Abstract
Autoreactive T cells play a pivotal role in the pathogenesis of autoimmune kidney disease. T cell vaccination (TCV) may limit autoimmune disease and induce CD8+ regulatory T cells (Tregs). We used Heymann nephritis (HN), a rat model of human membranous nephritis, to study the effects of TCV on autoimmune kidney disease. We harvested CD4+ T cells from renal tubular antigen (Fx1A) -immunized rats and activated these cells in vitro to express the MHC Class Ib molecule Qa-1. Vaccination of Lewis rats with these autoreactive Fx1A-induced T cells protected against HN, whereas control-primed T cells did not. Rats that underwent TCV had lower levels of proteinuria and serum creatinine and significantly less glomerulosclerosis, tubular damage, and interstitial infiltrates. Furthermore, these rats expressed less IFN-γ and IL-6 in splenocytes, whereas the numbers of Tregs and the expression of Foxp3 were unchanged. In vitro cytotoxicity assays showed CD8+ T cell-mediated elimination of Qa-1–expressing CD4+ T cells. In vivo, TCV abrogated the increase in Qa-1–expressing CXCR5+ TFH cells observed in HN compared with controls. Taken together, these results suggest that TCV protects against autoimmune kidney disease by targeting Qa-1–expressing autoreactive CD4+ cells.
CD4+ regulatory T cells (Tregs) have been shown to be critically involved in limiting autoimmune diseases such as multiple sclerosis, diabetes, and autoimmune renal disease.1 The importance of CD8+ T cells with regulatory function has been less studied until recently. However, the recognition that T cell vaccination (TCV) induces regulatory CD8 T cells and that these CD8 T cells seem to use the MHC class Ib molecule Qa-1 (or HLA-E in humans) has reignited interest in this subset.2–5 Furthermore, the identification that T follicular helper cells, a subset of splenic CD4 T cells that is responsible for assisting B cell antibody production and class switching, express high levels of Qa-1 suggests that CD8 Tregs may play a role in limiting antibody-mediated disease.6
Mouse Qa-1 and its human equivalent (HLA-E) are non-classic MHC class I molecules that bind hydrophobic peptides, including the leader sequences of class I HLA molecules and components of the T cell receptor (TCR). These molecules are transiently upregulated on the surface of activated T and antigen-presenting cells.7,8 Earlier studies of CD8+ suppressor cells suggested an important role for Qa-1 in mediating the function of these cells.9 Qa-1 gene-deficient mice showed enhanced CD4+ T cell responses to self and foreign antigens and increased susceptibility to experimental autoimmune encephalomyelitis (EAE), the mouse model of multiple sclerosis.10,11 Mice with mutations in Qa-1 to limit CD8 binding but with inhibitory natural killer binding have severe autoimmune disease, including nephritis.6 TCV with activated CD4+ T cells expressing Qa-1 can induce Qa-1–restricted CD8+ Tregs.4,10,12 In addition, CD8αα+ TCRаβ+ Treg clones recognize a TCR-derived peptide in the context of Qa-1 molecules.13 More recently, a number of markers of CD8 Tregs that target Qa-1–expressing T follicular helper (TFH) cells have been identified, including CD44, inducible T cell costimulator ligand, CXCR5, very late antigen-4, and CD122.6
CD8+ Tregs specific for Qa-1–expressing, intermediate-affinity CD4 effector T cells have been found in EAE and type 1 diabetes models induced by vaccination with antigen but targeted at T cells with intermediate avidity distinct from antigen specificity in keeping with a nonantigen-driven mechanism of suppression.14 In humans, increased expression of HLA-E is found on effector cells in multiple sclerosis. Mice can be protected from EAE by TCV.15 This finding has led to TCV in clinical trials in patients with multiple sclerosis.16–18 In glomerulonephritis, the work by Trivedi et al.19 using an HLA DR4 transgenic humanized mouse model with peptide immunization has induced nephritis that is limited by TCV. However, the protection against disease in this model is limited by its variability.19
Human idiopathic membranous nephritis is an autoimmune renal disease characterized by severe proteinuria and progression to renal failure.20 It is frequently caused by sensitization to phospholipase A2 receptor, an antigen expressed on glomerular podocytes,21 and associated with antibody deposition in the glomerulus. It is a major cause of ESRD worldwide.22 Active Heymann nephritis (HN), an experimental rat model of human autoimmune-mediated membranous nephritis, is used to study this disease and its potential therapies. HN is induced in Lewis rats by immunization with a crude renal tubular antigen (Fx1A), and it reproduces clinical features of human idiopathic membranous glomerulonephritis.23 Although autoantibodies particularly against megalin are observed in HN, the disease also involves T cell-mediated injury with a major role of CD4+ and CD8+ T cells.24–26 Although some studies have suggested that complement is important in HN,27 recent studies find that C6-deficient rats develop equivalent passive HN, excluding antibody-bound complement as a major component of injury.28
We have identified TCR restriction to TCRVβ-2 and -16 in HN and have shown that TCR DNA vaccines directed against the Vβ-sequence of the TCR can protect against nephritis29–31 A slowly progressive model of autoimmune disease with tissue-specific antibody production and the involvement of specific TCRs by pathogenic T cells that may be presented on their surface by Qa-1 to CD8 make Tregs HN an excellent candidate for TCV and evaluation of its mechanism of protection. Here, for the first time, we evaluate the role of CD8 Tregs induced by TCV in autoimmune renal disease, evaluate the role of Qa-1, and assess the potential role of TFH cells in autoimmune renal disease.
Our studies showed that TCV protects rats from developing active HN, with a major reduction in renal pathology and Fx1A antibodies. Qa-1 was expressed in vaccinating CD4+ cells and TFH cells, which were both increased in HN. Qa-1–restricted CD8+ cytotoxicity was shown in TCV rats. These findings suggest a therapeutic role for TCV and CD8+ Tregs in limiting autoimmune renal disease by deleting autoreactive CD4 T cells.
Results
Autoreactive CD4+ T Cells from HN Rats Used for Cellular Vaccination Express Qa-1
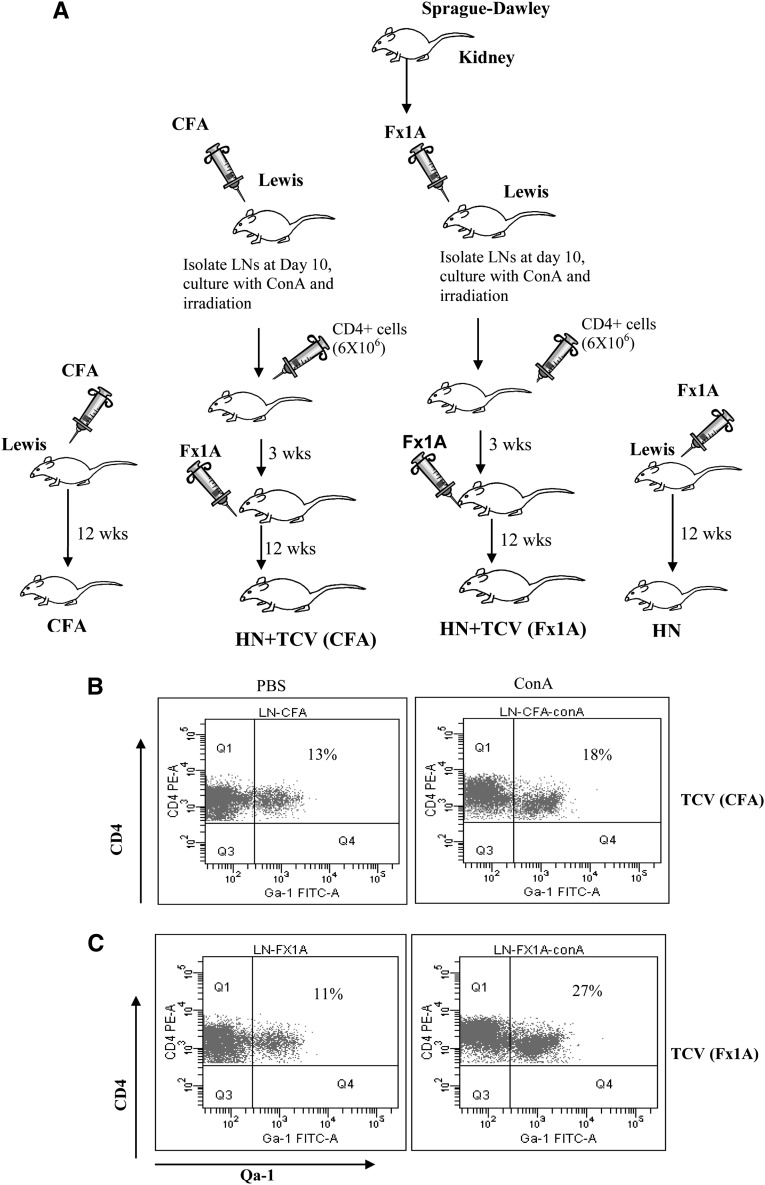
HN is induced in Lewis rats by immunization with a renal tubular antigen (Fx1A), leading to the induction of autoantibodies and immune injuries to the kidney. It has been shown previously in other disease models that TCV limits autoimmune disease through suppressive CD8+ T cells that target Qa-1–expressing CD4+ cells. We, therefore, sought to determine if TCV would be protective in the HN model. CD4+ T cells from draining lymph nodes (LNs) of the hind feet of rats immunized with Fx1A or CFA 10 days before were isolated and cultured in vitro with concanavalin A (conA), and then, they were irradiated and used to vaccinate Lewis rats (Figure 1A). For the control group, CD4+ T cells from rats immunized with CFA were cultured under the same conditions as the Fx1A group. CD4+ T cells used for TCV and controls were examined for surface expression of Qa-1 after conA stimulation. Increased expression of Qa-1 was shown (Figure 1 B and C). Qa-1 expression on CD4+ T cells was increased from 13% (PBS) to 18% (conA) in CFA-immunized rats (Figure 1B). However, there was a larger increase in Qa-1 expression on CD4 T cells from 11% to 27% in Fx1A-immunized rats after conA stimulation compared with the control group (Figure 1C, P<0.05, n=6).
Figure 1.
TCV through autoreactive CD4+ T cells increased the expression of Qa-1. (A) Fx1A was generated from Sprague–Dawley rat kidneys as previously described. Lewis rats were immunized with Fx1A as the experimental group and CFA as the control group. CD4+ T cells were purified from draining LNs of the two groups of rats 10 days after footpad injection of Fx1A or CFA and cultured with conA for 40 hours. The CD4+ T cells from Fx1A- and CFA-immunized rats were irradiated at 30 Gy and injected into two groups of Lewis rats through the tail vein (5×106 cells/rat). Fx1A was injected into each of their hind footpads 3 weeks after TCV. (B and C) CD4+ T cells were isolated from draining LNs from both (B) CFA- and (C) Fx1A-immunized rats and cultured on PBS or conA for 40 hours. Qa-1 expression on CD4+ T cells was increased from 13% (PBS) to 18% (conA) for CFA-immunized rats and 11% to 27% for Fx1A-immunized rats after conA stimulation (P<0.05, n=6).
TCV Reduces Proteinuria, Renal Dysfunction, and Histologic Damage in HN
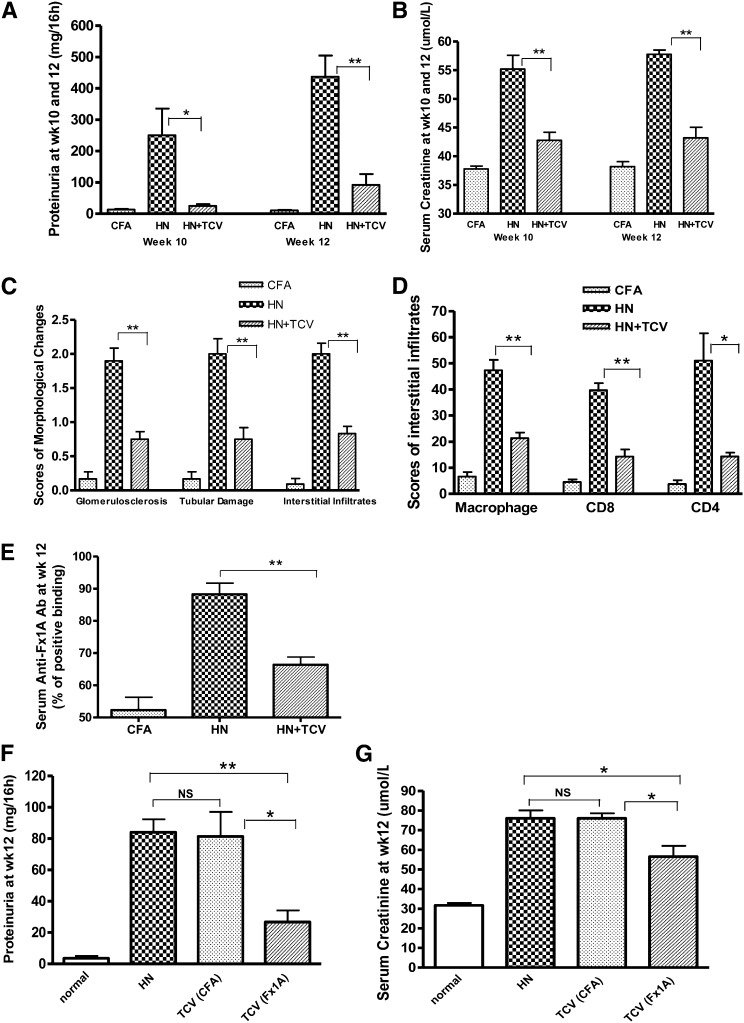
T cell-vaccinated HN rats (HN + TCV) showed significant protection against disease at weeks 10 and 12 compared with nonvaccinated HN rats that had already developed severe HN with more severe proteinuria and elevation of serum creatinine (P<0.05 or P<0.01) (Figure 2, A and B). Quantitative analysis showed significantly less glomerulosclerosis, tubular damage, and interstitial monocyte infiltration in the HN + TCV group compared with the HN group (P<0.05 or P<0.01) (Figure 2, C and D). Anti-Fx1A antibody levels expressed as percentage binding of known positive serum were measured by ELISA. The level of serum anti-Fx1A antibody (total IgG) in HN rats was significantly suppressed by TCV (Figure 2E). However, although the TCV (Fx1A) group significantly reduced proteinuria and serum creatinine, the control TCV (CFA) group was not protected and was equivalent to standard HN (Figure, 2 F and G), and the histologic renal injury reflected the same specific protection by TCV (Fx1A) (Figure 3C).
Figure 2.
TCV reduced proteinuria, inflammation, and protected renal function in HN, and the protection was induced by antigen-specific TCV. HN + TCV rats had significantly reduced (A) proteinuria excretion at weeks 10 and 12 and (B) serum creatinine compared with HN rats. (C) Semiquantitative scores of morphologic changes at week 12 showed significantly less damage in glomeruli and tubules and reduced interstitial monocyte infiltration in HN + TCV rats compared with HN rats. (D) Interstitial infiltration scores of macrophage and CD8+ and CD4+ cells on immunohistochemical sections showed reduced kidney infiltration in HN + TCV rats. (E) Comparison of Fx1A IgG responses in the three groups of rats; anti-Fx1A antibody levels expressed as percentage binding of known positive serum (anti-Fx1A titer; 1:200) measured by ELISA were significantly reduced by TCV in the course of HN. (F) Proteinuria and (G) serum creatinine were significantly reduced in Fx1A-primed TCV rats compared with CFA-primed TCV (n=6 for each group, mean ± SD). *P<0.05; **P<0.01.
Figure 3.
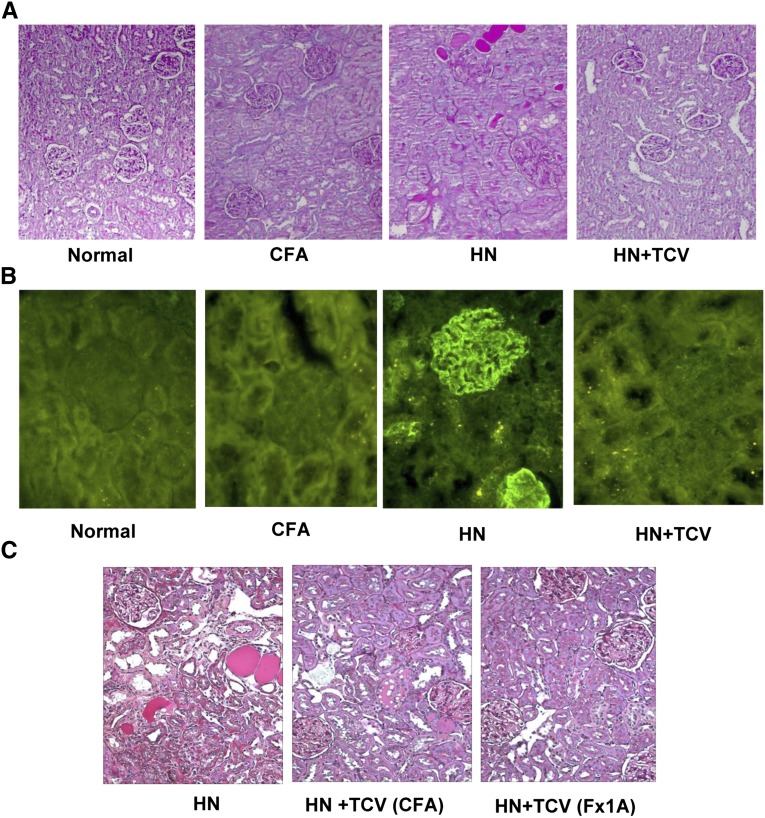
TCV reduced renal damage in HN. (A) Histology of renal representative sections under PAS staining showed reduced renal damage and cellular infiltration in rats that received TCV. Magnification, ×200. (B) Representative sections of kidneys showed that TCV reduced subepithelial glomerular immune deposits. Magnification, ×400. (C) In the TCV (CFA) control group, the renal injury was equivalent to the standard HN rats group, whereas TCV (Fx1A) showed less injury. Magnification, ×200.
Histologic renal injury at week 12 was significantly ameliorated in HN + TCV compared with HN rats (Figure 3A). Furthermore, the HN group showed significant immune deposition along the glomerular basement membrane, which was not detected in the HN + TCV or control group (Figure 3B).
TCV Reduces Infiltration of Macrophages and CD4+ and CD8+ T Cells
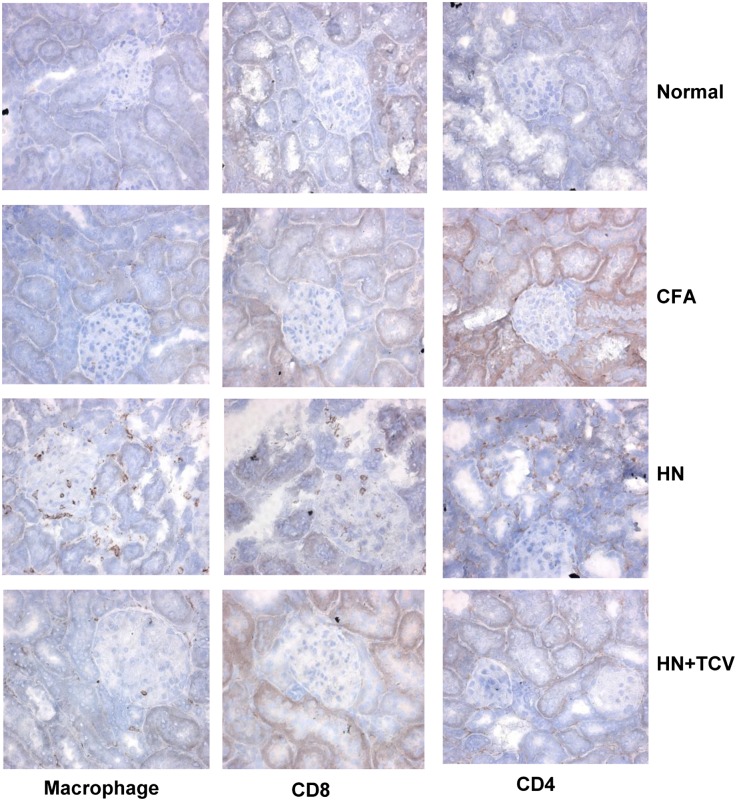
To assess the cellular infiltrations, we performed immunohistochemistry of week 12 kidney sections. There was a marked reduction of infiltrating macrophages, CD8+ T cells, and CD4+ T cells in the HN + TCV group compared with the HN group (Figure 4).
Figure 4.
Photomicrographs of immunohistochemical sections from normal, CFA, HN, and HN + TCV groups of rats showing reduction in infiltration with macrophages, CD4+ T cells, and CD8+ T cells in the HN + TCV group. Magnification, ×400.
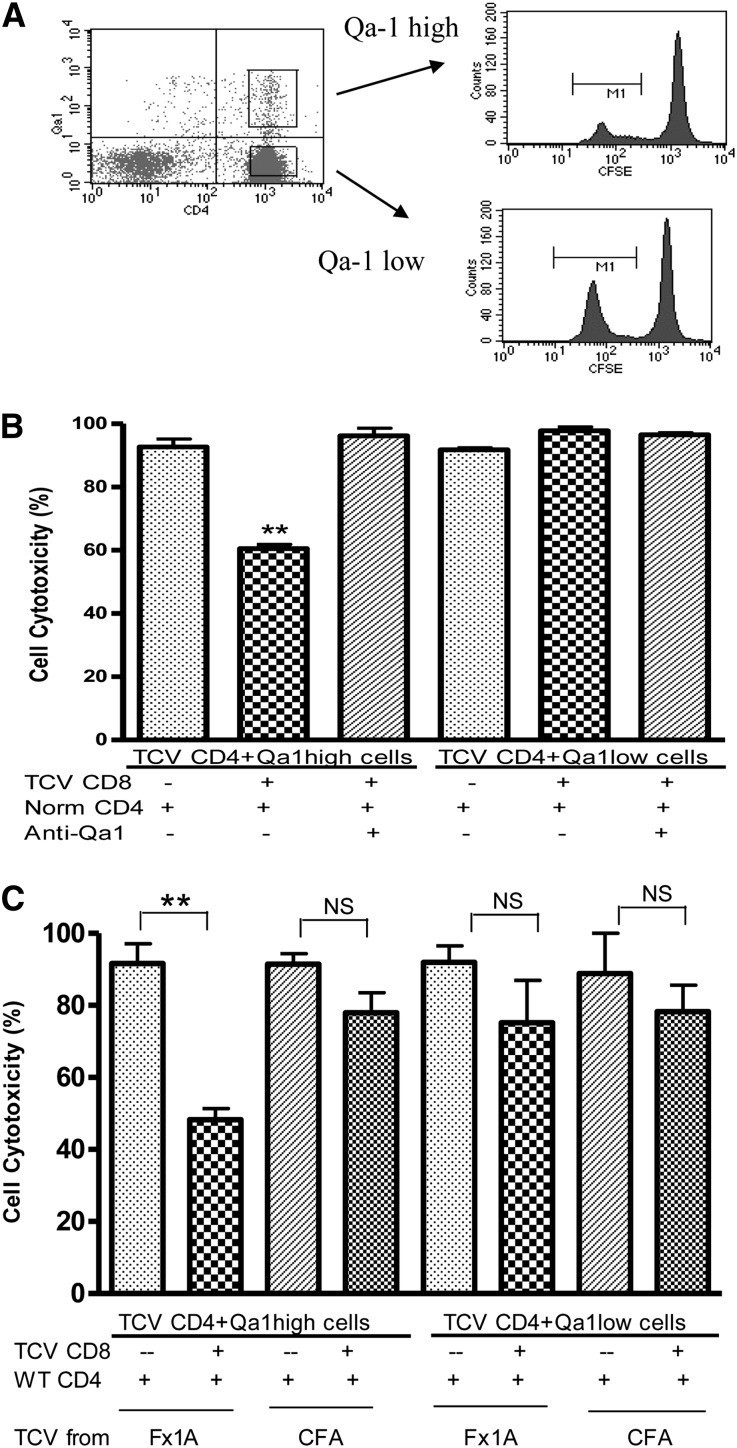
Qa-1–Expressing CD4+ T Cells Can Be Eliminated by CD8+ T Cells In Vitro
To investigate the possible role of CD8+ Tregs in protection of renal function by TCV, we conducted in vitro cytotoxicity assays. The studies were carried out to more directly define the potential inhibitory interaction between CD4+ and CD8+ cells (Concise Methods). CD4+ splenic T cells isolated from HN + TCV rats were sorted by flow cytometry into Qa-1 high and low populations and stained with a low concentration of carboxyfluorescein succinimidyl ester (CFSE); an equal number of control CD4 T cells from nonimmunized rats were stained with a high concentration of CFSE to act as a reference number of cells (Figure 5A). CFSE staining (high and low) was used to distinguish between the control and test populations. The relative CD4 T cell survival was reduced to about 60% when CD8+ T cells isolated from TCV rats were added to Qa-1 high and normal CD4+ T cells in coculture (P<0.01). The effect of CD8+ T cells was abrogated by the addition of blocking mouse anti-rat Qa-1 antibody (Figure 5B). These results suggest that CD8+ T cells specifically eliminate Qa-1–expressing CD4+ T cells. This result is also shown by the absence of effect on Qa-1 low CD4 cells. Thus, the induced CD8 T cells seem restricted by Qa-1 and able to eliminate Qa-1–expressing autoreactive CD4 T cells.
Figure 5.
In vitro cytotoxicity analysis showed the induction of cytotoxic CD8+ T cells by antigen-specific TCV. (A) CD4+ cells isolated from TCV or nonimmunized rats were stained with high or low concentrations of CFSE, sorted depending on Qa-1 high or low by flow cytometry, and cocultured alone or with TCV CD8+ T cells. (B) Qa-1 high CD4+ cells were significantly eliminated when cocultured with TCV CD8+ cells (**P<0.01), but CD4+ Qa-1 low cells were not eliminated. Anti–Qa-1 antibody inhibited the cytotoxic effects of TCV-derived CD8+ T cells on Qa-1 high CD4 T cells. (C) In vitro cytotoxicity assays were performed using CD4 target cells and CD8 effector cells from rats that had received either Fx1A TCV or CFA TCV using the same method as in A and B. There was no effect for CFA-derived CD8+ T cells to eliminate Qa-1–expressing CD4+ T cells in vitro compared with the cytotoxicity of CD8+ T cells isolated from Fx1A-derived TCV rats that were added to Qa-1 high CD4+ T cells in coculture. These results are representative of three independent experiments.
To assess specificity, we conducted in vitro cytotoxicity assays using CD4 target cells and CD8 effector cells from rats that had received either Fx1A TCV or CFA TCV using the same method as described above. We found no effect for CFA-derived CD8+ T cells to eliminate Qa-1–expressing CD4+ T cells in vitro (Figure 5C). This finding was compared with the cytotoxicity that was again found when CD8+ T cells isolated from Fx1A-derived TCV rats were added to Qa-1 high CD4+ T cells in coculture.
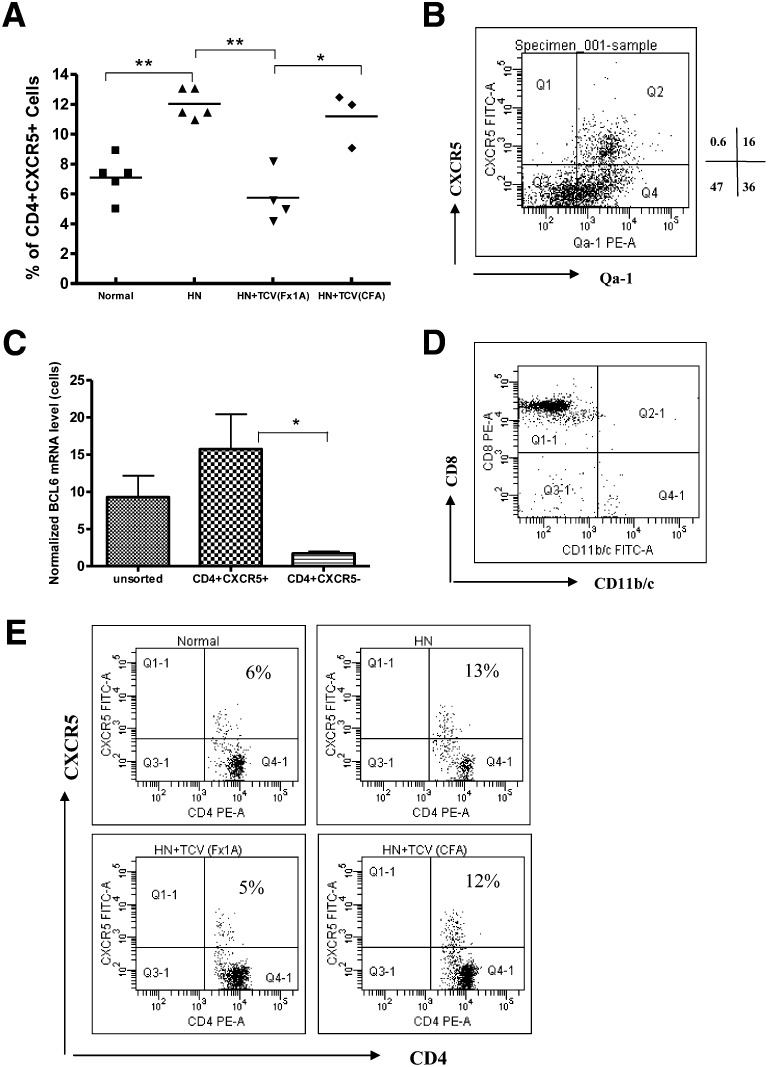
Increase in TFH Cells (CD4+ CXCR5+) in HN and Strong Expression of Qa-1 on TFH Cells
We compared the percentage of TFH cells between normal rats (CFA injected) and HN rats (Fx1A treated). There were significantly higher numbers of CD4+ CXCR5+ TFH cells in HN rats than the CFA group (Figure 6, A and E, P<0.01). There was a significant decrease in TFH cells after treatment with Fx1A-derived TCV in HN rats. However, TFH cells were not reduced in HN rats receiving CFA-derived TCV (Figure 6A, P<0.05). When gated on CD4+ cells, Qa-1 was highly expressed on CXCR5+ TFH cells (96% of TFH cells) (Figure 6B). Confirmation of the CXCR5+ CD4 T cells as TFH cells was done by assessing the level of the TFH-specific transcription factor B cell lymphoma (BCL)6 in CD4+/CXCR5+ and CD4+/CXCR5− cells from TCV rats. CD4+/CXCR5+ cells had greatly increased expression of BCL6 compared with CD4+/CXCR5− cells by real-time PCR consistent with a TFH phenotype (Figure 6C, P<0.05, n=6). To ensure that our CD8 population did not include DCs, we costained the sorted CD8 T cells with CD11b/c and confirmed that they were not of myeloid/dendritic cell lineage (Figure 6D).
Figure 6.
Characterization of TFH cells in HN. (A) Splenocytes were isolated from CFA and HN rats and analyzed by flow cytometry to assess the percentage of TFH cells between control (CFA injected) and HN (Fx1A treated) rats. There were significantly higher numbers of the CD4+ CXCR5+ TFH cells in HN rats than in the CFA group (*P<0.05; **P<0.01). (B) After gating on CD4+ cells, Qa-1 was highly expressed on CXCR5+ TFH cells (96% of total CD4+ cells). (C) Sorted CD4+ CXCR5+ TFH cells expressed a higher level of BCL6 mRNA than CD4+CXCR5− T cells by real-time PCR (*P<0.05). (D) Splenocytes were isolated from rats with HN and TCV, and then, sorted CD8+ T cells were analyzed by flow cytometry (purity>92%). The sorted CD8+ T cells were then stained with CD11b/c with limited CD11b/c expression. (E) Flow cytometric analysis showed TFH cells (CXCR5+ CD4+ T cells) increased to 12% after HN induction compared with normal rats (5% CXCR5+ of total CD4+ T cells); there is a significant decrease in TFH numbers in HN rats receiving TCV induced with Fx1A. However, HN rats receiving TCV induced with CFA had a similar increase in TFH cells (10%) as HN rats alone (n=6).
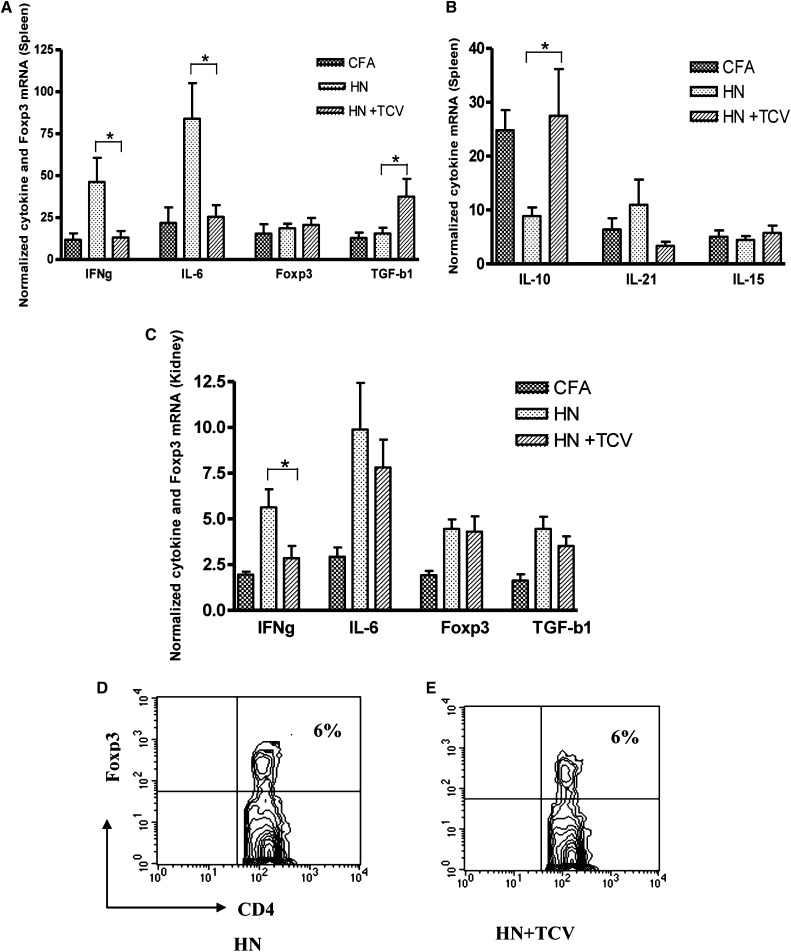
TCV Downregulates Inflammatory Cytokines and Upregulates TGF-β
To determine the mechanisms by which TCV protects renal function, we examined splenic and kidney cytokine levels at 12 weeks post-Fx1A/CFA immunization. We observed a significant reduction in IFN-γ and IL-6 and increase in TGF-β mRNA levels in the HN + TCV group compared with the HN group in spleen (Figure 7A, P<0.05). There was a similar expression pattern in the kidney, which showed a significant reduction in IFN-γ mRNA (P<0.05), but there was no significant difference for TGF-β in kidney (Figure 7C). However, there was a significant increase in IL-10 expression in the spleen of HN rats receiving TCV compared with HN rats alone (Figure 7B, P<0.05). Thus, TCV limits inflammatory cytokines, including IL-6 and IFN-γ, and induces the anti-inflammatory cytokines, such as TGF-β and IL-10. IL-21, a major TFH cytokine, was increased (but not significantly) in the spleens of HN rats (Figure 7B).
Figure 7.
Real-time quantitative PCR of cytokine and Foxp3 mRNA levels from splenocytes and kidney 12 weeks postimmunization with Fx1A. (A and B) HN + TCV rats had significantly lower levels of IL-6 and IFN-γ and higher levels of TGF-β and IL-10 than (A) splenocytes from HN rats (*P<0.05). (C) There was higher level of IFN-γ in kidney for HN + TCV rats compared with HN rats (*P<0.05). Splenic Foxp3 mRNA level was not different among the three groups of rats. Flow cytometric analysis showed no difference in Foxp3+ Treg numbers in circulation between (D) HN and (E) HN + TCV groups.
Foxp3+ CD4+ Tregs Remained Unchanged with or without TCV in Both Spleen and Circulation
To investigate whether TCV-induced protection was because of the expansion of Foxp3+ CD4+ Tregs, we examined Foxp3 mRNA level in the spleen and the number of Foxp3+ Tregs in circulation. As shown in Figure 7, D and E, the Foxp3 mRNA level remained unchanged with or without TCV (Figure 7D), and there was no significant difference of the numbers of CD4+ Foxp3+ T cells in circulation between HN and HN + TCV rats (Figure 7, D and E). Thus, our results suggest that Foxp3+ CD4+ Tregs are not involved in protecting TCV rats from renal injury, because Treg numbers and Foxp3 expression were not altered by TCV.
Discussion
TCV has been shown to induce protection in a variety of experimental autoimmune diseases.32–34 We described the important role of TCV in limiting autoimmune nephritis induced by Fx1A immunization. Rats were protected from renal injury by TCV, with normal renal function and significantly reduced proteinuria and autoantibodies. Histologically, glomerulosclerosis, tubular damage, and tubulointerstitial infiltrates were also significantly ameliorated by TCV.
Previous studies have suggested that T cells play an important role in the HN rat model and that they are associated with glomerular immune deposits and infiltration of glomeruli and interstitium by mononuclear cells.23 T cells activated with Fx1A seem crucial to the pathogenesis of HN.24,25,31 Treatment with depleting monoclonal antibodies to CD4 before Fx1A administration has been shown to completely prevent proteinuria in this model.26 The existence of T cells in the glomeruli at 8 weeks, coincident with the development of proteinuria, also implies a role for glomerular T cells in the pathogenesis of this disease model.25 Furthermore, the disease can be induced even in rats lacking components of the complement pathway, suggesting a more limited role of antibody compared with T cells in mediating injury.25,26 Although the potential epitopes expressed in the context of Qa-1 in this model have not been determined, Fx1A, which contains a mixture of renal tubular protein extract, may contain appropriate epitopes that can be expressed by autoreactive T cells in the context of Qa-1. The reductions in serum Ig level and immune deposition in the glomeruli of TCV-treated rats suggest either a loss of T cell help or a direct effect on antibody generation in the TCV-treated HN rats.
We have evaluated specificity using CFA TCV derived from CFA-treated rats and compared the effect with Fx1A-derived TCV in in vivo experiments. We have found that the protective effects of TCV (Fx1A primed) were not achieved by CD4+ T cells (CFA primed), with higher proteinuria, higher serum creatinine, and histologic renal injury that was equivalent to standard HN. We again found an increase in TFH cells in HN, and this increase was limited by TCV but only from TCV derived from Fx1A-immunized rats. We then assessed specificity with in vitro cytotoxicity assays; CD8+ T cells immunized by CFA did not eliminate Qa-1–expressing CD4+ T cells, whereas CD8+ T cells from Fx1A-immunized rats were effective in eliminating these Qa-1–expressing CD4 T cells.
Although we showed the elimination of Qa-1–expressing potentially autoreactive CD4 T cells by CD8 T cells from vaccinated rats in vitro, there is still a possibility that TCV can induce CD4 Tregs. However, the Foxp3 mRNA level remained unchanged with or without TCV, and there was no significant difference of the numbers of CD4+ Foxp3+ T cells in circulation between HN and HN + TCV rats. These results suggest that Foxp3+ CD4+ Tregs are not involved in the protection of TCV rats. Although we and others have found a role for CD4 Tregs in limiting renal injury by either inhibiting pathogenic T cells or possibly limiting innate immune injury in the adriamycin nephropathy model, these Tregs are not involved in the mechanism of TCV protection.35 In HN treated with TCV, CD8+ Tregs limit autoreactive CD4 T cells potentially through their actions on TFH cells.33,36
Our studies show that CD8+ Tregs induced by TCV are effective in limiting T cell-mediated renal disease. This protective effect is mediated through CD8 effector mechanisms that are Qa-1–restricted rather than through the MHC, potentially targeting TFH cells expressing high levels of Qa-1 but also potentially through other Qa-1–expressing target cells. We conclude that the range of autoimmune diseases that can be treated with TCV should be extended to autoimmune renal disease, and this finding also raises the intriguing question about the role of TFH cells in autoimmune disease such as membranous nephritis.
Concise Methods
Rats and Induction of Active HN
Male Lewis rats at the age of 8 weeks (weighing 180–200 g) were purchased from the Animal Resources Centre in Perth, Australia. Outbred male Sprague–Dawley rats were used for producing Fx1A as described previously.29,37,38 To induce HN, Lewis rats were immunized subcutaneously in both hind footpads. Each footpad was injected with 200 μl (100 μl in each footpad) emulsion containing 15 mg Fx1A, 1 mg Mycobacterium tuberculosis HRa37 (Difco, Detroit, MI), 100 µl Incomplete Freund’s Adjuvant (Sigma-Aldrich, St. Louis, MO), and 100 µl PBS. The CFA control rats were immunized with emulsion without Fx1A.
CD4+ TCV and Induction of Active HN
CD4+ T cells were purified from draining LNs of rats using MACS CD4+ MicroBeads (Miltenyi Biotec, Germany) 10 days after immunization with Fx1A. Purified CD4+ T cells were cultured in the presence of conA (5 μg/ml) for 40 hours. The cultured CD4+ T cells (of approximately 97% purity by FACS analysis) were then washed three times with Roswell Park Memorial Institute medium, irradiated at 30 Gy, and resuspended at 2.5×107/ml in Roswell Park Memorial Institute medium. Lewis rats were injected in the tail vein with 200 µl cell suspension (6×106 cells/rat). HN was induced 3 weeks after TCV.
Flow Cytometry
Antibodies used for flow cytometry included PE-conjugated anti-rat CD4+ antibody, biotin-conjugated Qa-1 antibody, Streptavidin-PE (BD Bioscience, Australia), and Blr1 (Santa Cruz Biotechnology). Staining was performed following the manufacturer’s instructions. All samples were analyzed on a FACScan analyzer (Becton Dickinson, Mountain Vies, CA). CellQuest software Version V was used for acquisition and analysis (Becton Dickinson, Australia).
Renal Function, Histology, and Morphometric Evaluation
Renal function was assessed by measurement of proteinuria excretion and serum albumin and creatinine as described previously.35 Histologic staining was performed, and the degree of renal injury was graded as described previously.35 In brief, for each biopsy, a semiquantitative score from two blinded trained observers was used to evaluate the degree of renal injury, and a minimum of 10 consecutive fields at a magnification of ×200 was assessed and scored in each section. The degree of renal injury was estimated by evaluating the percentage of renal injury per field, and it was graded on a scale of zero to four: 0, normal glomeruli, tubules, and interstitial volume; 0.5, small focal area of glomerular and tubular injury and interstitial infiltration; 1, involvement of <10% of the cortex; 2, involvement of up to 25% of the cortex; 3, involvement of 50%–75% of the cortex; 4, extensive damage involving >75% of the cortex.
Autoantibody Determination
Anti-Fx1A autoantibody (total IgG) was measured by ELISA as described previously.26 Briefly, an immunoELISA microtiter plate (NUNC, In Vitro Technology, Australia) was coated with soluble Fx1A (50 μg/ml). A control serum with a high level of anti-Fx1A antibody was used as standard, and serial dilutions of the positive serum were performed. Sample sera were diluted 8 and 64 times. Normal rat serum was used as the negative control. All samples and controls were added in triplicate to the plates. Absorbance was read at 405 nm on an ELISA plate reader (Multiskan Ascent, Pathtech, Australia) and corrected for a control sample of known strongly positive serum OD. The results are expressed as log2 titers.
Immunohistochemistry
Immunohistochemical staining was performed to look at infiltration of CD4+ and CD8+ T cells and macrophages in the kidney. Primary antibodies used in immunohistochemistry were mouse anti-rat W3/25 (CD4+ cells), mouse anti-rat MRC OX8 (CD8+ cells), and mouse anti-rat ED1 (macrophages; Serotec, Oxford, UK). The secondary antibody was biotinylated goat anti-mouse Ig (BD Biosciences, Australia). Immunohistochemical staining was performed, and interstitial infiltration was assessed as described previously.35
In Vitro Cytotoxicity Assay of CD8 T Cells against CD4 T Cell Targets
CD4+ and CD8+ cells were purified from normal, TCV, or HN rats as indicated. The target cells were either Qa-1–expressing or Qa-1–nonexpressing CD4+ T cells. CD8+ T cells isolated from both TCV and normal rats were tested in two different groups. Specificity for Qa-1 was tested using blocking antibodies. CD4+ T cells isolated from TCV were stained with biotin-conjugated Qa-1 monoclonal antibody followed by Streptavidin-PE, and Qa-1–positive CD4+ T cells were then sorted by flow cytometry according to the level of Qa-1expression on their surface. Qa-1 high and low CD4+ T cells labeled with CFSE low or normal CD4+ T cells labeled with CFSE high were cultured alone or cocultured with TCV CD8+ T cells for 4 days. In blocking experiments, coculture was performed in the presence of mouse anti-rat Qa-1 antibody. CD4+ cells were measured with FACS for their Qa-1 expression or sorted according to the level of Qa-1 expression on their surface.
Real-Time PCR for IL-6, Foxp3, IFN-γ, and TGF-β
Total RNA was isolated from rat spleen and was reverse-transcribed.35 cDNA was subjected to quantitative PCR analysis using commercial primers and Taqman probes specific for IL-6, Foxp3, IFN-γ, TGF-β, and glyceraldehyde-3-phosphate dehydrogenase (Applied Biosystem, Australia)35; n=6 from each group, and each sample was run in triplicate.
Statistical Analyses
Statistical analyses were performed using one-way ANOVA for multiple comparisons. Results are expressed as the group mean ± SD. Two group differences were analyzed by t test, with a P value (two-tailed) <0.05 considered statistically significant.
Disclosures
None.
Acknowledgments
We thank Prof. Carola Vinuesa of ANU for advice. We thank the animal house staff at the Children’s Hospital Research Institute for care of the animals. We thank Mary Sartor and Sanda Lum for help in cell sorting.
This work was supported by National Health and Medical Research Council of Australia Grants 249414 and 457408.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC: The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol 7: 231–237, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Cantor H, Shen FW, Boyse EA: Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: After immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med 143: 1391–1440, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YM, Alexander SI: CD8 regulatory T cells: What’s old is now new. Immunol Cell Biol 87: 192–193, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Kashleva H, Xu LX, Forman J, Flaherty L, Pernis B, Braunstein NS, Chess L: T cell vaccination induces T cell receptor Vbeta-specific Qa-1-restricted regulatory CD8(+) T cells. Proc Natl Acad Sci USA 95: 4533–4537, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panoutsakopoulou V, Huster KM, McCarty N, Feinberg E, Wang R, Wucherpfennig KW, Cantor H: Suppression of autoimmune disease after vaccination with autoreactive T cells that express Qa-1 peptide complexes. J Clin Invest 113: 1218–1224, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H: Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 467: 328–332, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Werneck MB, Cantor H: The immunoregulatory effects of Qa-1. Immunol Rev 212: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kumar V: Homeostatic control of immunity by TCR peptide-specific Tregs. J Clin Invest 114: 1222–1226, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantor H, Hugenberger J, McVay-Boudreau L, Eardley DD, Kemp J, Shen FW, Gershon RK: Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med 148: 871–877, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H: Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol 5: 516–523, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Correale J, Farez M, Gilmore W: Vaccines for multiple sclerosis: Progress to date. CNS Drugs 22: 175–198, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Fujii T, Okada M, Fujita Y, Sato T, Tanaka M, Usui T, Umehara H, Mimori T: Vaccination with autoreactive CD4(+)Th1 clones in lupus-prone MRL/Mp-Fas(lpr/lpr) mice. J Autoimmun 33: 125–134, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston T, Jensen P, Kumar V: Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+TCRalphabeta+ T cells. J Immunol 177: 7645–7655, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Zheng Z, Jiang Y, Chess L, Jiang H: The specificity of T cell regulation that enables self-nonself discrimination in the periphery. Proc Natl Acad Sci USA 106: 534–539, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Zhang L, Liang B, Saenger Y, Li J, Chess L, Jiang H: Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci USA 104: 20472–20477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loftus B, Newsom B, Montgomery M, Von Gynz-Rekowski K, Riser M, Inman S, Garces P, Rill D, Zhang J, Williams JC: Autologous attenuated T-cell vaccine (Tovaxin) dose escalation in multiple sclerosis relapsing-remitting and secondary progressive patients nonresponsive to approved immunomodulatory therapies. Clin Immunol 131: 202–215, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Correale J, Lund B, McMillan M, Ko DY, McCarthy K, Weiner LP: T cell vaccination in secondary progressive multiple sclerosis. J Neuroimmunol 107: 130–139, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Medaer R, Stinissen P, Truyen L, Raus J, Zhang J: Depletion of myelin-basic-protein autoreactive T cells by T-cell vaccination: Pilot trial in multiple sclerosis. Lancet 346: 807–808, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Trivedi S, Zang Y, Culpepper S, Rosenbaum E, Fernandez I, Martinez L, Hoffman RW, Greidinger EL: T cell vaccination therapy in an induced model of anti-RNP autoimmune glomerulonephritis. Clin Immunol 137: 281–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cattran D: Management of membranous nephropathy: When and what for treatment. J Am Soc Nephrol 16: 1188–1194, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G: End-stage renal disease in the Asian-Pacific region. Semin Nephrol 23: 107–114, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Heymann W, Hackel DB, Harwood S, Wilson SG, Hunter JL: Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med 100: 660–664, 1959 [DOI] [PubMed] [Google Scholar]
- 24.Penny MJ, Boyd RA, Hall BM: Permanent CD8(+) T cell depletion prevents proteinuria in active Heymann nephritis. J Exp Med 188: 1775–1784, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penny MJ, Boyd RA, Hall BM: Role of T cells in the mediation of Heymann nephritis. ii. Identification of Th1 and cytotoxic cells in glomeruli. Kidney Int 51: 1059–1068, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Quiza CG, Leenaerts PL, Hall BM: The role of T cells in the mediation of glomerular injury in Heymann’s nephritis in the rat. Int Immunol 4: 423–432, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Schiller B, He C, Salant DJ, Lim A, Alexander JJ, Quigg RJ: Inhibition of complement regulation is key to the pathogenesis of active Heymann nephritis. J Exp Med 188: 1353–1358, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spicer ST, Tran GT, Killingsworth MC, Carter N, Power DA, Paizis K, Boyd R, Hodgkinson SJ, Hall BM: Induction of passive Heymann nephritis in complement component 6-deficient PVG rats. J Immunol 179: 172–178, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Wu H, Walters G, Knight JF, Alexander SI: DNA vaccination against specific pathogenic TCRs reduces proteinuria in active Heymann nephritis by inducing specific autoantibodies. J Immunol 171: 4824–4829, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Wu H, Zhang GY, Knight JF: T cell receptor BV gene usage in interstitial cellular infiltrates in active Heymann nephritis. Nephrol Dial Transplant 16: 1374–1381, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Walters G, Wu H, Knight JF: Glomerular T cells in Heymann nephritis. Clin Exp Immunol 126: 319–325, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friese MA, Fugger L: Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol 66: 132–141, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Smith TR, Kumar V: Revival of CD8+ Treg-mediated suppression. Trends Immunol 29: 337–342, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Lu L, Cantor H: Generation and regulation of CD8(+) regulatory T cells. Cell Mol Immunol 5: 401–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YM, Zhang GY, Wang Y, Hu M, Wu H, Watson D, Hori S, Alexander IE, Harris DC, Alexander SI: Foxp3-transduced polyclonal regulatory T cells protect against chronic renal injury from adriamycin. J Am Soc Nephrol 17: 697–706, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Madakamutil LT, Maricic I, Sercarz E, Kumar V: Regulatory T cells control autoimmunity in vivo by inducing apoptotic depletion of activated pathogenic lymphocytes. J Immunol 170: 2985–2992, 2003 [DOI] [PubMed] [Google Scholar]
- 37.de Heer E, Daha MR, van Es LA: Lymph node cells from rats with Heymann’s nephritis produce in vitro autoantibodies directed against purified renal tubular antigen. Immunology 52: 743–752, 1984 [PMC free article] [PubMed] [Google Scholar]
- 38.Edgington TS, Glassock RJ, Dixon FJ: Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med 127: 555–572, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]