Abstract
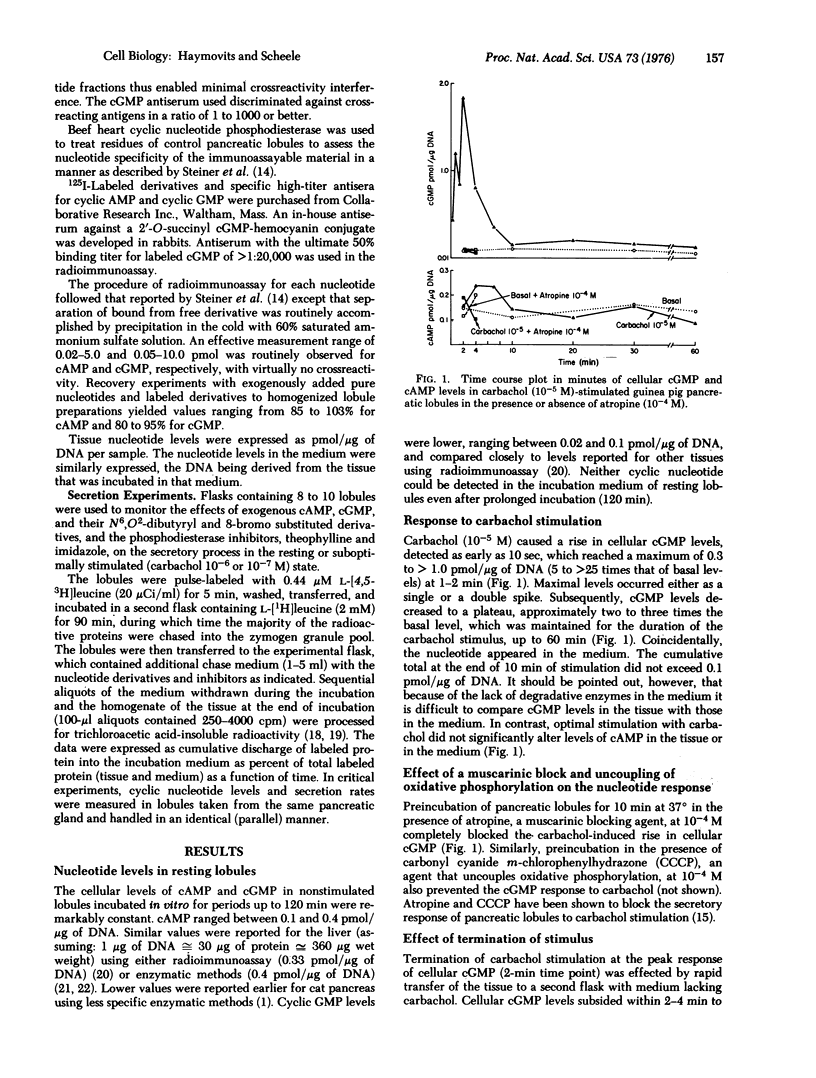
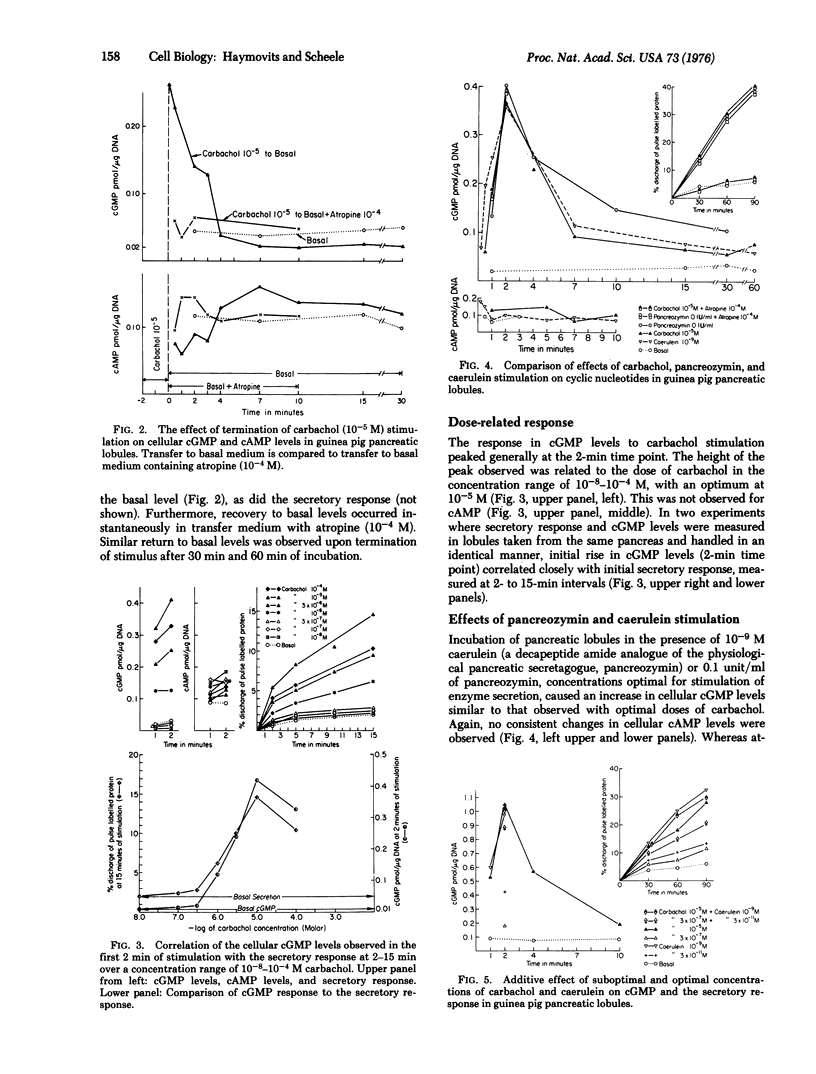
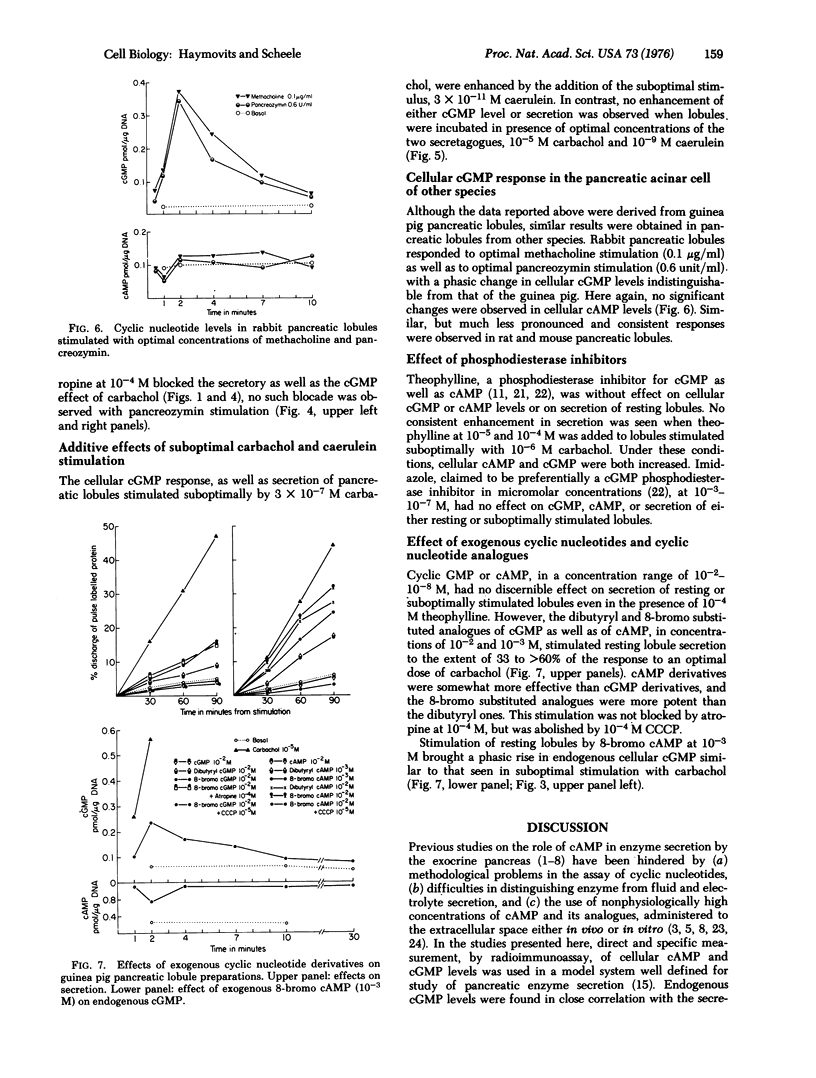
Cellular levels of cAMP and cGMP were measured in guinea pig pancreatic lobules incubated in vitro, during basal or stimulated secretion. Stimulation with optimal concentrations of carbamylcholine (carbachol) (10(-5) M), pancreozymin (0.1 unit/ml), and caerulein (10(-9) M) resulted within seconds in a sharp rise in cGMP levels, from five to more than 20 times that of basal levels. cAMP levels did not change significantly. cGMP increases were maximal at 2 min then subsided by 4-7 min to a plateau about two to three times that of basal level. This plateau was maintained for the duration of the secretagogue stimulus. Removal of the carbachol stimulus resulted in a rapid decrease in cGMP levels to that of the basal state. The cellular cGMP levels observed within the first 2 min of stimulation correlated closely with the dose of carbachol and the secretory response. Atropine at 10(-4) M blocked the cGMP elevation due to carbachol but not that due to pancreozymin, while carbonyl cyanide m-chlorophenyl hydrazone, an uncoupler of oxidative phosphorylation, blocked the response to both secretagogues. Similar though less extensive findings were observed using rabbit pancreatic lobules incubated in vitro. High concentrations (10(-2)-10(-3) M) of the dibutyryl and 8-bromo analogues of both nucleotides were effective, though suboptimal, secretagogues. In the case of the cAMP analogues, the secretory response was associated with a rise in endogenous cGMP levels, similar to that observed during suboptimal carbachol stimulation. These findings suggest that cGMP may be an intracellular mediator in the process of stimulus secretion coupling in the acinar cell of the exocrine pancreas.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauduin H., Rochus L., Vincent D., Dumont J. E. Role of cyclic 3',5'-amp in the action of physiological secretagogues on the metabolism of rat pancreas in vitro. Biochim Biophys Acta. 1971 Oct;252(1):171–183. doi: 10.1016/0304-4165(71)90106-1. [DOI] [PubMed] [Google Scholar]
- Benz L., Eckstein B., Matthews E. K., Williams J. A. Control of pancreatic amylase release in vitro: effects of ions, cyclic AMP, and colchicine. Br J Pharmacol. 1972 Sep;46(1):66–67. doi: 10.1111/j.1476-5381.1972.tb06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Case R. M., Johnson M., Scratcherd T., Sherratt H. S. Cyclic adenosine 3',5'-monophosphate concentration in the pancreas following stimulation by secretin, cholecystokinin-pancreozymin and acetylcholine. J Physiol. 1972 Jun;223(3):669–684. doi: 10.1113/jphysiol.1972.sp009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T. The actions of dibutyryl cyclic adenosine 3',5'-monophosphate and methyl xanthines on pancreatic exocrine secretion. J Physiol. 1972 Jun;223(3):649–667. doi: 10.1113/jphysiol.1972.sp009867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Greengard P. Guanosine 3':5'-cyclic monophosphate-dependent phosphorylation of endogenous substrate proteins in membranes of mammalian smooth muscle. Proc Natl Acad Sci U S A. 1974 May;71(5):1891–1895. doi: 10.1073/pnas.71.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Larner J., Sasko H., O'Toole A. G. Enzymic analysis of cyclic 3', 5'-AMP in mammalian tissues and urine. Anal Biochem. 1969 Apr 4;28(1):523–544. doi: 10.1016/0003-2697(69)90208-5. [DOI] [PubMed] [Google Scholar]
- Heisler S., Fast D., Tenenhouse A. Role of Ca 2+ and cyclic AMP in protein secretion from rat exocrine pancreas. Biochim Biophys Acta. 1972 Oct 25;279(3):561–572. doi: 10.1016/0304-4165(72)90178-x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lint T. F., George W. J. Hormonal control of lysosomal enzyme release from human neutrophils. Effects of autonomic agents on enzyme release, phagocytosis, and cylic nucleotide levels. J Exp Med. 1974 Jun 1;139(6):1395–1414. doi: 10.1084/jem.139.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa E., Ishikawa S., Davis J. W., Sutherland E. W. Determination of guanosine 3',5'-monophosphate in tissues and of guanyl cyclase in rat intestine. J Biol Chem. 1969 Dec 10;244(23):6371–6376. [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Condensing vacuole conversion and zymogen granule discharge in pancreatic exocrine cells: metabolic studies. J Cell Biol. 1971 Mar;48(3):503–522. doi: 10.1083/jcb.48.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodell R. G., Toskes P. P., Reber H. A., Brooks F. P. Significance of cyclic AMP in the regulation of exocrine pancreas secretion. Experientia. 1970 May 15;26(5):515–517. doi: 10.1007/BF01898480. [DOI] [PubMed] [Google Scholar]
- Kulka R. G., Sternlicht E. Enzyme secretion in mouse pancreas mediated by adenosine-3'5'-cyclic phosphate and inhibited by adenosine-3'-phosphate. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1123–1128. doi: 10.1073/pnas.61.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. P., Kuo J. F., Greengard P. Role of muscarinic cholinergic receptors in regulation of guanosine 3':5'-cyclic monophosphate content in mammalian brain, heart muscle, and intestinal smooth muscle. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3287–3291. doi: 10.1073/pnas.69.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S. H., Whitley T. H., Stowe N. W., Steiner A. L. Immunohistochemical localization of 3': 5'-cyclic AMP and 3': 5'-cyclic GMP in rat liver, intestine, and testis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2022–2026. doi: 10.1073/pnas.72.6.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderstap A. S., Bonting S. L. Cyclic AMP and enzyme secretion by the isolated rabbit pancreas. Pflugers Arch. 1969;313(1):62–70. doi: 10.1007/BF00586329. [DOI] [PubMed] [Google Scholar]
- Scheele G. A., Palade G. E. Studies on the guinea pig pancreas. Parallel discharge of exocrine enzyme activities. J Biol Chem. 1975 Apr 10;250(7):2660–2670. [PubMed] [Google Scholar]
- Smith P. A., Case R. M. Effects of cholera toxin on cyclic adenosine 3',5'-monophosphate concentration and secretory processes in the exocrine pancreas. Biochim Biophys Acta. 1975 Aug 13;399(2):277–290. doi: 10.1016/0304-4165(75)90258-5. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]


