history of diabetes at baseline | 1 | No screening | 0 | 3716 | 3716 | 118912 | 122,628 | 0 |
| 2 | Screening is discontinued after age 50 | 1089 | 2533 | 3662 | 119621 | 123,283 | 655 | |
| 3 | Screening is discontinued after age 60 | 1552 | 2271 | 3823 | 119785 | 123,608 | 980 | |
| 4 | Screening is discontinued after age 70 | 1753 | 2183 | 3936 | 119871 | 123,807 | 1179 | |
| 5 | Screening is discontinued after age 80 | 1799 | 2192 | 3991 | 119878 | 123,869 | 1241 | |
| 6 | No stop age | 1813 | 2195 | 4008 | 119888 | 123,896 | 1268 | |
| Without history of diabetes at baseline | 1 | No screening | 0 | 5445 | 5445 | 100,961 | 106,406 | 0 |
| 2 | Screening is discontinued after age 50 | 1376 | 3328 | 4704 | 101,559 | 106,263 | -143 | |
| 3 | Screening is discontinued after age 60 | 2046 | 2692 | 4738 | 101,753 | 106,491 | 85 | |
| 4 | Screening is discontinued after age 70 | 2410 | 2389 | 4799 | 101,852 | 106,651 | 245 | |
| 5 | Screening is discontinued after age 80 | 2563 | 2317 | 4880 | 101,879 | 106,759 | 353 | |
| 6 | No stop age | 2599 | 2306 | 4905 | 101,885 | 106,790 | 384 | |
While screening for CRC reduces the cost of cancer treatment (Table 3), it is expensive, reaching a life-time cost of $2599 in patients without diabetes at baseline. It also increases other medical costs (Table 3), since individuals screened for CRC live longer than their unscreened counterparts and incur higher medical costs for other diseases.
Figure 1 plots QALYs gained and the increase in cost for each of the screening strategies. QALYs gained, cost, and incremental cost-effectiveness ratios (ICERs) depend strongly on whether a person has diabetes at age 50 and the upper age at which screening is stopped. For a population of 1,000 patients diagnosed with diabetes at baseline, extending screening stop age from 70 years old to 80 years old increased quality-adjusted life years (QALYs) by 0.3, with an ICER of $206,671/QALY (Table 4). The corresponding figures for 1,000 patients without diabetes at age 50 are an increase in 2.3 QALYs and an ICER of $46,957/QALY (Table 4).
Table 4.
Incremental Cost-Effectiveness Ratios (ICER) as Function of Screening Stop Age in 1000 Individuals, with and Without Diabetes Diagnosis at age 50
| Population | Screening strategy | Difference in total medical cost as compared with no screening (in $1000) | QALY gained as compared with no screening | ICER ($ per QALY saved) | |
|---|---|---|---|---|---|
| # | Name | ||||
| With history of diabetes at baseline | 1 | No screening | 0 | 0.0 | -- |
| 2 | Screening is discontinued after age 50 | 655 | 105.5 | 6,209 | |
| 3 | Screening is discontinued after age 60 | 980 | 123.9 | 17,663 | |
| 4 | Screening is discontinued after age 70 | 1179 | 129.5 | 35,563 | |
| 5 | Screening is discontinued after age 80 | 1241 | 129.8 | 206,671 | |
| 6 | No stop age | 1268 | 129.9 | 270,005 | |
| Without history of diabetes at baseline | 1 | No screening | 0 | 0.0 | -- |
| 2 | Screening is discontinued after age 50 | -143 | 152.4 | -- | |
| 3 | Screening is discontinued after age 60 | 85 | 190.8 | 5,937 | |
| 4 | Screening is discontinued after age 70 | 245 | 206.0 | 10,526 | |
| 5 | Screening is discontinued after age 80 | 353 | 208.3 | 46,957 | |
| 6 | No stop age | 384 | 208.7 | 77,500 | |
Table 5 shows the influence of several parameters in a one-way sensitivity analysis of the ICER of increasing stop age from 70 to 80 for patients diagnosed with diabetes at age 50 or younger. The cost-effectiveness of strategy #5 extending stop age beyond age 70 for this population is most sensitive to cost of colonoscopy, and adherence to colonoscopy screening.
Table 5.
One-way sensitivity analysis of key parameters
| Subject of sensitivity analysis | Base case value | Value(s) used for sensitivity analysis | ICER of extending stop age from 70 to 80 (Base value: $206,671/QALY) |
|---|---|---|---|
| Colonoscopy sensitivity to large adenomas | 95% | 90% | $94,081 |
| Fraction of colonoscopy reaching the cecum | 97% | 70% | $103,137 |
| Costs of CRC treatment | See Table 1 | -50% compared with base case values | $191,669 |
| +50% compared with base values | $221,672 | ||
| Costs of colonoscopy and polypectomy | See Table 1 | -50% compared with base case values | $130,003 |
| +50% compared with base case values | $283,336 | ||
| Adherence to scheduled screening or surveillance colonoscopy | 100% | 80% | $72,655 |
| 60% | $43,901 | ||
| Risk of colonoscopy perforation | See Table 1 | +50% compared with base case values | $215,853 |
Discussion
By examining CRC screening in patients with and without diabetes, and capturing the impact of screening stop age on the overall health and economic outcomes, the current study emphasizes the importance of considering comorbidity, such as diabetes, when individualizing cancer screening recommendations. We demonstrated that health benefits of CRC screening are substantially different for patients with and without diabetes and screening for CRC in patients diagnosed with diabetes at age 50 or younger is not cost-effective beyond age 70.
We found that individuals with diabetes at age 50 derive less survival benefit from CRC screening than those without diabetes. In individuals without diabetes at age 50, screening until age 80 saves 0.29 life years per person as compared with no screening, approximately 1.8 times more than that in individuals diagnosed with diabetes at age 50. In addition, the incremental gains as a result of extending screening to older ages are also much smaller in individuals with diabetes as compared with those without.
Our study highlights the importance of accounting for medical costs of other diseases in a cost analysis. Colorectal cancer screening reduces the risk of dying from CRC, which consequently increases the probability of developing and dying from other diseases such as CVD, which might be more costly than if a person dies at a younger age of CRC. This leads to an overall increase in the total medical cost for all screening strategies as compared to no-screening. Previous cost-effectiveness analyses40,41 do not take other medical costs into account and likely underestimate the economic costs of CRC screening. These increased costs still fall within conventional criteria for cost-effectiveness, but represent a more realistic assessment of actual costs associated with a CRC screening program.
We identified several cost-effective screening strategies for patients with and without diabetes. For individuals without diabetes at age 50, the incremental cost-effectiveness ratios are below the benchmark of $50,000/QALY for CRC screening up to age 80. The incremental cost-effectiveness ratio of stopping at age 80 (strategy #5) as compared with age 70 (strategy #4) was $46,957. For individuals with diabetes at baseline, it is reasonably cost-effective to screen for CRC up to age 70 and not cost effective if screening is extended to age 80. The costs per QALY saved are not very sensitive to changes in the reference assumptions about costs of colonoscopy screening, costs of CRC treatment, and costs of other diseases.
The strength of the current study lies in the ability of the model to account for all demographic variables, biomarkers, risk factors, disease evolution, and healthcare processes pertinent to colorectal cancer and diabetes in an integrated framework. Both the colorectal cancer and the diabetes sub-models have been extensively calibrated and validated against large-scale studies and clinical trials.
Our analysis has several limitations. First, our results apply to the U.S. population, as the Archimedes Model is calibrated to the U.S. healthcare system, so it is unknown to what extent it generalizes to other countries. Second, in the base-case analysis, we assumed 100% adherence to colonoscopy screening. In real-world settings, adherence is less than 100%. In our sensitivity analysis, we showed that less-than-perfect adherence to colonoscopy screening favors stopping screening at later ages. Third, although the Archimedes Model has been rigorously validated against the pertinent clinical trials, there is no way to ensure its accuracy at predicting events that have never been studied empirically with trials.
While individualized screening recommendations based on patient and family history of cancer and adenoma detection, inflammatory bowel disease, and known or suspected predisposition to CRC are included in current guidelines, different screening recommendations based on gender and race and age only have been suggested in the literature,42 with the exception of the American College of Gastroenterology’s recommendation that average risk African Americans begin screening at age 4543 and the USPSTF’s recommendation against routine screening between ages 76-84, and against screening altogether after age 85, using age as a proxy for comorbidity.44 Lansdorp-Vogelaar et al.45 showed that the improvements in costs and effects of individualizing CRC screening based on gender and race on a population level were only marginal. This is consistent with current simulation results. With respect to an age to stop screening, the current study emphasizes the need to individualize screening recommendations based on comorbidities and life expectancy. In addition, a recent study reported a CRC screening rate of 41% in patients with severe comorbidity who had life expectancies less than 5 years.5 More consideration of comorbidity is needed if we want to improve screening rates in patients with substantial life expectancies and reduce potentially harmful screening in older patients with limited life expectancies.46
The results of the study should not be used as a basis to discontinue screening beyond age 70 years for populations with diabetes. Rather, it argues against a one-size-fits-all recommendation based on age and highlights the pitfalls of judging older patients of similar ages as a homogenous population. We advocate for the development of a more individualized approach to CRC screening decisions that accounts for comorbid disease and other relevant risk factors impacting CRC screening outcomes. Individualized guidelines have been shown to improve care and reduce costs in management of hypertension47 and are expected to produce similar benefits for cancer screening. The increasing use of information technology in primary care should facilitate the integration of computerized risk-benefit calculators with patients’ electronic health records, which will enable primary care physicians to individualize CRC screening recommendations in an efficient way, and enhance shared decision making.
Electronic Supplementary Material
Acknowledgments
Conflict of Interest Declaration
The authors declare no conflict of interest. This study was carried out by Archimedes in collaboration with Drs. Smith and Walter. Dr. Walter is supported by a grant 1R01CA134425 from the National Cancer Institute.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. [DOI] [PubMed]
- 2.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326(10):653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 4.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet;375(9726):1624-33. [DOI] [PubMed]
- 5.Walter LC, Lindquist K, Nugent S, Schult T, Lee SJ, Casadei MA, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465–73. doi: 10.7326/0003-4819-150-7-200904070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–69. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkins E, Boult C, Wagner E. New ways to care for older people. Building systems based on evidence. New York: Springer; 1999. [Google Scholar]
- 8.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 9.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167(11):1145–51. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 10.Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145(9):646–53. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care. 2005;28(7):1805–7. doi: 10.2337/diacare.28.7.1805. [DOI] [PubMed] [Google Scholar]
- 12.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(22):1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 13.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95(3):230–6. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 14.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Kahn R, Alperin P, Eddy D, Borch-Johnsen K, Buse J, Feigelman J, et al. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet;375(9723):1365-74. [DOI] [PubMed]
- 16.Schlessinger L, Eddy DM. Archimedes: a new model for simulating health care systems–the mathematical formulation. J Biomed Inform. 2002;35(1):37–50. doi: 10.1016/S1532-0464(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 17.Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32(2):361–6. doi: 10.2337/dc08-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eddy DM, Schlessinger L. Archimedes: a trial-validated model of diabetes. Diabetes Care. 2003;26(11):3093–101. doi: 10.2337/diacare.26.11.3093. [DOI] [PubMed] [Google Scholar]
- 19.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143(4):251–64. doi: 10.7326/0003-4819-143-4-200508160-00006. [DOI] [PubMed] [Google Scholar]
- 20.Eddy DM, Schlessinger L. Validation of the archimedes diabetes model. Diabetes Care. 2003;26(11):3102–10. doi: 10.2337/diacare.26.11.3102. [DOI] [PubMed] [Google Scholar]
- 21.Clinical Outcomes Research Initiative, www.cori.org. 2007 (Accessed December 2011)..
- 22.Surveillance Epidemiology and End Results (SEER) Program Populations (1969-2006) (www.seer.cancer.gov/popdata) NCI, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released February 2009. (Accessed December 2011).
- 23.Tobi M. Polyps as biomarkers for colorectal neoplasia. Front Biosci. 1999;4:D329–38. doi: 10.2741/Tobi. [DOI] [PubMed] [Google Scholar]
- 24.Rutter CM, Yu O, Miglioretti DL. A hierarchical non-homogenous Poisson model for meta-analysis of adenoma counts. Stat Med. 2007;26(1):98–109. doi: 10.1002/sim.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welin S, Youker J, Spratt JS., Jr The Rates and Patterns of Growth of 375 Tumors of the Large Intestine and Rectum Observed Serially by Double Contrast Enema Study (Malmoe Technique) Am J Roentgenol Radium Ther Nucl Med. 1963;90:673–87. [PubMed] [Google Scholar]
- 26.Bolin S, Nilsson E, Sjodahl R. Carcinoma of the colon and rectum–growth rate. Ann Surg. 1983;198(2):151–8. doi: 10.1097/00000658-198308000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umetani N, Masaki T, Watanabe T, Sasaki S, Matsuda K, Muto T. Retrospective radiographic analysis of nonpedunculated colorectal carcinomas with special reference to tumor doubling time and morphological change. Am J Gastroenterol. 2000;95(7):1794–9. doi: 10.1111/j.1572-0241.2000.02174.x. [DOI] [PubMed] [Google Scholar]
- 28.Matsui T, Tsuda S, Yao K, Iwashita A, Sakurai T, Yao T. Natural history of early colorectal cancer: evolution of a growth curve. Dis Colon Rectum. 2000;43(10 Suppl):S18–22. doi: 10.1007/BF02237221. [DOI] [PubMed] [Google Scholar]
- 29.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 30.Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993;85(16):1311–8. doi: 10.1093/jnci/85.16.1311. [DOI] [PubMed] [Google Scholar]
- 31.Chao A, Connell CJ, Cokkinides V, Jacobs EJ, Calle EE, Thun MJ. Underuse of screening sigmoidoscopy and colonoscopy in a large cohort of US adults. Am J Public Health. 2004;94(10):1775–81. doi: 10.2105/AJPH.94.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higginbotham S, Zhang ZF, Lee IM, Cook NR, Giovannucci E, Buring JE, et al. Dietary glycemic load and risk of colorectal cancer in the Women's Health Study. J Natl Cancer Inst. 2004;96(3):229–33. doi: 10.1093/jnci/djh020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):643–54. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 34.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 36.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'Brien MJ, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56(3):143–59. doi: 10.3322/canjclin.56.3.143. [DOI] [PubMed] [Google Scholar]
- 37.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276(16):1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 38.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–42. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 39.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46(4):349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 40.Lansdorp-Vogelaar I, Ballegooijen M, Zauber AG, Habbema JD, Kuipers EJ. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst. 2009;101(20):1412–22. doi: 10.1093/jnci/djp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129(4):1151–62. doi: 10.1053/j.gastro.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 42.Flottemesch T. C-A5-02: Is Tailored Screening for Colorectal Cancer based on Gender and Race Cost-Effective? Clin Med Res. 2011;9(3–4):174. doi: 10.3121/cmr.2011.1020.c-a5-02. [DOI] [Google Scholar]
- 43.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 44.Force USPST. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 45.Lansdorp-Vogelaar I, Ballegooijen M, Zauber AG, Boer R, Wilschut J, Winawer SJ, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009;70(1):96–108. doi: 10.1016/j.gie.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walter LC, Lewis CL, Barton MB. Screening for colorectal, breast, and cervical cancer in the elderly: a review of the evidence. Am J Med. 2005;118(10):1078–86. doi: 10.1016/j.amjmed.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 47.Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: the potential for increasing quality and reducing costs. Ann Intern Med. 2011;154(9):627–34. doi: 10.7326/0003-4819-154-9-201105030-00008. [DOI] [PubMed] [Google Scholar]
- 48.Villavicencio RT, Rex DK. Colonic adenomas: prevalence and incidence rates, growth rates, and miss rates at colonoscopy. Semin Gastrointest Dis. 2000;11(4):185–93. [PubMed] [Google Scholar]
- 49.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 50.Cottet V, Pariente A, Nalet B, Lafon J, Milan C, Olschwang S, et al. Colonoscopic screening of first-degree relatives of patients with large adenomas: increased risk of colorectal tumors. Gastroenterology. 2007;133(4):1086–92. doi: 10.1053/j.gastro.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 51.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7(2):253–63. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 52.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 53.Elwing JE, Gao F, Davidson NO, Early DS. Type 2 diabetes mellitus: the impact on colorectal adenoma risk in women. Am J Gastroenterol. 2006;101(8):1866–71. doi: 10.1111/j.1572-0241.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 54.Wilson LS, Lightwood J. Model of estimated rates of colorectal cancer from polyp growth by year of surveillance. J Med Screen. 2001;8(4):187–96. doi: 10.1136/jms.8.4.187. [DOI] [PubMed] [Google Scholar]
- 55.Butterly LF, Chase MP, Pohl H, Fiarman GS. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4(3):343–8. doi: 10.1016/j.cgh.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 56.O'Brien MJ, Winawer SJ, Zauber AG, Gottlieb LS, Sternberg SS, Diaz B, et al. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98(2):371–9. [PubMed] [Google Scholar]
- 57.Shinya H, Wolff WI. Morphology, anatomic distribution and cancer potential of colonic polyps. Ann Surg. 1979;190(6):679–83. doi: 10.1097/00000658-197912000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheiden R, Sand J, Pandin M, Wagener Y, Capesius C. Colorectal high-grade adenomas: incidence, localization and adenoma-adenocarcinoma ratio in a retrospective and comparative population-based study of 225 consecutive cases between 1988 and 1996. Int J Colorectal Dis. 2000;15(1):29–34. doi: 10.1007/s003840050004. [DOI] [PubMed] [Google Scholar]
- 59.Hermanek P KK. Illustrierte Synopsis kolorektaler Tumore. Pharmazeutische Verlagsanstalt, Munich. 1983.
- 60.Hofstad B, Vatn M. Growth rate of colon polyps and cancer. Gastrointest Endosc Clin N Am. 1997;7(3):345–63. [PubMed] [Google Scholar]
- 61.Stein KB, Snyder CF, Barone BB, Yeh HC, Peairs KS, Derr RL, et al. Colorectal Cancer Outcomes, Recurrence, and Complications in Persons With and Without Diabetes Mellitus: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2009. [DOI] [PMC free article] [PubMed]
- 62.Rex DK. Colonoscopy: a review of its yield for cancers and adenomas by indication. Am J Gastroenterol. 1995;90(3):353–65. [PubMed] [Google Scholar]
- 63.Loeve F, Brown ML, Boer R, Ballegooijen M, Oortmarssen GJ, Habbema JD. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000;92(7):557–63. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 64.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849–57. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 65.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 66.Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94(6):1650–7. doi: 10.1111/j.1572-0241.1999.01157.x. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sullivan PW, Ghushchyan V. Mapping the EQ-5D index from the SF-12: US general population preferences in a nationally representative sample. Med Decis Making. 2006;26(4):401–9. doi: 10.1177/0272989X06290496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.