Abstract
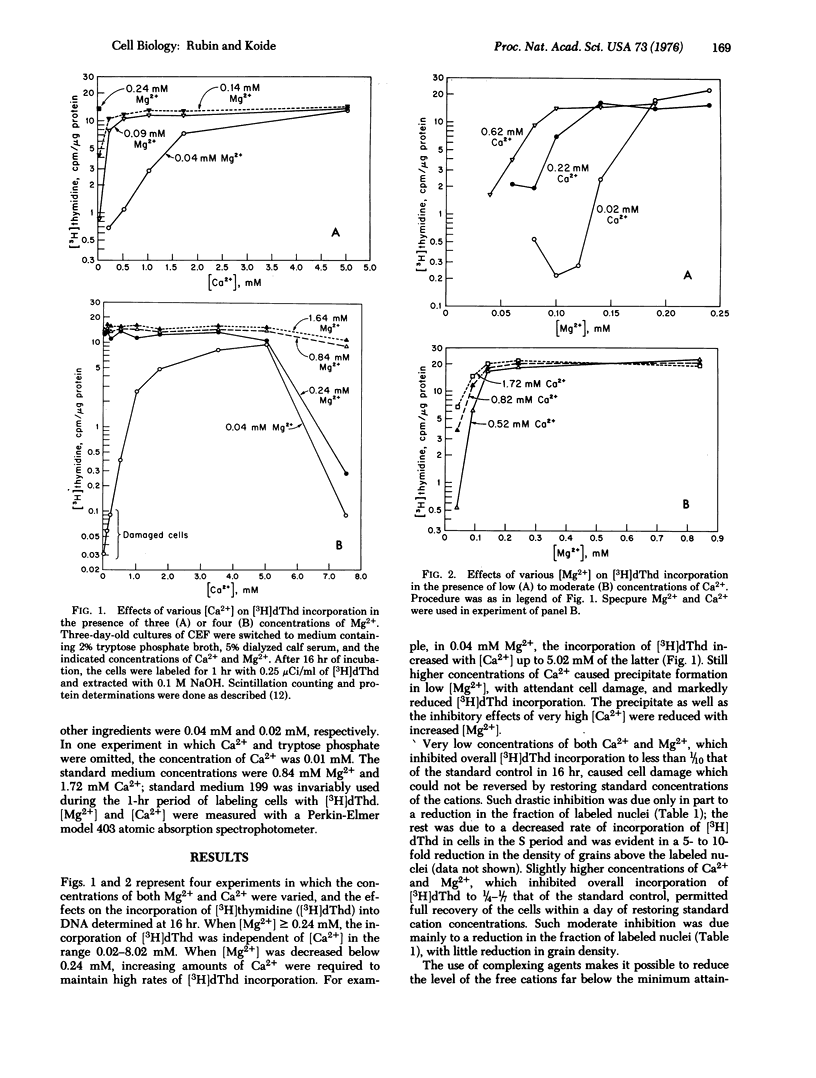
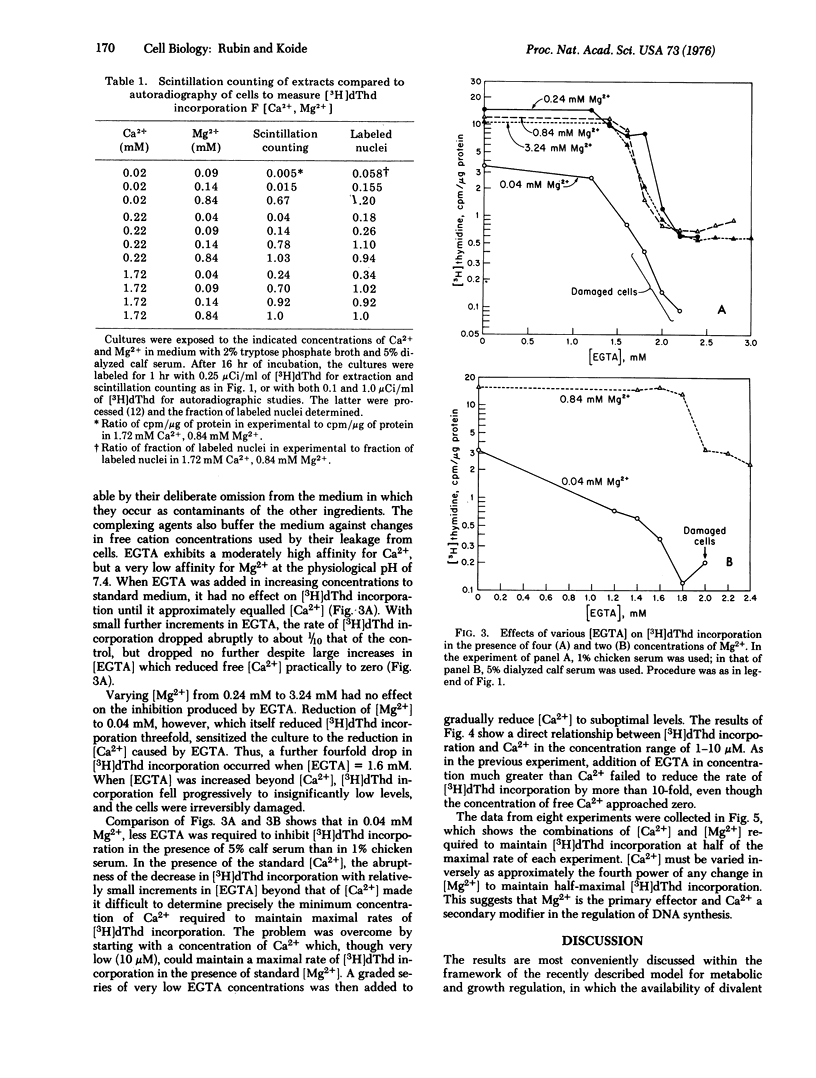
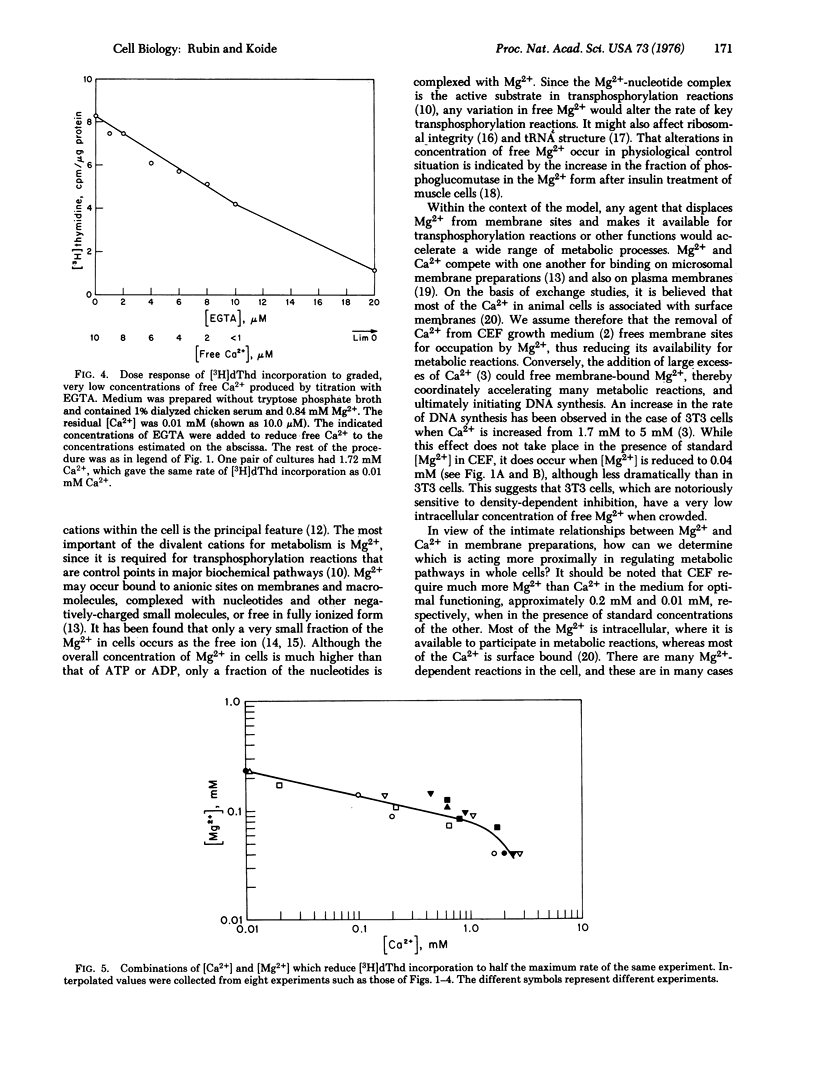
The effects on DNA synthesis of various combinations of Mg2+ and Ca2+ in cultures of chick embryo cells have been studied. When [Mg2+] larger than or equal to 0.24 mM, reduction of Ca2+ from the standard concentration of 1.72 mM to 0.01 mM had no effect on the incorporation of [3H]thymidine ([3H]dThd) into DNA over a 16-hr period. When Mg2+ was reduced to 0.04 mM, [3H]dThd incorporation into DNA decreased directly with [Ca2+] below 1.72 mM and increased slightly up to [Ca2+] = 5.02 mM, where cell damage began to occur. The change in [Ca2+] necessary to maintain a half-maximal rate of [3H]dThd incorporation was found to depend inversely on the fourth power of the change in [Mg2+]. Chelation of Ca2+ with approximately equimolar ethylene glycol-bis(beta-aminoethyl ether)-N,N'-tetraacetic acid (EGTA) in the presence of [Mg2+] larger than or equal to 0.24 mM reduced [3H]dThd incorporation about 10-fold, and large excesses of EGTA did not further reduce it. The amount of EGTA required to produce a detectable inhibition of [3H]dThd incorporation was independent of [Mg2+] larger than or equal to 0.24 mM, as was the level of residual incorporation in excess EGTA. When [Mg2+] was reduced to 0.04 mM, however, [3H]dThd incorporation declined even when [EGTA] less than [Ca2+], and vanished when EGTA was in large excess. The results are discussed within the framework of a model for the regulation of cell metabolism and growth in which the availability of free Mg2+ is the central coordinating factor. The metabolic effects of Mg2+ depend on its distribution between elements such as ATP and binding sites on membranes. We propose that the major metabolic effects of varying [Ca2+] are produced indirectly through its competition with Mg2+ for membrane sites, thereby making more or less Mg2+ available for rate-limiting transphosphorylation reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARVALHO A. P., SANUI H., PACE N. CALCIUM AND MAGNESIUM BINDING PROPERTIES OF CELL MEMBRANE MATERIALS. J Cell Physiol. 1963 Dec;62:311–317. doi: 10.1002/jcp.1030620311. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Elkington J. Induction of growth in resting fibroblastic cell cultures by Ca++. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1584–1588. doi: 10.1073/pnas.72.4.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Activation of phosphofructokinase by stimulants of cell multiplication. Nat New Biol. 1973 Dec 12;246(154):181–183. doi: 10.1038/newbio246181a0. [DOI] [PubMed] [Google Scholar]
- Gail M. Time lapse studies on the motility of fibroblasts in tissue culture. Ciba Found Symp. 1973;14:287–310. doi: 10.1002/9780470719978.ch14. [DOI] [PubMed] [Google Scholar]
- Hickie R. A., Kalant H. Effects of perfusion media on the Ca++ and Mg++ content of rat liver. Can J Physiol Pharmacol. 1966 Nov;44(6):893–900. doi: 10.1139/y66-111. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Fresco J. R. Renaturation of transfer ribonucleic acids through site binding of magnesium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):941–948. doi: 10.1073/pnas.55.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESELSON M., NOMURA M., BRENNER S., DAVERN C., SCHLESSINGER D. CONSERVATION OF RIBOSOMES DURING BACTERIAL GROWTH. J Mol Biol. 1964 Sep;9:696–711. doi: 10.1016/s0022-2836(64)80176-5. [DOI] [PubMed] [Google Scholar]
- Morrill G. A., Robbins E. The role of calcium in the regulation of the steady-state levels of sodium and potassium in the HeLa cell. J Gen Physiol. 1967 Mar;50(4):781–792. doi: 10.1085/jgp.50.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Rubin H. Increased hyaluronic acid production on stimulation of DNA synthesis in chick embryo fibroblasts. Nature. 1975 Mar 6;254(5495):65–66. doi: 10.1038/254065a0. [DOI] [PubMed] [Google Scholar]
- Peck E. J., Jr, Ray W. J., Jr Metal complexes of phosphoglucomutase in vivo. Alterations induced by insulin. J Biol Chem. 1971 Feb 25;246(4):1160–1167. [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Rose I. A. The state of magnesium in cells as estimated from the adenylate kinase equilibrium. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1079–1086. doi: 10.1073/pnas.61.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Central role for magnesium in coordinate control of metabolism and growth in animal cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3551–3555. doi: 10.1073/pnas.72.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Inhibition of DNA synthesis in animal cells by ethylene diamine tetraacetate, and its reversal by zinc. Proc Natl Acad Sci U S A. 1972 Mar;69(3):712–716. doi: 10.1073/pnas.69.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Koide T. Early cellular responses to diverse growth stimuli independent of protein and RNA synthesis. J Cell Physiol. 1975 Aug;86(1):47–58. doi: 10.1002/jcp.1040860107. [DOI] [PubMed] [Google Scholar]
- Rubin H., Koide T. Early cellular responses to diverse growth stimuli independent of protein and RNA synthesis. J Cell Physiol. 1975 Aug;86(1):47–58. doi: 10.1002/jcp.1040860107. [DOI] [PubMed] [Google Scholar]
- Sanui H. pH dependence of the effect of adenosine triphosphate and ethylenediaminetetraacetate on sodium and magnesium binding by cellular membrane fragments. J Cell Physiol. 1970 Jun;75(3):361–368. doi: 10.1002/jcp.1040750312. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Stimulation of glucose transport in cultures of density-inhibited chick embryo cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3154–3157. doi: 10.1073/pnas.68.12.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. N., Singh M., August J. T., Horecker B. L. Alterations in glucose metabolism in chick-embryo cells transformed by Rous sarcoma virus: intracellular levels of glycolytic intermediates. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4129–4132. doi: 10.1073/pnas.71.10.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow M. P. Interaction of 28Mg with Ca and K in the smooth muscle of guinea-pig taenia coli. J Physiol. 1969 Nov;205(1):19–38. doi: 10.1113/jphysiol.1969.sp008948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. S., Armstrong P. B., Granger R. E. On the recovery of adhesiveness by trypsin-dissociated cells. J Membr Biol. 1973;13(2):97–128. doi: 10.1007/BF01868223. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Rixon R. H., MacManus J. P., Balk S. D. Calcium, cyclic adenosine 3',5'-monophosphate, and the control of cell proliferation: a review. In Vitro. 1973 Jan-Feb;8(4):257–278. doi: 10.1007/BF02615905. [DOI] [PubMed] [Google Scholar]


