Abstract
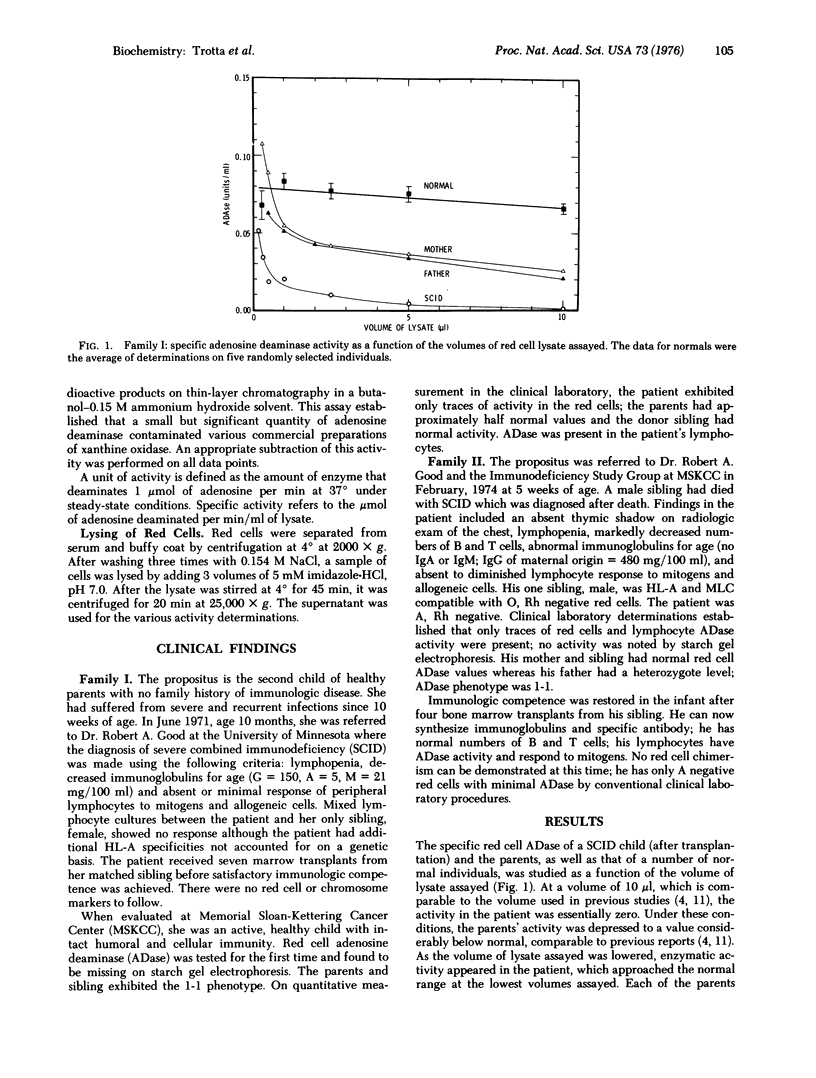
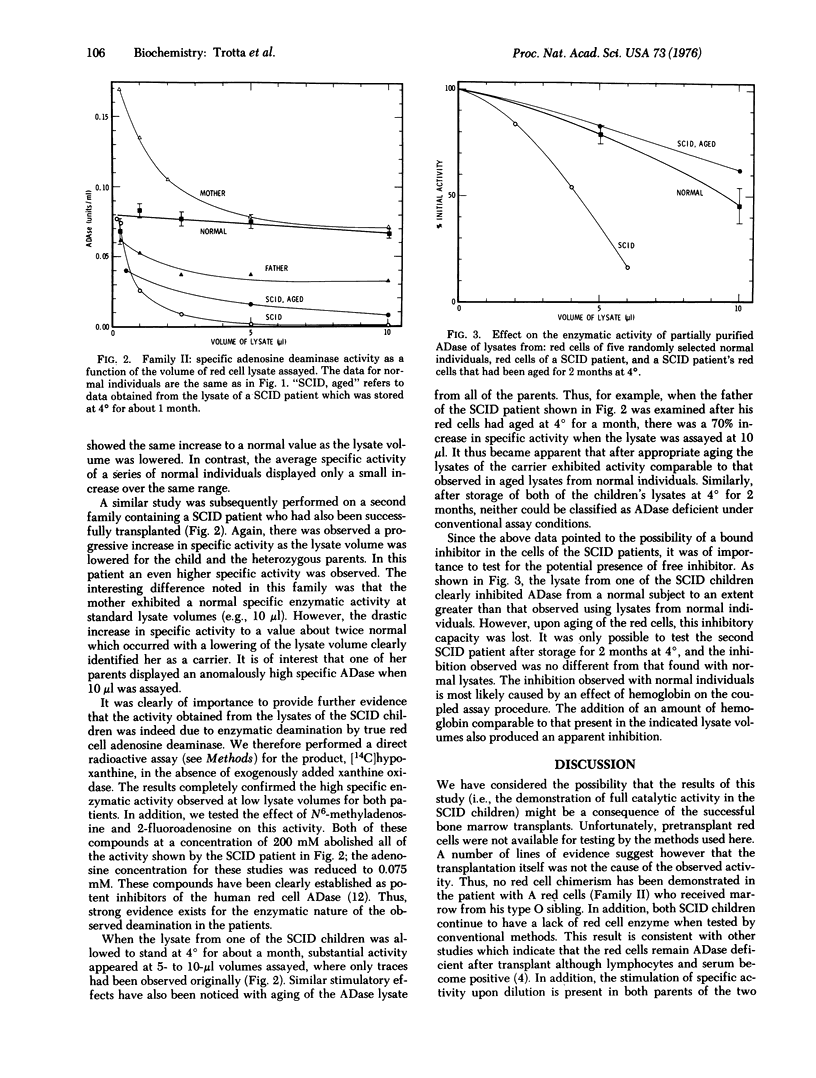
The red cell lysates of two children with severe combined immunodeficiency disease (SCID) exhibited a virtually total absence of adenosine deaminase (adenosine aminohydrolase, EC 3.5.4.4) when standard volumes were assayed. Under these conditions the parents exhibited depressed specific activity except for one mother, whose lysate showed a normal value for activity. Upon storage of the lysate at 4 degrees, a significant amount of activity appeared in one of the SCID children, and the activity of the heterozygous carriers was stimulated. With the use of a sensitive spectrophotometric assay based on conversion of inosine to uric acid, it was shown that the specific enzymatic activity in each of the SCID patients increased progressively as the volume of lysate assayed was lowered. With the smallest amount of lysate this specific activity was in the normal range. Similarly, the specific activity of each of the parents' lysates increased to the level of normal (or, in one case, about twice normal) as smaller volumes were assayed. The activity in the SCID patient was inhibitable by 2-fluoroadenosine and N6-methyladenosine, known competitive inhibitors of human red cell adenosine deaminase. The lysate from the SCID patient was also shown to inhibit adenosine deaminase partially purified from a normal individual. The results are interpreted in terms of a genetically programmed production of an adenosine deaminase inhibitor in at least one variant of the severe combined immunodeficiency disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Sagar S. M., Parks R. E., Jr Adenosine deaminase from human erythrocytes: purification and effects of adenosine analogs. Biochem Pharmacol. 1975 Mar 15;24(6):693–701. doi: 10.1016/0006-2952(75)90245-2. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Scott C. R., Swedberg D. R. Heterogeneity for adenosine deaminase deficiency: Expression of the enzyme in cultured skin fibroblasts and amniotic fluid cells. Am J Hum Genet. 1975 Jan;27(1):46–52. [PMC free article] [PubMed] [Google Scholar]
- Creagan R. P., Tischfield J. A., Nichols E. A., Ruddle F. H. Letter: Autosomal assignment of the gene for the form of adenosine deaminase which is deficient in patients with combined immunodeficiency syndrome. Lancet. 1973 Dec 22;2(7843):1449–1449. doi: 10.1016/s0140-6736(73)92850-x. [DOI] [PubMed] [Google Scholar]
- Dissing J., Knudsen B. Adenosine-deaminase deficiency and combined immunodeficiency syndrome. Lancet. 1972 Dec 16;2(7790):1316–1316. doi: 10.1016/s0140-6736(72)92692-x. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Adenosine deaminase isozymes in human tissues. Ann Hum Genet. 1971 Oct;35(2):207–219. doi: 10.1111/j.1469-1809.1956.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Green H., Chan T. Pyrimidine starvation induced by adenosine in fibroblasts and lymphoid cells: role of adenosine deaminase. Science. 1973 Nov 23;182(4114):836–837. doi: 10.1126/science.182.4114.836. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Levytaka V., Pollara B., Meuwissen H. J. Evidence for control of several different tissue-specific isozymes of adenosine deaminase by a single genetic locus. Nat New Biol. 1973 Dec 19;246(155):200–202. doi: 10.1038/newbio246200a0. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Cook P. J., Harris H. Further data on the adenosine deaminase (ADA) polymprphism and a report of a new phenotype. Ann Hum Genet. 1969 May;32(4):361–367. doi: 10.1111/j.1469-1809.1969.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Ishii K., Green H. Lethality of adenosine for cultured mammalian cells by interference with pyrimidine biosynthesis. J Cell Sci. 1973 Sep;13(2):429–439. doi: 10.1242/jcs.13.2.429. [DOI] [PubMed] [Google Scholar]
- Jenkins T. Red-blood-cell adenosine deaminase deficiency in a "healthy" Kung individual. Lancet. 1973 Sep 29;2(7831):736–736. doi: 10.1016/s0140-6736(73)92568-3. [DOI] [PubMed] [Google Scholar]
- Scott C. R., Chen S. H., Giblett E. R. Detection of the carrier state in combined immunodeficiency disease associated with adenosine deaminase deficiency. J Clin Invest. 1974 Apr;53(4):1194–1196. doi: 10.1172/JCI107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Weyden M. B., Buckley R. H., Kelley W. N. Molecular form of adenosine deaminase in severe combined immunodeficiency. Biochem Biophys Res Commun. 1974 Apr 8;57(3):590–595. doi: 10.1016/0006-291x(74)90587-7. [DOI] [PubMed] [Google Scholar]


