Abstract
Asthma is an inflammatory disorder of the airways, characterized by infiltration of mast cells, eosinophils, and Th2-type CD4+ T cells in the airway wall. Airway epithelium constitutes the first line of interaction with our atmospheric environment. The protective barrier function of the airway epithelium is likely impaired in asthma. Furthermore, recent studies suggest critical immunogenic and immunomodulatory functions of airway epithelium. In particular, a triad of cytokines, including IL-25, IL-33 and TSLP, is produced and released by airway epithelial cells in response to various environmental and microbial stimuli or by cellular damage. These cytokines induce and promote Th2-type airway inflammation and cause remodeling and pathological changes in the airway walls, suggesting their pivotal roles in the pathophysiology of asthma. Thus, the airway epithelium can no longer be regarded as a mere structural barrier, but must be considered an active player in pathogenesis of asthma and other allergic disorders.
Keywords: asthma, epithelium, IL-25, IL-33, TSLP
1. Introduction
Asthma is a syndrome, which is characterized by chronic inflammation of conducting airways, remodeling of airway walls, airway hyperresponsiveness (AHR) to non-specific stimuli, and episodic exacerbations of airway obstruction. Infiltration of mast cells, eosinophils, type 2 CD4+ T cells (Th2 cells) and Th17 cells in the airway walls is observed in patients with asthma, even those with mild severity or without symptoms. Because airway challenge of sensitized patients or experimental animals that are immunized with antigens induces Th2-type inflammation [1], it has been considered that Th2-type immune responses to allergens play a major role in the pathogenesis of asthma. However, not all asthma involves allergic sensitization or presence of serum IgE antibodies. The patients with non-allergic (called intrinsic) asthma share most of the pathologies and, indeed, Th2-type airway inflammation as those patients with IgE antibodies (called extrinsic). Considerable evidence exists that Th2 cells are responsible for many of the features of asthma. For example, Th2 cells and Th2-type cytokines are detected in bronchoalveolar-lavage (BAL) fluids or lung tissues from patients with asthma [1]. Treatment of patients with asthma by targeting Th2 cytokines can be effective, especially in patients with severe and refractory asthma [2]. In addition, IL-17 expression is also observed in patients with asthma, and the severity of AHR correlates with IL-17 expression levels [3, 4]. A novel subset of memory “Th2 cells” that produces IL-17 and exacerbates airway inflammation has also been identified [5]. This evidence suggests the importance of Th2- and perhaps Th17-type T cells in driving airway inflammation and pathological changes in asthma; however, our knowledge is limited concerning how asthma patients develop such dysregulated Th2 and Th17 immune responses in their airways.
Our understanding of the pathogenesis of asthma has undergone great transformation throughout the years. We have moved from an understanding of asthma as a disease primarily of airway caliber to the concept of dysregulation of airway immunity that drives chronic inflammation and structural anomalies. More recently, the immune response in asthmatic airways is considered a result of aberrant functions of the airway epithelium [6]. That is, damage to the airway epithelium or dysregulated activation of airway epithelium by exposure to atmospheric insults, allergens, and viruses and bacteria may play central roles in driving Th2-type immune response and chronic airway inflammation. In this regard, there has been particular interest in new cytokines, including thymic stromal lymphopoietin (TSLP), IL-25 and IL-33. These cytokines are produced by airway epithelial cells as well as other immune and structural cells and are uniformly implicated in Th2-type immune responses. Several genetic studies found strong associations with risk of developing asthma and single nucleotide polymorphisms (SNPs) in genes for these cytokines and their receptors, including IL1RL1/IL18R1, TSLP and IL33 [7–9]. Therefore, the focus of this review is to highlight recent advances in our understanding of the biology and the roles of these epithelial cell-derived cytokines, TSLP, IL-25 and IL-33, in the airway immune response and discuss how abnormalities of airway epithelial functions may impact airway inflammation, airway structure, and inception and progression of asthma.
2. Functions of airway epithelium
The epithelial cell layer that is lining the airway wall provides the first line of protection against environmental pathogens by several mechanisms. Structurally, it acts as a physical barrier, excluding potential invaders from the underlying tissues [10]. It also secretes a number of anti-microbial peptides, such as defensins, cathelicidins and collectins, and reactive oxygen species (ROS), which are directly toxic to pathogens [11]. Anti-microbial peptides also recruit and activate inflammatory cells, such as neutrophils [10, 11]. Airway epithelium also acts as an immunological barrier. Airway epithelial cells recruit and activate immune cells by releasing cytokines and chemokines, such as IL-6, IL-8, eotaxins, and GM-CSF [11]. Indeed, airway epithelial cells are poised to respond rapidly to the microbial organisms and environmental molecules in the airway lumen, due both to proximity to the external environment and by expression of the receptors for them. For example, protease-activated receptors (PARs) on epithelial cells can be triggered by a subset of environmental allergens that contain proteolytic activity, such as fungi, house dust mites, and cockroaches. Likewise, lipolysaccharide (LPS) and other microbial components can activate Toll-like receptors (TLRs), prompting epithelial cells to release inflammatory mediators [12]. Among these mediators, there has been considerable interest in novel epithelial cell-derived cytokines, including IL-25, IL-33 and TSLP. Indeed, upon activation through PARs and TLRs, epithelial cells produce and release these cytokines [13]. Furthermore, IL-33 is released upon damage to the epithelium, such as mechanical injury [14, 15] or UVB light treatment [16], and is considered an “alarmin” that provides a link between tissue damage/injury and immune responses. Thus, airway epithelial cells are no longer considered a mere barrier and may play a pivotal role in regulating the immune responses and inflammation in airway mucosa.
3. Epithelial-derived cytokines that regulate Th2 immunity
IL-25, IL-33 and TSLP are all implicated in promoting Th2-type immune responses. However, these cytokines are quite dissimilar and belong to different cytokine families. To understand better how these cytokines are involved in Th2-type immune responses, it is important to understand the cellular sources as well as biological responses to these cytokines. The information is summarized in Table 1. This section serves to highlight the key characteristics of these cytokines.
Table 1.
Direct Effects of Epithelium-Derived Cytokines on Immune and Structural Cells
| TSLP | IL-25 | IL-33 | |
|---|---|---|---|
| Immune Cells | |||
| Innate lymphoid cells | induce IL-5, IL-13 [50–52] | induce IL-5, IL-13, GM-CSF [50–52, 55] induce proliferation [51, 55] promote blasting [50] |
|
| DC | upregulate CD40, CD80, OX40L [60, 67, 90, 91, 145] drive Th2 polarization [91] drive stronger Th2 polarization when co-primed with antigen [146] induce MDC and TARC [60, 67] |
induce IL-5, IL-13 [76] | induce IL-6, TNFα, IL-1β, CCL17 [147, 148] upregulate CD40, CD80, CD86, OX40L, MHC II [147, 148] |
| Mast cell/mast cell precursors | augment IL-1/ TNFα-induced IL5, IL-6, IL-13, IL-8, GM-CSF, CCL1 [70] | augment SP-induced VEGF [102] activate NF-κB [39] induce IL-5, IL-13, IL-6, IL-8, IL-13, IL-1β, TNFα, MCP-1, eotaxin [47, 149–153] synergistically with ag induce TNFα, IL-6, IL-13, MCP-1, MCP-3, MIP1α [154]enhance survival [151] enhance adhesion [151] cross-activate c-kit receptor [155] |
|
| Eosinophil | enhance survival [156] upregulate ICAM-1, CD18 [156] induce IL-6, IL-8, CXCL1, CCL2 [156] |
enhance survival [157, 158] induce IL-8, MIP-1β, IL-6, MCP-1 [158] upregulate ICAM-1 [157, 158] |
enhance survival [44, 159] induce IL-13, TGFβ, IL-6. IL-8, MCP-1 [44, 45, 99, 103, 160] upregulate ST2, CD11b [99, 159] induce ROS [44] induce degranulation [44] increase adhesion [44, 159] |
| Basophil | polarize to non-classical subtype [161] upregulate ST2 [161] |
upregulate IL17RB post ag challenge [27] induce IL-5, IL-13 [48] |
Induce IL-4, IL-5, IL-6, IL-8, IL-9, IL-13 [47, 48, 160, 162–165] release histamine [164] upregulate CD11b, enhance adhesion [165] induce degranulation [165] enhance migration toward eotaxin [165] |
| Macrophages | induce IL-5, IL-13 [76] | prime naïve to M1 [166] drive M1 to M2 [166] augment LPS-induced IL-6 [139] augment IL-13-mediated killing of P. muris [167] enhance IL-13 polarization to M2 [152] upregulate TLR-4, MD2 and augment LPS-induced responses [168] |
|
| NK | induce IL-4, IL-13 [169] | induce IFNγ [48, 170] | |
| NKT | induce IL-13 [171] | induce IL-13 [77] with α-galcer, induces IL-4, IL-5, IL-13 [172] |
induce IFNγ [170] augment α-galcer -induced IL-4, IL-5, IL-13, TNFα, IFNγ, IL-12 [48] with IL-12 induces IFNγ [48] |
| naïve CD4+ T | induce proliferation [93] suppress differentiation to iTreg [94] |
induce IL-4 [17, 173] | |
| Treg | inhibit IL-10 production, suppression [138] | ||
| Th2 | induce proliferation [174] induce IL-4 [174] activate Stat5 [175] |
induce IL-4, IL-5 [17] | induce IL-5, IL-9, IL- 13 [33, 39, 162, 176] chemoattractant [177] |
| Th9 | augment cytokine-mediated IL-9 [78] | ||
| Structural Cells | |||
| Epithelial cells | induce IL-13 [71] | increase gene transcription of eotaxin, RANTES, MUC5ac, TSLP [17, 178] | induce IL-8 [141] increase gene transcription of TSLP, TSLPR [179] |
| Fibroblast/fibrocytes | produce collagen [106] | induce eotaxin [180] | |
| Airway smooth muscle cells | induce [Ca2+]i [140] induce eotaxin, IL-8, IL-6 [181] |
||
| Endothelial cells | increase expressed of VEGF and VEGFR [101] increase proliferation [101] |
Induce IL-8, IL-6, MCP-1 [141] | |
| Progenitor Cells | |||
| BM Lin−Sca1+c-Kit− CD25+ | with IL-2 or IL-7, induce IL-5 and IL-13 [56] | ||
| Bone marrow progenitors | drive to eosinophils [99] | ||
| CD34+ cells | augment IL-33-induced IL-5, IL-13, GM-CSF [107] | induce mast cell maturation [107] induce IL-5, IL-13, GM-CSF [107] |
|
3.1. IL-25
IL-25 is a member of the IL-17 family. Most of Th17-family members are known to promote neutrophilic inflammation. In contrast, IL-17E, also known as IL-25, does not appear to play a role in neutrophilic responses, but rather is known to promote Th2 responses or eosinophilic inflammation [17, 18]. Indeed, IL-25 may serve as a counter regulator for other IL-17 family members. Increased levels of IL-25 inhibit Th17 development and blockade of IL-25 enhances IL-17 production [19, 20]. IL-25 signals through a heterodimeric complex of IL-17RA and IL-17RB [21] and induces TRAF6-mediated activation of NF-κB [22]. It also has been shown to activate NFATc1 and JunB [17].
IL-25 can be produced not only by epithelial cells, but also by Th2 cells, mast cells, macrophages, eosinophils and basophils [17, 18, 23–26]. The IL-25 receptor, IL-17RB, is expressed on basophils [27], NKT cells, and recently identified type 2 innate lymphoid cells ([28], and more details below); no IL-17RB has been identified on CD4+ T cells.
3.2. IL-33
IL-33 is a member of the IL-1 cytokine family, which includes IL-1β and IL-18. IL-33 was originally discovered as a nuclear factor of high endothelial venules (NF-HEV), [29] and is constitutively expressed by some tissue structural cells, including epithelial cells. Each of the IL-1 family members likely drives different immune responses. IL-33 is implicated in Th2-type immunity, and IL-1β and IL-18 are associated with Th17- and Th1-type immune responses, respectively [30]. The IL-33 receptor is dimeric, consisting of an IL-1R-like subunit (IL-1RL1, also known as ST2) that associates with the IL-1R accessory protein (IL-1RAcP). Similarly to other IL-1 family cytokines, IL-33 signals through MyD88, IRAK, JNK, p38, p44/42, JAK2 [31] and activates NF-kB. Importantly, ST2 was first identified as a marker of Th2 cells even before its ligand was identified [32, 33].
While IL-33 belongs to the IL-1 family, the regulatory mechanisms for production and release of IL-33 are distinct from those for IL-1β and IL-18. IL-33 is constitutively expressed and stored as a 30 kDa full-length precursor form. Proteolytic cleavage of the proforms of IL-1β and IL-18 by caspase-1 activates these cytokines and allows them to be secreted extracellularly. In contrast, full-length IL-33 has immunological activities and cleavage of IL-33 by caspase-1 inactivates the cytokine [34–36]. Therefore, it has been proposed that full-length IL-33 is released from nuclei of the cells by cellular injury or necrosis and triggers immune responses [37]; cellular apoptosis that initiates caspase cleavage of IL-33 may inactivate the protein and prevent unnecessary immune responses. Nonetheless, overexpression of full-length IL-33 recently resulted in secretion of a biologically active 18 kDa cleaved product that mimics the pathologies of IL-33-mediated inflammation [38]. A splice variant of IL-33 that lacks the proposed caspase-1 cleavage site activated a human mast cell line (HMC-1) and a mouse macrophage line (Raw 26.7) in an ST2-dependent manner [35]. Therefore, more studies are necessary to elucidate the structure and functional relationships of IL-33 and the mechanisms involved in production and extracellular release of “active” IL-33 during immune responses in vivo.
Besides epithelial cells [39], IL-33 is produced by other structural cells, such as endothelial cells, bronchial smooth muscle cells, fibroblasts, keratinocytes and adipocytes [39–41]. Immune cells, such as dendritic cells (DCs), macrophages, and mast cells may also produce IL-33 [39, 42]. IL-33 receptor, ST2, is expressed on a wide variety of cell types, including vascular endothelial cells, epithelial cells, eosinophils, mast cells, B cells, basophils, NK, NKT, and fully differentiated Th2 cells [43–49], implicating the potential roles of IL-33 in asthma as well as many other inflammatory disorders. In addition, novel innate lymphoid cells that produce large quantities of Th2-type cytokines in response to IL-25 or IL-33 have been identified recently in gastrointestinal organs [50–53]. Specifically, intraperitoneal injection of IL-25 or IL-33 into mice led to the expansion of cells that produce IL-4 and/or IL-13 in gut-associated lymphoid tissue (GALT) [53] and in mesenteric lymph nodes (LNs) and spleen [51]. Likewise, an IL-33-responsive cell type with a lymphoid morphology was identified in fat-associated lymphoid clusters (FALCs) in the mesentery layer [50]. These innate lymphoid cells lack conventional lineage markers but often express IL-7Ra, Sca-1, c-Kit, CD44, CD25, Thy1.2, and ICOS. Importantly, similar innate lymphoid cells have also been identified in the lungs and bone marrow of naïve mice and those mice infected with virus or exposed to allergens [54–58], suggesting roles for these innate cells in Th2-type airway inflammation and asthma.
3.3. TSLP
TSLP is a type I four α-helical bundle short-chain cytokine and is part of the IL-2 family [59, 60]. It signals through a heterodimeric receptor complex consisting of the IL-7Ra chain and a γc –like chain specific for TSLP (TSLPR) [61]. Signaling occurs through Stat5 phosphorylation [62]. However, requirements for JAK, specifically JAK1 and JAK2, are controversial [62, 63]. The primary source for TSLP is epithelial cells, but basophils, mast cells and keratinocytes have also been shown to produce TSLP [64–69]. TSLPR is found on DCs, mast cells, monocytes, macrophages, B cells, T cells, basophils and eosinophils [60, 69, 70] as well as on epithelial cells themselves [71].
4. Roles of IL-25, IL-33 and TSLP in Th2-type airway inflammation and asthma
The roles for IL-25, IL-33 and TSLP in Th2-type airway inflammation have been studied in a variety of mouse models, including systemic and airway administration of these cytokines, systemic or topical expression of cytokine genes, and Th2-type immune responses models with allergens, model antigens [e.g. ovalbumin (OVA)], and helminthes infection. These studies consistently find potent activities of these cytokines to mediate Th2 type immune responses. For example, transgenic overexpression of IL-25 [18, 72–74] induces Th2 cytokine expression in tissues, airway eosinophilia, increased serum levels of IgE antibodies and Th2 cytokines, mucus overproduction, goblet cell hyperplasia, airway wall thickening, and AHR; these inflammatory and pathological changes are consistent with human asthma. Similarly, systemic administration of exogenous IL-25 induces AHR, inflammation and mucus overproduction, which are dependent on IL-13, IL-4Rα or STAT6 [75]. Interestingly, IL-25-induced airway pathological changes are apparent in mice deficient in Rag1 gene, suggesting that innate immunity, rather than T cells or B cells, mediates the responses [18, 76]. Furthermore, a novel subset of natural killer T (NKT) cells that expresses the IL-17RB and predominantly produces IL-13 and chemokines upon stimulation with IL-25 was implicated in AHR induced by airway administration of lipid antigens [77]. In an OVA-induced Th2-type airway inflammation model, mice injected with soluble IL-17RB protein or anti-IL-25 antibody or those deficient in the IL-25 gene showed reduced production of IgE antibody and were protected from development of Th2-type airway inflammation [78–81]. These findings suggest that IL-25 is involved in Th2-type airway inflammation through both innate and adaptive immunity.
Similar biological activities are observed with IL-33. When IL-33 is administered systemically, mice develop airway eosinophilia as well as increased production of Th2-type cytokines and mucus in the lungs [39]; again, these pathological changes are retained in Rag2-deficient mice [47]. Indeed, airway administration of IL-33 induced marked increases in eosinophil number and IL-5 and IL-13 levels in the airways, which are mediated by novel type 2 innate lymphoid cells as described earlier [55]. Furthermore, in vivo airway administration of ubiquitous allergens, such as Alternaria alternata, induced hallmark features of Th2-type airway inflammation that is dependent on IL-33 and the novel type 2 innate lymphoid cells [55]. These findings suggest that airway inflammation induced by IL-33 is at least in part mediated by innate immunity, in particular the novel lymphoid cells. In addition, lack of the ST2 (i.e. IL-33 receptor) gene or administration of anti-ST2 Ab or ST2 fusion protein attenuated production of Th2 cytokines and eosinophil infiltration in various mouse models of airway inflammation [32, 82–84]; these studies used adoptive transfer of differentiated Th2 cells or helminthes or fungal infection models. On the other hand, in IL-33-deficient mice that were sensitized and challenged with OVA antigen, IL-33 was necessary for airway eosinophilia and AHR but not for production of IgE antibodies and Th2 cytokines, suggesting that IL-33 may be involved only in the early and innate Th2 response but may not be necessary for subsequent adaptive immune responses [85]. Similarly, in the OVA model, airway eosinophilia and Th2 cytokines were not affected or attenuated only during a narrow window of time in ST2-deficient mice [86, 87]. Altogether, IL-33 clearly mediates Th2-type airway inflammation by activating innate immune cells, but the contribution of IL-33 in antigen-specific Th2-type immunity may be dependent on the model.
Transgenic overexpression of TSLP in the airway also induces asthma-like pathologic changes in mice, consisting of airway eosinophilia, increases in serum IgE and BAL Th2-type cytokines, AHR, increased mucus production, and thickening of airway epithelium [88]. However, unlike IL-25 and IL-33, the ability of TSLP to induce innate Th2-type immunity may be limited. For example, overexpression of TSLP alone in the airways caused a weak innate response and was insufficient for development of robust airway inflammation [89]. Both overexpression of TSLP and airway administration of OVA antigen were required to induce disease development [89], suggesting that TSLP induces predisposition toward the development of aberrant responses against innocuous environmental antigens. Other evidence also suggests a role for TSLP as a link between innate and adaptive Th2-type immune responses. Airway eosinophilia and airway remodeling in an OVA-induced allergic airway inflammation model were attenuated in mice lacking the TSLPR [88, 90]. TSLP primes antigen-presenting cells, such as DCs, as treatment of mice with anti-TSLPR or soluble TSLPR prior to OVA sensitization inhibited DC maturation (expression of CD40, CD80, CD86, MHC II), decreased the number of OVA-bearing DCs in the draining lymph nodes and prevented Th2-type sensitization to the antigen [91, 92]. TSLP may also directly act on CD4+ T cells. Allergic airway inflammation was restored in TSLPR KO mice when they were reconstituted with wild-type CD4+ T cells [62, 90, 93]. TSLP inhibited development of induced regulatory T cells (iTregs) from naïve CD4+ T-cells [94], suggesting that TSLP may break immunological tolerance. Likewise, mice sensitized with OVA plus TSLP had fewer OVA-specific iTregs in the draining lymph nodes than did mice sensitized with OVA alone [94]. Altogether these findings suggest that TSLP plays pivotal roles in Th2-type immune responses and asthma-like airway inflammation likely through mechanisms involving the interface between the innate and adaptive immunity and/or adaptive immunity itself.
5. Effects of IL-25, IL-33 and TSLP on airway epithelium
While IL-25, IL-33 and TSLP are produced by airway epithelial cells, these cytokines affect the functions and integrity of airway epithelium itself either directly or indirectly through activation of airway immune cells. Thus, these cytokines may create a feedback loop between airway epithelial cells and immune cells in respiratory mucosa, which is likely important in the pathophysiology of asthma. Sections 5.1 – 5.3 will highlight current concepts and new discoveries about how these feedback loops drive and sustain Th2-type immunity and pathology in the airways.
5.1. Barrier function
In asthma, impaired epithelial barrier function may render the airways vulnerable to infection or colonization with microorganisms and increase epithelial permeability to environmental molecules, resulting in priming of DCs and development and/or exacerbation of Th2-type immune responses [6]. Physical damage to the epithelial cells as well as markers of stress and injury are observed in patients with asthma. Epithelial barrier function is provided by the structure of the epithelial cells and by the tight junctions between epithelial cells. Furthermore, Goblet cells and Clara cells secrete mucins and surfactants, respectively, that provide a surface coating that is able to both trap and carry away inhaled particles. Epithelial cells from patients with asthma are less efficient in formation of tight junctions than cells from normal individuals [95, 96]. The tight junction formation of airway epithelial cells is further reduced by exposure to environmental insults, such as tobacco smoke [96]. When skin barrier function is disrupted by a mutation of Notch in mice, the levels of skin tissue TSLP are increased [97]. Exposure of airway epithelium to a cysteine protease, papain, induces ROS generation by DCs and epithelial cells, resulting in oxidized phospholipids that stimulate epithelial cells to release TSLP through the TLR4-TRIF pathway [98]. These finding suggest that defective barrier or increased inflammatory mediators trigger epithelial cells to produce TSLP and potentially other cytokines, resulting priming of the mucosal tissue to develop Th2-type immunity.
5.2. Epithelial remodeling
Thickening of the basement membrane and airway walls and altered mucus production by epithelial cells are all hallmarks of chronic asthma and lead to narrowing of the airways. Mucin, produced in excess by epithelial cells (i.e. goblet cell metaplasia), combined with DNA and eosinophil proteins, creates viscous mucus that can plug the airways. These are several pieces of evidence to suggest that IL-33 and TSLP are involved in these epithelial remodeling and pathologic changes. Thickening of the basal lamina, part of the basement membrane, can be induced by subjecting the epithelium to chronic injury or stress, to infection and inflammation, and to profibrotic cytokines, such as IL-13, VEGF, and TGF-β. IL-33 may play a role in basement membrane thickening by inducing IL-13 and TGF-β production by infiltrating eosinophils [99]. Furthermore, gut epithelial cells in response to pathogens produce TSLP; this initial TSLP drives IL-13 production by immune cells and IL-13 then further enhances TSLP expression [100], suggesting a positive feedback between TSLP and IL-13 in epithelium. IL-33 also enhances substance P-induced release of VEGF by mast cells [101, 102]. In a mouse model of chronic airway inflammation, anti-ST2 when combined with CpG attenuated mucus cell metaplasia [83], suggesting potential therapeutic benefit of blocking the IL-33 pathway.
The pathological changes in asthma also involve the airway walls, smooth muscle tissues (ASM) and vascular structure. These tissue cells can be directly activated by epithelial cell-derived cytokines or indirectly by the cytokines (e.g. IL-13) that are produced by immune cells in response to these cytokines. For example, fibroblasts in the airway walls may contribute to remodeling by secreting matrix proteins, such as collagens and fibronectins, in response to IL-13 or TGF-β mediated by IL-25, IL-33 or TSLP. IL-25 directly induces VEGF and VEGFR expression by vascular endothelial cells [101]. Subcutaneous administration of IL-33 induces cutaneous fibrosis in a mechanism that appears to involve the release of IL-13 by IL-33-stimulated eosinophils [103]; anti-IL-5 treatment and subsequent reduction of eosinophils numbers in airway mucosa markedly reduced deposition of collagen and other matrix proteins in human asthma [104, 105]. In atopic dermatitis, TSLP causes fibroblasts to produce collagen [106]. Similarly, IL-33 drives maturation of mast cells precursors [107], and mast cell-derived mediators may act on airway smooth muscle cells to induce airway pathology including remodeling [108].
5.3. Viral-induced airway epithelial changes
Early viral infections, in particular respiratory viral infections in children, have been linked to subsequent development of asthma. In addition, at least half of all asthma exacerbations in adults and children are the result of upper respiratory tract infections. Convincing evidence suggests that viral infection, viral ssRNA and viral dsRNA induce IL-25, TSLP, and IL-33 release by epithelial cells in vitro [109–112]. These viral-mediated epithelial cytokines may contribute to Th2-type airway inflammation in the airways in vivo. For example, infection of mice with influenza virus induces IL-33 release from alveolar macrophages, resulting in production of IL-13 by type 2 innate lymphoid cells and induction of AHR [57]. A recent series of studies by Siegle et al have attempted to utilize airway epithelial changes in response to viral infection to create a “natural” model for inducing chronic asthma in mice. Mice subjected to frequent inhalation exposure to low levels of aerosolized OVA developed tolerance to OVA. In contrast, if the mice were first infected with pneumonia virus of mice (PVM), mice developed features of chronic asthma and subsequent challenge with OVA elicited asthma-like exacerabations [113]. Moreover, PVM infection enhanced airway IL-25 production, and antibody blockade of IL-25 prior to antigen sensitization attenuated airway remodeling and eosinophilia [113], suggesting a critical role for IL-25 in priming the airways to antigen sensitization in this model. Furthermore, IL-33-induced lung inflammation is inhibited by the presence of iNKT cells through their production of IFN-γ [114]. Severe RSV infection in infants reduces the number of circulating NK cells, and is correlated with increased incidence of asthma later in life [115]. Therefore, respiratory viral infection in particular early in life may mediate Th2-type immunity in the airways by several mechanisms by modulating the IL-25 and IL-33 pathways.
While production of IL-25 and IL-33 by airway epithelium in response to viral infection may be detrimental to the host by promoting Th2-type immune responses, these cytokines can also be protective against extensive lung damage and may facilitate the healing process in certain settings. For example, IL-25- and IL-33-responsive innate type 2 immune cells accumulated in the lungs after infection with influenza virus [116]. Depletion of these innate lymphoid cells resulted in loss of airway epithelial integrity, diminished lung function and impaired airway remodeling; these defects were restored by administration of amphiregulin, a product of innate lymphoid cells. These results suggest roles for Th2-type innate lymphoid cells in restoring airway epithelial integrity and tissue homeostasis after infection with influenza virus. Thus, epithelial cell-derived cytokines may be a “two-edged sword” during airway infection with respiratory viruses.
6. Links to human asthma
There is accumulating evidence available to suggest that the IL-25, IL-33 and TSLP pathways are associated with human asthma. For example, patients with asthma demonstrate increased IL-25 and IL-17RB mRNA transcription in their lung biopsy specimens [26]; IL-25+ cells are evident in the submucosal tissues [117]. When patients are challenged with inhaled allergens, the number of IL-25+ and IL-25R+ cells in bronchial biopsies increases, and the increase in IL-25 expression correlates with severity of bronchial constriction [118]. In addition, basophils from patients with seasonal allergic rhinitis show increased surface IL-17RB after allergen challenge [27]. Similarly, serum from asthmatic patients contains increased levels of soluble ST2 as compared to control individuals and those levels are enhanced during asthma exacerbation [119]. In patients with chronic rhinosinusitis, airway epithelial cells from patients who are resistant to treatment expressed increased levels of IL-33 mRNA [120] as compared to those responsive to treatments. IL-33 was increased in epithelial cells from bronchial biopsies and in BAL fluids from patients with moderate to severe asthma as compared to normal controls [121]. Expression of IL-33 by airway epithelial cells is also increased in patients with allergic rhinitis [122].
A strong connection between TSLP and human allergic diseases was originally made with patients with atopic dermatitis [67]. TSLP was also overexpressed in the skin of individuals with Netherton syndrome, a severe skin disease characterized by atopic dermatitis-like lesions that result from mutations in the serine peptidase inhibitor Kazal-type 5 (SPINK5) gene [64]. Likewise, increased evidence is available regarding the roles for TSLP in human asthma. Epithelial cells from asthmatic subjects constitutively express more TSLP than do healthy subjects [71], and the levels of TSLP in the airways of asthmatic individuals are correlated with disease severity [123, 124]. Bronchial epithelial cells from individuals with asthma release more TSLP upon exposure to dsDNA than do those from healthy controls [125]. Human airway epithelial cells produce TSLP in response to environmental factors, such as fungal proteases and respiratory viruses [110, 112, 126] and the response is significantly enhanced in the presence of inflammatory cytokines, such IL-4, IL-13, IL-25, TNFα, TGFβ, and IFNβ [110, 127].
Genetics links have been made between asthma and allergic diseases and polymorphisms in the genes encoding IL-25, IL-33 and TSLP and their receptors. For example, polymorphisms in the IL33 gene are associated with Japanese patients with allergic rhinitis [128]. SNPs in the distal promoter of the IL1RL1 (or ST2) gene are associated with atopic dermatitis and children with asthma presenting acute symptoms [129, 130]. Genetic studies also support a critical role for TSLP in asthma. Several SNPs at the TSLP genomic locus were associated with increased asthma susceptibility or protection in multiple ethnic backgrounds [131–133]. One such SNP present in the genomic TSLP locus creates a novel AP-1 transcription factor binding site that could potentially lead to increased TSLP transcription [131]. SNPs at IL17RB (i.e. IL-25 receptor gene) also have been associated with asthma [134]. Importantly, IL33, IL1RL1 (i.e. ST2) and TSLP genes have been implicated in asthma in recent large-scale genome-wide association scan studies [8, 9].
7. Conclusion and future directions
Current evidence suggests potent activities of IL-25, IL-33, and TSLP in promoting Th2-type immune responses by affecting various cell types involved in innate and adaptive immune responses such as DCs, CD4+ T cells, innate type 2 lymphoid cells and NKT cells (Figure 1). Th2-type cytokines produced by these immune cells may induce eosinophilic inflammation of the airways and cause structural changes in airway epithelium and other tissue components, consistent with the pathology of asthma. Human clinical studies and genetic studies also suggest dysregulation of the IL-25, IL-33 and TSLP pathways in patients with asthma. Thus, by producing these cytokines, airway epithelium may play central roles in pathophysiology of asthma and allergic disease. In addition, the roles for TSLP and IL-25 in inhibiting iTreg functions underscore the importance of airway epithelium in breaking tolerance in asthma as well as a variety of other human diseases [94, 135–138]. Furthermore, epithelial cell-derived cytokines, which are released in response to environmental allergens or microbes, may act to augment continued activation of airway epithelial cells themselves in an autrocrine fashion [139–141]. Such self-perpetuating mechanisms mediated by cytokines in the airway epithelium may explain the chronic and persistent inflammation that is observed in patients with asthma.
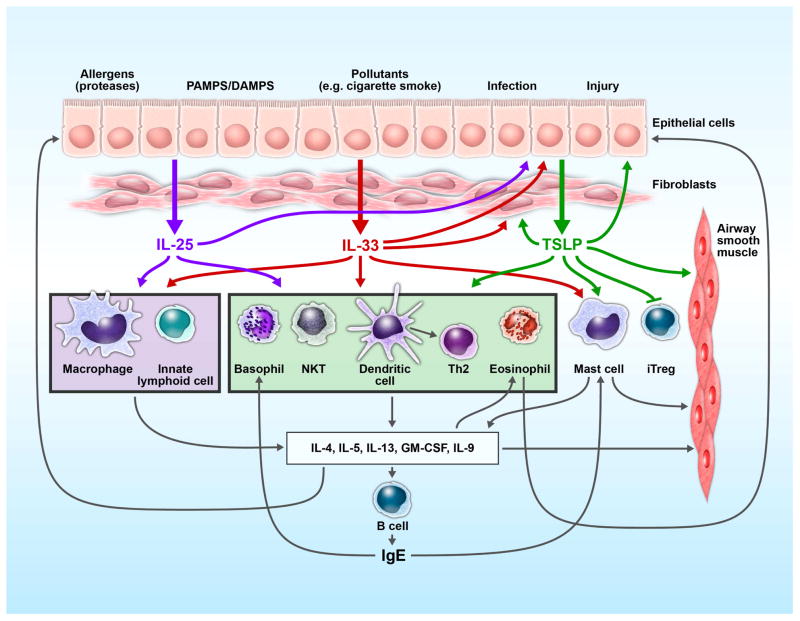
Figure 1.
Interplay between epithelium, immune cells and epithelium-derived cytokines in asthma. Environmental insults (allergens/proteases, microbes, viruses, etc.) stimulate the release of IL-25, IL-33 and TSLP from airway epithelial cells. This triad of cytokines mediates both overlapping and unique effects on immune and structural cells in the airways. They activate DCs, basophils, mast cells, eosinophils, differentiated Th2 cells and NKT cells and drive Th2-type inflammation. Additionally, TSLP induces DC maturation (i.e. upregulation of MHC II and co-stimulatory molecules) capable of polarizing naïve CD4+ cells to the Th2-type. IL-25 and IL-33 induce Th2 cytokine production by innate lymphoid cells and drive macrophages to release IL-13. Th2 cytokines elicit multiple effects on airway tissues, including remodeling, fibrosis and proliferation of smooth muscle cells. Epithelium-derived cytokines, IL-25, IL-33 and TSLP, and the products of Th2-type immune responses provide a positive feedback to airway epithelial cells by enhancing production of mucus and matrix proteins and by stimulating production of cytokines, such as TSLP. Thus, the airway epithelium and its cytokines may play a central role in controlling the immune network within the airway mucosa in asthma.
Several questions still need to be addressed to fully understand the roles of epithelial-derived cytokines in asthma, however. First, what are the immunological triggers that cause the dysregulated activation of airway epithelium and production of epithelium-derived cytokines? One potential break point could be viral infection. The link between viral infection and development and/or exacerbation of asthma is strong in human studies [6]. In an intriguing mouse model mimicking chronic asthma in children, early exposure to respiratory virus induced acute and chronic inflammatory changes mimicking human asthma [19]; airway production of IL-25 is likely involved in this process. Although we do not fully understand how respiratory viral infection in early life affects the functions of airway epithelial cells later in life, these studies provide an excellent starting point for further research into the mechanisms of virus-induced asthma and development of strategies to prevent the inflammatory pathways.
Second, could these epithelial cytokines be effective and safe therapeutic targets for human diseases? The potent abilities of these cytokines to activate and polarize the immune responses to the Th2 pathway suggest that targeting these cytokines may be more efficacious than targeting downstream cytokines, such as IL-5 and IL-13. Recent demonstration of synergistic effects when suboptimal doses of ST2 antibody and CpG-oligodeoxynucleotide (a TLR9 agonist that skews responses toward Th1) were administered in a mouse model of chronic asthma suggests such an approach is promising [83]. The presence of innate immune receptors on the epithelium suggests that targeting these receptors and their ligands has potential both in preventing disease and in ameliorating exacerbations of the established disease. On the other hand, these epithelial cytokines may be critical to maintain immune homeostasis in respiratory mucosa. For example, IL-25 may counterbalance the proinflammatory effects of IL-17 [142]. The epithelium and its cytokines may exist in a delicate balance and caution will need to taken when manipulating these cytokines.
Finally, we need to better understand the immunobiology of IL-25, IL-33, and TSLP and their roles in diseases. While all three cytokines are released by epithelial cells in response to both exogenous and endogenous signals, distinct differences in regulation have been observed. For examples, several triggers are common for all three (e.g. viral infection [109–112]); however, several others uniquely activate release of only one cytokine (e.g. oxidized phospholids and TSLP [98]). Timing and magnitude of release after stimulation also may vary [55]. Accordingly, several key questions need to be addressed in this regard. For instance, in the context of allergic inflammation, what are the relationships among these cytokines? IL-33 and IL-25 are distinguished by their unique activities to activate innate type 2 immune responses. However, as discussed earlier, it is not totally clear to what extent and in which situations IL-25/IL-33 are involved in the generation of Th2-type immune responses. Is IL-25/IL-33 activity more important early in a Th2 response? Is TSLP important later? Further studies are necessary to define the hierarchy and interaction of these cytokines during Th2-type immune responses and inflammation. Questions also remain how the activities of these cytokines, in particular IL-33, are generated. In most cells, IL-33 is found constitutively in the nucleus as a full-length molecule. As described earlier, IL-33 is considered an “alarmin” that is released by cellular injury or tissue damage. On the other hand, recent studies suggest that IL-33 is released by airway epithelial cells in a non-cytotoxic manner [143, 144]. Thus, it is unclear what are the important physiologic triggers to induce extracellular release of biologically active IL-33 and, if so, what molecular and cellular mechanisms are involved. Finally, what is the fate of these cytokines after they are released into extracellular space? Are soluble receptors involved in regulation of IL-33 similarly to other IL-1-family cytokines? The answers to these questions will be important to fully understand the physiologic and pathologic situations in which these cytokines are engaged. In closing, during the past several years, considerable amounts of information have been generated regarding the roles for IL-25, IL-33 and TSLP in Th2-type immunity. We are now close to understanding the complex mechanisms of chronic Th2-type airway inflammation in asthma and related disorders and to develop novel strategies to treat patients with these diseases.
Highlights.
Airway epithelium plays barrier and immune regulatory roles in asthma
Epithelial-derived cytokines, IL-25, IL-33 and TSLP, promote type 2 immune responses
They may create a positive feedback loop promoting airway inflammation and remodeling
Therapy targeting these cytokines may be effective to prevent or attenuate asthma
Acknowledgments
This work was supported by NIH grants, AI49235 and AI71106, Mayo Graduate School and Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Corren J. Cytokine inhibition in severe asthma: current knowledge and future directions. Curr Opin Pulm Med. 2011;17:29–33. doi: 10.1097/MCP.0b013e3283413105. [DOI] [PubMed] [Google Scholar]
- 3.Chakir J, Shannon J, Molet S, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 4.Molet S, Hamid Q, Davoine F, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 5.Wang YH, Voo KS, Liu B, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 7.Hunninghake GM, Soto-Quiros ME, Avila L, et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010;65:1566–1575. doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torgerson DG, Ampleford EJ, Chiu GY, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413–435. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 13.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickel H, Gambichler T, Kamphowe J, Altmeyer P, Skrygan M. Standardized tape stripping prior to patch testing induces upregulation of Hsp90, Hsp70, IL-33, TNF-alpha and IL-8/CXCL8 mRNA: new insights into the involvement of ‘alarmins’. Contact Dermatitis. 2010;63:215–222. doi: 10.1111/j.1600-0536.2010.01769.x. [DOI] [PubMed] [Google Scholar]
- 15.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126:976–984. 984 e971–975. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne SN, Beaugie C, O’Sullivan C, Leighton S, Halliday GM. The immune-modulating cytokine and endogenous Alarmin interleukin-33 is upregulated in skin exposed to inflammatory UVB radiation. Am J Pathol. 2011;179:211–222. doi: 10.1016/j.ajpath.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angkasekwinai P, Park H, Wang YH, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 19.Siegle JS, Hansbro N, Dong C, Angkasekwinai P, Foster PS, Kumar RK. Blocking induction of T helper type 2 responses prevents development of disease in a model of childhood asthma. Clin Exp Immunol. 2011;165:19–28. doi: 10.1111/j.1365-2249.2011.04392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaph C, Du Y, Saenz SA, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rickel EA, Siegel LA, Yoon BR, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 22.Maezawa Y, Nakajima H, Suzuki K, et al. Involvement of TNF receptor-associated factor 6 in IL-25 receptor signaling. J Immunol. 2006;176:1013–1018. doi: 10.4049/jimmunol.176.2.1013. [DOI] [PubMed] [Google Scholar]
- 23.Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J Immunol. 2009;183:5705–5715. doi: 10.4049/jimmunol.0901666. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K, Nakajima H, Suzuki K, et al. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 25.Kang CM, Jang AS, Ahn MH, et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005;33:290–296. doi: 10.1165/rcmb.2005-0003OC. [DOI] [PubMed] [Google Scholar]
- 26.Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Mobini R, Fang Y, et al. Allergen challenge of peripheral blood mononuclear cells from patients with seasonal allergic rhinitis increases IL-17RB, which regulates basophil apoptosis and degranulation. Clin Exp Allergy. 2010;40:1194–1202. doi: 10.1111/j.1365-2222.2010.03542.x. [DOI] [PubMed] [Google Scholar]
- 28.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Baekkevold ES, Roussigne M, Yamanaka T, et al. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L, Wei G, Zhu J, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funakoshi-Tago M, Tago K, Sato Y, Tominaga S, Kasahara T. JAK2 is an important signal transducer in IL-33-induced NF-kappaB activation. Cell Signal. 2011;23:363–370. doi: 10.1016/j.cellsig.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Lohning M, Stroehmann A, Coyle AJ, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu D, Chan WL, Leung BP, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong J, Bae S, Jhun H, et al. Identification of constitutively active interleukin 33 (IL-33) splice variant. J Biol Chem. 2011;286:20078–20086. doi: 10.1074/jbc.M111.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamkanfi M, Dixit VM. IL-33 raises alarm. Immunity. 2009;31:5–7. doi: 10.1016/j.immuni.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Zhiguang X, Wei C, Steven R, et al. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol Lett. 2010;131:159–165. doi: 10.1016/j.imlet.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384:105–109. doi: 10.1016/j.bbrc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 42.Nile CJ, Barksby E, Jitprasertwong P, Preshaw PM, Taylor JJ. Expression and regulation of interleukin-33 in human monocytes. Immunology. 2010;130:172–180. doi: 10.1111/j.1365-2567.2009.03221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoki S, Hayakawa M, Ozaki H, et al. ST2 gene expression is proliferation-dependent and its ligand, IL-33, induces inflammatory reaction in endothelial cells. Mol Cell Biochem. 2010;335:75–81. doi: 10.1007/s11010-009-0244-9. [DOI] [PubMed] [Google Scholar]
- 44.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow JY, Wong CK, Cheung PF, Lam CW. Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation. Cell Mol Immunol. 2010;7:26–34. doi: 10.1038/cmi.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, Liew FY. IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol. 2011;186:2584–2591. doi: 10.4049/jimmunol.1002103. [DOI] [PubMed] [Google Scholar]
- 47.Kondo Y, Yoshimoto T, Yasuda K, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 48.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 49.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 51.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saenz SA, Siracusa MC, Perrigoue JG, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barlow JL, Bellosi A, Hardman CS, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. e194. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 55.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-Responsive Lineage-CD25+CD44hi Lymphoid Cells Mediate Innate Type 2 Immunity and Allergic Inflammation in the Lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage(−)Sca1+c-Kit(−)CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol. 2011;187:5795–5804. doi: 10.4049/jimmunol.1102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang YJ, Kim HY, Albacker LA, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HY, Chang YJ, Subramanian S, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227. e216. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leonard WJ. TSLP: finally in the limelight. Nat Immunol. 2002;3:605–607. doi: 10.1038/ni0702-605. [DOI] [PubMed] [Google Scholar]
- 60.Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 61.Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 62.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–5977. [PubMed] [Google Scholar]
- 63.Rochman Y, Kashyap M, Robinson GW, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Briot A, Deraison C, Lacroix M, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le TA, Takai T, Vu AT, et al. Flagellin induces the expression of thymic stromal lymphopoietin in human keratinocytes via toll-like receptor 5. Int Arch Allergy Immunol. 2011;155:31–37. doi: 10.1159/000318679. [DOI] [PubMed] [Google Scholar]
- 66.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 68.Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vu AT, Baba T, Chen X, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol. 2010;126:985–993. 993 e981–983. doi: 10.1016/j.jaci.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Semlali A, Jacques E, Koussih L, Gounni AS, Chakir J. Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J Allergy Clin Immunol. 2010;125:844–850. doi: 10.1016/j.jaci.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 72.Hurst SD, Muchamuel T, Gorman DM, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 73.Kim MR, Manoukian R, Yeh R, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 74.Pan G, French D, Mao W, et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 75.Sharkhuu T, Matthaei KI, Forbes E, et al. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy. 2006;36:1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- 76.Claudio E, Sonder SU, Saret S, et al. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terashima A, Watarai H, Inoue S, et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ballantyne SJ, Barlow JL, Jolin HE, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 80.Mueller C, Keeler A, Braag S, Menz T, Tang Q, Flotte TR. Modulation of exaggerated-IgE allergic responses by gene transfer-mediated antagonism of IL-13 and IL-17e. Mol Ther. 2010;18:511–518. doi: 10.1038/mt.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tamachi T, Maezawa Y, Ikeda K, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 82.Coyle AJ, Lloyd C, Tian J, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramaprakash H, Shibata T, Duffy KE, et al. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. Am J Pathol. 2011;179:104–115. doi: 10.1016/j.ajpath.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oboki K, Ohno T, Kajiwara N, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoshino K, Kashiwamura S, Kuribayashi K, et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999;190:1541–1548. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kurowska-Stolarska M, Kewin P, Murphy G, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 88.Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 89.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi L, Leu SW, Xu F, et al. Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin Immunol. 2008;129:202–210. doi: 10.1016/j.clim.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 92.Zhang F, Huang G, Hu B, Song Y, Shi Y. A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin Exp Immunol. 2011;164:256–264. doi: 10.1111/j.1365-2249.2011.04328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Shami A, Spolski R, Kelly J, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lei L, Zhang Y, Yao W, Kaplan MH, Zhou B. Thymic stromal lymphopoietin interferes with airway tolerance by suppressing the generation of antigen-specific regulatory T cells. J Immunol. 2011;186:2254–2261. doi: 10.4049/jimmunol.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86:105–112. doi: 10.1139/y08-004. [DOI] [PubMed] [Google Scholar]
- 96.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc. 2009;6:655–659. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 97.Demehri S, Liu Z, Lee J, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang H, Cao W, Kasturi SP, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 100.Biton M, Levin A, Slyper M, et al. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nat Immunol. 2011;12:239–246. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 101.Corrigan CJ, Wang W, Meng Q, et al. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci U S A. 2011;108:1579–1584. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rankin AL, Mumm JB, Murphy E, et al. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2010;184:1526–1535. doi: 10.4049/jimmunol.0903306. [DOI] [PubMed] [Google Scholar]
- 104.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 106.Oh MH, Oh SY, Yu J, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186:7232–7242. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 108.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 109.Brandelius A, Yudina Y, Calven J, et al. dsRNA-induced expression of thymic stromal lymphopoietin (TSLP) in asthmatic epithelial cells is inhibited by a small airway relaxant. Pulm Pharmacol Ther. 2011;24:59–66. doi: 10.1016/j.pupt.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kawasaki J, Ushio H, Kinoshita H, et al. Viral infection induces Thymic stromal lymphopoietin (TSLP) in human keratinocytes. J Dermatol Sci. 2011;62:131–134. doi: 10.1016/j.jdermsci.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 112.Qiao J, Li A, Jin X. TSLP from RSV-stimulated rat airway epithelial cells activates myeloid dendritic cells. Immunol Cell Biol. 2011;89:231–238. doi: 10.1038/icb.2010.85. [DOI] [PubMed] [Google Scholar]
- 113.Siegle JS, Hansbro N, Herbert C, et al. Early-life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir Res. 2010;11:14. doi: 10.1186/1465-9921-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bourgeois EA, Levescot A, Diem S, et al. A natural protective function of invariant NKT cells in a mouse model of innate-cell-driven lung inflammation. Eur J Immunol. 2011;41:299–305. doi: 10.1002/eji.201040647. [DOI] [PubMed] [Google Scholar]
- 115.Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J Immunol. 2010;185:4681–4690. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 116.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Letuve S, Lajoie-Kadoch S, Audusseau S, et al. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J Allergy Clin Immunol. 2006;117:590–596. doi: 10.1016/j.jaci.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 118.Corrigan CJ, Wang W, Meng Q, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. 2011;128:116–124. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 119.Oshikawa K, Kuroiwa K, Tago K, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 120.Reh DD, Wang Y, Ramanathan M, Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy. 2010;24:105–109. doi: 10.2500/ajra.2010.24.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prefontaine D, Nadigel J, Chouiali F, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 122.Kamekura R, Kojima T, Takano K, Go M, Sawada N, Himi T. The role of IL-33 and its receptor ST2 in human nasal epithelium with allergic rhinitis. Clinical and Experimental Allergy. 2012 doi: 10.1111/j.1365-2222.2011.03867.x. Electronic publication ahead of print. [DOI] [PubMed] [Google Scholar]
- 123.Shikotra A, Choy DF, Ohri CM, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–111. e109. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 124.Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 125.Uller L, Leino M, Bedke N, et al. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma. Thorax. 2010;65:626–632. doi: 10.1136/thx.2009.125930. [DOI] [PubMed] [Google Scholar]
- 126.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sakashita M, Yoshimoto T, Hirota T, et al. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy. 2008;38:1875–1881. doi: 10.1111/j.1365-2222.2008.03114.x. [DOI] [PubMed] [Google Scholar]
- 129.Ali M, Zhang G, Thomas WR, et al. Investigations into the role of ST2 in acute asthma in children. Tissue Antigens. 2009;73:206–212. doi: 10.1111/j.1399-0039.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 130.Shimizu M, Matsuda A, Yanagisawa K, et al. Functional SNPs in the distal promoter of the ST2 gene are associated with atopic dermatitis. Hum Mol Genet. 2005;14:2919–2927. doi: 10.1093/hmg/ddi323. [DOI] [PubMed] [Google Scholar]
- 131.Harada M, Hirota T, Jodo AI, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:368–374. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 132.Harada M, Hirota T, Jodo AI, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44:787–793. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hirota T, Takahashi A, Kubo M, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet. 2011;43:893–896. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jung JS, Park BL, Cheong HS, et al. Association of IL-17RB gene polymorphism with asthma. Chest. 2009;135:1173–1180. doi: 10.1378/chest.08-1595. [DOI] [PubMed] [Google Scholar]
- 135.Blazquez AB, Mayer L, Berin MC. Thymic stromal lymphopoietin is required for gastrointestinal allergy but not oral tolerance. Gastroenterology. 2010;139:1301–1309. doi: 10.1053/j.gastro.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 136.Duan W, Mehta AK, Magalhaes JG, et al. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J Allergy Clin Immunol. 2010;126:1284–1293. e1210. doi: 10.1016/j.jaci.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haas J, Korporal M, Schwarz A, Balint B, Wildemann B. The interleukin-7 receptor alpha chain contributes to altered homeostasis of regulatory T cells in multiple sclerosis. Eur J Immunol. 2011;41:845–853. doi: 10.1002/eji.201041139. [DOI] [PubMed] [Google Scholar]
- 138.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin Immunol. 2010;6:4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ohno T, Oboki K, Morita H, et al. Paracrine IL-33 stimulation enhances lipopolysaccharide-mediated macrophage activation. PLoS One. 2011;6:e18404. doi: 10.1371/journal.pone.0018404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol. 2010;185:3035–3040. doi: 10.4049/jimmunol.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yagami A, Orihara K, Morita H, et al. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–5750. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 142.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–268. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 143.Kakkar R, Hei H, Dobner S, Lee R. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M111.298703. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hulse KE, Reefer AJ, Engelhard VH, et al. Targeting allergen to FcgammaRI reveals a novel T(H)2 regulatory pathway linked to thymic stromal lymphopoietin receptor. J Allergy Clin Immunol. 2010;125:247–256. e241–248. doi: 10.1016/j.jaci.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- 148.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ali S, Nguyen DQ, Falk W, Martin MU. Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem Biophys Res Commun. 2010;391:1512–1516. doi: 10.1016/j.bbrc.2009.12.107. [DOI] [PubMed] [Google Scholar]
- 150.Ho LH, Ohno T, Oboki K, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 151.Iikura M, Suto H, Kajiwara N, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 152.Kurowska-Stolarska M, Stolarski B, Kewin P, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 153.Moulin D, Donze O, Talabot-Ayer D, Mezin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 154.Mildner M, Storka A, Lichtenauer M, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res. 2010;87:769–777. doi: 10.1093/cvr/cvq104. [DOI] [PubMed] [Google Scholar]
- 155.Drube S, Heink S, Walter S, et al. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood. 2010;115:3899–3906. doi: 10.1182/blood-2009-10-247411. [DOI] [PubMed] [Google Scholar]
- 156.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 157.Cheung PF, Wong CK, Ip WK, Lam CW. IL-25 regulates the expression of adhesion molecules on eosinophils: mechanism of eosinophilia in allergic inflammation. Allergy. 2006;61:878–885. doi: 10.1111/j.1398-9995.2006.01102.x. [DOI] [PubMed] [Google Scholar]
- 158.Wong CK, Cheung PF, Ip WK, Lam CW. Interleukin-25-induced chemokines and interleukin-6 release from eosinophils is mediated by p38 mitogen-activated protein kinase, c-Jun N-terminal kinase, and nuclear factor-kappaB. Am J Respir Cell Mol Biol. 2005;33:186–194. doi: 10.1165/rcmb.2005-0034OC. [DOI] [PubMed] [Google Scholar]
- 159.Suzukawa M, Koketsu R, Iikura M, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–1253. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 160.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blom L, Poulsen BC, Jensen BM, Hansen A, Poulsen LK. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS One. 2011;6:e21695. doi: 10.1371/journal.pone.0021695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schneider E, Petit-Bertron AF, Bricard R, et al. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J Immunol. 2009;183:3591–3597. doi: 10.4049/jimmunol.0900328. [DOI] [PubMed] [Google Scholar]
- 165.Suzukawa M, Iikura M, Koketsu R, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–5989. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 166.Joshi AD, Oak SR, Hartigan AJ, et al. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52. doi: 10.1186/1471-2172-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nelson MP, Christmann BS, Werner JL, et al. IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina. J Immunol. 2011;186:2372–2381. doi: 10.4049/jimmunol.1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Espinassous Q, Garcia-de-Paco E, Garcia-Verdugo I, et al. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J Immunol. 2009;183:1446–1455. doi: 10.4049/jimmunol.0803067. [DOI] [PubMed] [Google Scholar]
- 169.Wu WH, Park CO, Oh SH, et al. Thymic stromal lymphopoietin-activated invariant natural killer T cells trigger an innate allergic immune response in atopic dermatitis. J Allergy Clin Immunol. 2010;126:290–299. e291–294. doi: 10.1016/j.jaci.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 170.Bourgeois E, Van LP, Samson M, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 171.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 172.Stock P, Lombardi V, Kohlrautz V, Akbari O. Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J Immunol. 2009;182:5116–5122. doi: 10.4049/jimmunol.0804213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Swaidani S, Bulek K, Kang Z, et al. T cell-derived Act1 is necessary for IL-25-mediated Th2 responses and allergic airway inflammation. J Immunol. 2011;187:3155–3164. doi: 10.4049/jimmunol.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41:1862–1871. doi: 10.1002/eji.201041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reefer AJ, Hulse KE, Lannigan JA, et al. Flow cytometry imaging identifies rare T(H)2 cells expressing thymic stromal lymphopoietin receptor in a “proallergic” milieu. J Allergy Clin Immunol. 2010;126:1049–1058. 1058 e1041–1010. doi: 10.1016/j.jaci.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 177.Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 178.Xu G, Zhang L, Wang DY, et al. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy. 2010;65:581–589. doi: 10.1111/j.1398-9995.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 179.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 180.Kurokawa M, Matsukura S, Kawaguchi M, et al. Expression and effects of IL-33 and ST2 in allergic bronchial asthma: IL-33 induces eotaxin production in lung fibroblasts. Int Arch Allergy Immunol. 2011;155(Suppl 1):12–20. doi: 10.1159/000327259. [DOI] [PubMed] [Google Scholar]
- 181.Shan L, Redhu NS, Saleh A, Halayko AJ, Chakir J, Gounni AS. Thymic stromal lymphopoietin receptor-mediated IL-6 and CC/CXC chemokines expression in human airway smooth muscle cells: role of MAPKs (ERK1/2, p38, and JNK) and STAT3 pathways. J Immunol. 2010;184:7134–7143. doi: 10.4049/jimmunol.0902515. [DOI] [PubMed] [Google Scholar]