Abstract
Background
General agreement exists in the literature that individuals with aphasia can exhibit a working memory deficit that contributes to their language processing impairments. Though conceptualized within different working memory frameworks, researchers have suggested that individuals with aphasia have limited working memory capacity, impaired attention-control processes as well as impaired inhibitory mechanisms. However, across studies investigating working memory ability in individuals with aphasia, different measures have been used to quantify their working memory ability and identify the relationship between working memory and language performance.
Aims
The primary objectives of this article are to (1) review current working memory theoretical frameworks, (2) review tasks used to measure working memory, and (3) discuss findings from studies that have investigated working memory as they relate to language processing in aphasia.
Main Contribution
Though findings have been consistent across studies investigating working memory ability in individuals with aphasia, discussion of how working memory is conceptualized and defined is often missing, as is discussion of results within a theoretical framework. This is critical, as working memory is conceptualized differently across the different theoretical frameworks. They differ in explaining what limits capacity and the source of individual differences as well as how information is encoded, maintained, and retrieved. When test methods are considered within a theoretical framework, specific hypotheses can be tested and stronger conclusions that are less susceptible to different interpretations can be made.
Conclusions
Working memory ability has been investigated in numerous studies with individuals with aphasia. To better understand the underlying cognitive constructs that contribute to the language deficits exhibited by individuals with aphasia, future investigations should operationally define the cognitive constructs of interest and discuss findings within theoretical frameworks.
Working memory (WM) ability in adults with aphasia has received a great deal of attention in the literature in recent years (e.g., Hula & McNeil, 2008; Murray, 1999; Shuster, 2004; Wright & Shisler, 2005). WM can be operationalized as the ability to store representations while concurrently performing a task (Baddeley, 2003, 2007; Baddeley, Chincotta, & Adlam, 2001). General agreement exists that adults with aphasia present with WM deficits and it has been hypothesized that these deficits may partly account for the language characteristics present in adults with aphasia. The purposes of this article are to review current WM frameworks, present tasks used to measure WM, discuss findings from studies that have investigated WM as it contributes to language processing performance in individuals with aphasia, and identify gaps in the literature that warrant consideration in future studies.
Theoretical Frameworks of Working Memory
Though working memory is a relatively recent concept in aphasiology, it has a long history in cognitive psychology. In the early history of memory research, several researchers suggested that the human information processing system included a mechanism, referred to as short-term memory (STM), for storing small amounts of information for brief periods of time (Adams & Dijksstra, 1966; Brown 1958; Peterson, 1959; Pillsbury & Sylvester, 1940). One of the first and most influential models that attempted to capture this mechanism was the Atkinson and Shiffrin’s Modal Model (1968). The Modal Model consisted of a series of sensory registers (Crowder & Morton, 1968; Sperling, 1960) that fed into a short-term store (STS) of limited capacity that depended on control processes to prevent the information from decaying. The STS was responsible for both encoding the information in and retrieving it from long-term memory (LTM). The model was hypothesized to work sequentially, with the product of each phase being forwarded to the next for further processing.
Even though the Modal Model was able to account for several phenomena, it soon became clear that there were several limitations associated with it. Conceptually, the serial nature of the model appeared illogical and overly simplified (Anderson & Bower, 1973; Bower & Hilgard, 1981). One of the problems with the Modal Model, which eventually led to its disuse, was its inability to account for certain neuropsychological data. Based on the Modal Model, STS was a necessary link between the sensory experiences and encoding that information in LTM. However, Shallice and Warrington (1970) demonstrated that participants who had deficits in STS did not demonstrate deficits in their ability to encode new information in LTM; and, in contrast to what the Modal Model would predict the individual’s performance on several cognitive tasks was unimpaired. Baddeley and Hitch’s prototypical model of WM (1974) was introduced in an attempt to address some of the limitations of the Modal Model (Baddeley, 2007).
Baddeley’s Working Memory Model
Baddeley and Hitch (1974) published the seminal work in WM proposing a multicomponent model. Baddeley and Hitch’s original WM model included a central executive system (CES) and two slave systems: the visuospatial sketch pad and the phonological loop. The CES originally included a central store for holding abstract information but was eliminated when the model was revised (Baddeley, 1986; Cowan, 2008). Later, an episodic buffer was added to the model to serve as a storage component (Baddeley, 2000).
Though the CES was part of the first instantiation of the model (i.e., Baddeley & Hitch, 1974), it was not fully developed until later. Baddeley (1986) further developed and modeled the CES after Norman and Shallice’s (1980, 1986) supervisory attentional system (SAS). SAS is a limited capacity mechanism responsible for suppressing habitual responses by inhibiting inappropriately activated schemata if they are incompatible with a person’s current goals. The primary role of the CES is to delegate attentional control in a similar fashion to the SAS. Further, the CES has been implicated in linking WM with LTM (Baddeley, 1996, 2007), which is considered architecturally distinct from the WM model.
The slave systems include the visuospatial sketchpad and the phonological loop. The visuospatial sketchpad is responsible for retaining what appear to be at least two separate types of information, visual and spatial (Repovs & Baddeley, 2006). The phonological loop, which is of particular interest to aphasiologists, is responsible for rehearsing verbal information and recycling it to refresh the memory trace. Its role is central, because if the trace decays then the information is lost. The phonological loop is comprised of two subsystems: the phonological input store and an articulatory rehearsal process.
Based on prior research, Baddeley and Hitch (1974) argued for the existence of a structure that stores verbal information, i.e., the phonological input store. For example, in earlier studies, researchers have found that individuals make “acoustic” errors when they recall lists of phonologically similar consonants. Further, it was possible to predict their ability to recall the sequences based on the consonants’ phonological similarity, i.e., the phonemic similarity effect (Conrad, 1964; Conrad & Hull, 1964). These findings suggested that the to-be-recalled information in such tasks is coded phonologically, at least partially. Baddeley (1966) demonstrated that the phonemic similarity effects also influenced word recall. For example, word sequences such as man cat cap map can were more error prone than lists that consisted of phonologically dissimilar items such as pit day cow pen sup.
Word length has also been found to be a crucial factor that limits the number of items that can be held in memory, i.e., the word length effect. Baddeley, Thomson, and Buchanan (1975) found that lists containing short, monosyllabic words were better retained compared to lists containing polysyllabic words that required more time to articulate. Baddeley et al. argued that the number of distinct elements that can be actively maintained in memory is a function of the rate at which the elements dissolve and also the speed of the rehearsal process. Baddeley (1986) assumed that the elements in the phonological store decay in about 2 seconds unless they are refreshed. The nature of the mechanism underlying the phonological loop was further demonstrated under conditions that prevent subvocal rehearsal. Baddeley, Lewis, and Vallar (1984) had individuals perform an articulatory suppression task (Murray, 1967, 1968); the participants repeated an irrelevant word during input, and then recalled the word list. During the task, they found that the word length effect was eliminated and concluded that their results were consistent with the idea of an articulatory rehearsal process.
The episodic buffer (Baddeley, 2000, 2007) was later added to the model to serve as the interface among the two slave systems and LTM; it also serves as a workspace for integrating currently activated items. Before the episodic buffer was added, there was no mechanism in place that served as an interface between the two slave systems. Adding the episodic buffer was an attempt to address a number of limitations that were identified after stripping the CES of its storage capabilities and limiting it only to attentional control processes. Consider the following scenario: you plan to make a phone call and the number is written on the refrigerator. You need to walk to the phone that is in another room. The number has to be retained (through rehearsal) as well as the goal (going to the other room to make the phone call). During such a process we typically do not repeat “Going to the living room to make a phone call. Going to the living room to make a phone call”…. In the absence of the episodic buffer, there would be no mechanism for combining the phonological code that is repeated (i.e., the phone number) and the goal-oriented task (i.e., going to the living room to make the phone call). However, the episodic buffer allows for combining different types of information (e.g., visual, phonological, and/or semantic) and storing the product using a multimodal code. With the inclusion of the episodic buffer, then, the model is greatly extended and can be applied to other activities beyond recalling word lists, such as potentially accounting for how individuals are able to process discourse. It can also be used to explain how the ability to retain words increases dramatically when they are presented as part of a sentence (Baddeley, 2009).
Cowan’s Embedded Processes Model
Cowen proposed an information-processing model, the Embedded Processes Model, within which he cast a new light on WM. The model was influenced by Hebbian theory (Hebb, 1949), in which elements can be activated outside conscious awareness (Balota, 1983; Moray, 1959). The model was also proposed in response to some limitations of Baddeley and Hitch’s (1974) original multicomponent WM model. Cowan (1988) argued that the specialized nature of the stimuli (i.e., verbal and visuospatial) that could be accommodated in Baddeley’s model were too restrictive. Cowan proposed the notion of activated, generic representational formats to replace the specialized buffers and eliminate the need for domain-specific storage structures. However, it should be noted that by adding the episodic buffer, Baddeley resolved this limitation by allowing storage and processing of complex stimuli and linking information to LTM (Baddeley, 2009; Cowan, 2005).
The Embedded Processes Model assumes hierarchically arranged subsets of elements represented in memory (Cowan, 1988, 1995, 2005). First, it includes an activated subset of traces in LTM, which is conceptualized as a vast store of knowledge and prior events. These traces become activated in response to external stimuli or due to spreading activation and constitute the contents of STM. Then, there is a significantly smaller subset of elements that are in the focus of attention. When activated elements are in the focus of attention, they receive the maximum activation and are readily available for cognitive processing. Information that is activated but remains outside the focus of attention is usually processed only superficially. In other words, only perceptual features are activated (Broadbent, 1958; Conway, Cowan, & Bunting, 2001).
Two mechanisms come into play to compete for attention and determine what representations will enter conscious awareness. These mechanisms include the attention orienting system and the central executive. The attention orienting system is driven by novel stimuli. If elements in the perceptual present are important, novel, unpredictable or intense enough, some of their features become activated. If a critical threshold is exceeded, attention is recruited and the element is fully encoded and realized (Sokolov, 1963). Using the cocktail party effect as an example, a conversation is occurring across the room and your name is said. Though the conversation is not in your focus of attention, hearing your name, which has a low critical threshold, orients your attention to the conversation. Alternatively, the attention orienting system can also partly account for how representations blend in with the context: if a stimulus is presented but remains unchanged for a period of time, habituation may occur and the stimulus may “slip out” of conscious awareness. The second mechanism – the central executive – is a goal-oriented mechanism and requires involvement of voluntary and controlled attentional processes and is directly related to WM capacity.
Similar to Baddeley (1986), Cowan agrees that memory activation is time-limited and is subject to interference from similarly encoded items and decay if items are not reactivated. Interference may include retroactive or proactive interference. Retroactive interference refers to memory breakdowns for target items (e.g., where you ate lunch last Tuesday) that are caused by learning new material between the time period of initial encoding and tested recall (e.g., ate lunch at multiple places since last Tuesday). Proactive interference refers to memory breakdowns for recently learning items (e.g., new zip code) because of interference from previously learned items (e.g., previous zip code) (Anderson & Neely, 1996). Further, the focus of attention is capacity-limited both in terms of processing as well as storage (Cowan, 2006). For example, cognitively-healthy adults have a capacity for maintaining 4 +/−1 separate items; the number is less for children, older adults, and adults with aphasia (Broadbent, 1975; Cowan, 1999, 2001, 2005; Ronnberg, Larsson, Fogelsjoo, Nilsson, Lindberg, & Angquist, 1996; Sperling, 1960; Watkins, 1974; Ween, Verfaellie, & Alexander, 1996). Interference from previously presented stimuli and products of concurrent executive processing tasks can also “displace” contents from the focus of attention.
Cowan (1988, 1995) has applied the Embedded Processes Model to account for results found in WM studies. For example, the model can explain similarity effects such as phonemic similarity (e.g., ‘D’ and ‘B’ v. ‘A’ and ‘X’); but is also applicable to other stimulus types. He argued that representations held in working memory can be distorted in the presence of similar representations depending on how many features they share. Cowan also argued that maintenance of information can be achieved through different processes and these include rehearsal and recycling. Rehearsal is achieved by placing the items in the focus of attention. Alternatively, recycling items through the focus of attention can occur by volitionally searching through a set of items in the memory store to reactivate the desired item.
Hasher and Zacks Theoretical Framework
Similar to the previously presented theoretical frameworks, Hasher and Zacks (1988) agree that attention-control processes are central to explaining individual differences in WM. However, they place a greater emphasis on the inhibitory processes that restrict attention to task-relevant information (Hasher & Zacks, 1988; Hasher, Zacks, & May, 1999). They argue that activation of information occurs automatically; however, three different mechanisms may be engaged depending on the nature of the task, to “shield” the contents of WM. The mechanisms include access, deletion, and restraint.
The first mechanism, access, pertains to the ability of the cognitive apparatus to selectively attend to information (e.g., Simons & Chabris, 1999). Specifically, access is responsible for directing attention to goal-related information by suppressing distracting, goal-irrelevant elements from entering conscious awareness. Access operates early on in the processing sequence when activation spreads through representations in response to external or internal stimuli (Hasher, Tonev, Lustig, & Zacks, 2001).
Access has been empirically demonstrated in studies investigating the relationship between age and the efficiency of selective attention. For example, it has been shown that older adults’ processing times for familiar, well-learned tasks (e.g., reading) increase differentially in the presence of distraction compared to young adults (Connelly, Hasher, & Zacks, 1991). Further, the effect depends on whether the distractors are conceptually related to the targets (Carlson, Hasher, Connelly, & Zacks, 1995). These example findings have been interpreted as evidence of less efficient inhibitory skills for older individuals. Similar results have been reported in several studies in the selective attention literature (e.g., Gazzaley, Cooney, Rissman, & D’Esposito, 2005; Plude & Hoyer, 1986; Rabbitt, 1965; for extensive reviews on the topic see McDowd & Shaw, 2000 and Kramer & Madden, 2008).
The second inhibitory mechanism is deletion. Typically, activated representations are removed or deleted from conscious awareness once the represented information is no longer relevant. However, if the activated representations are not removed then the individual’s focus of attention would become cluttered with irrelevant information resulting in inefficient processing. Hasher and Zacks (1988) and Hamm and Hasher (1992) found that older adults demonstrated increased access to alternative interpretations following garden path passages. However, older participants were not able to remove the alternative interpretations when subsequently presented information demonstrated that a single interpretation was correct. Hamm and Hasher argued that this finding reflected older individuals’ limited efficiency of regulating/suppressing the alternative interpretations that were activated due to the nature of the garden path passages. Kim, Hasher, and Zacks (2007) extended Hamm and Hasher’s findings by testing another prediction made by Hasher and Zack’s model; if information was deemed irrelevant during one task but became relevant in a subsequent task, older individuals would show a benefit because the information required for the second task would not have been completely inhibited. Indeed, Kim and colleagues found that younger adults were less susceptible to distracting information. However, older adults were more likely to take advantage of the distracting information, to which they were exposed to during the first task, to perform better on the subsequent task (see also May & Hasher, 1998; Rowe, Valderrama, Hasher, & Lenartowicz, 2006).
The third mechanism – restraint, is responsible for controlling strong responses. Older individuals’ difficulty to inhibit overlearned responses has been demonstrated in a variety of tasks including the Stroop task (e.g., Spieler, Balota, & Faust, 1996; Wright, Capilouto, Srinivasan, & Fergadiotis, 2011), the antisaccade task (e.g., Butler, Zacks, & Henderson, 1999; Campbell, Al-Aidroos, Fatt, Pratt, & Hasher, 2010), and the “Moses Illusion” (May, Hasher & Bhatt, 1994). Using the Stroop task, Spieler et al. (1996) demonstrated that older individuals had more difficulty naming the colors while simultaneously inhibiting their tendency to process the words. This was evident both in terms of larger interference scores for the older group and a greater proportion of slower response times, although overall mean correct scores were similar. In the antisaccade task, participants have to overcome the natural tendency to look towards a visual distractor and instead look in the opposite direction to detect a briefly presented target. Butler et al. (1999) found that older adults had disproportionally greater difficulty looking in the correct direction compared to the young group. Finally, May et al. (1994) utilized the “Moses illusion” (Erickson & Mattson, 1981; Reder & Kusbit, 1991) and demonstrated older individuals’ difficulty overcoming strong contextual information. In this paradigm, participants answer general-knowledge questions; such as, “How many animals of each did Moses take on the ark?” Due to the misleading contextual information that acts as a cue (i.e., “animals”, “ark”, a biblical name) people have a strong propensity to respond “two” disregarding that Moses was probably never on the ark. May et al. (1994) argued that older adults’ higher probability of ignoring the incongruence was directly associated with their ability to regulate habituated responses.
In summary, several working memory theoretical frameworks have been developed in the literature to account for individual variation on WM tasks, age-related differences on WM tasks, as well as poor performance on WM tasks by neurologically impaired populations (e.g., adults with aphasia). There are some commonalities among the frameworks (See Table 1). For example, within the frameworks, WM has a limited capacity. Also, attention-control processes are central to explaining variance in WM performance. However, there are significant differences as well. Both Baddeley’s WM model and Cowan’s Embedded Processes Model emphasize the importance of the central executive in keeping representations in a readily accessible state. However, Baddeley’s model has a more rigid, crystallized structure with specialized buffers; whereas, Cowan’s model stresses the generality of representational formats that can be handled within his framework. Alternatively, Hasher and Zacks emphasize the ability to regulate attention through inhibitory mechanisms and place less emphasis on capacity to explain performance variation.
Table 1.
Summary of the similarities and differences among Baddeley’s, Cowan’s, and Hasher and Zacks’ theoretical frameworks of working memory.
| Baddeley’s Working Memory Model (2000) | Cowan’s Embedded Processes Model (2005) | Hasher & Zacks’ Theoretical Framework (1988) |
|---|---|---|
| The model consists of: (i) the central executive system, (ii) two slave systems: the visuospatial sketchpad and the phonological loop, and (iii) the episodic buffer. | Hierarchically embedded subsets of memory: (i) activated portions of LTM in response to internal and/or external cues (STM) and (ii) a subset of STM that is in the focus of attention. | |
| The central executive delegates attentional control and keeps representations in a rapidly accessible state for cognitive processing. | Inhibitory mechanisms (access, deletion, and restraint) down-regulate activation to achieve goals. WM depends on the efficiency of inhibiting goal-irrelevant information. | |
| The visuospatial sketchpad and the phonological loop are passive, domain-specific structures responsible for retaining visuospatial and verbal information, respectively. The episodic buffer serves (i) as the interface among the two slave systems and LTM, and (ii) as a workspace for integrating currently activated items. | Domain-general representational format instead of specialized buffers. | |
Working Memory Measures
Complex Span Tasks
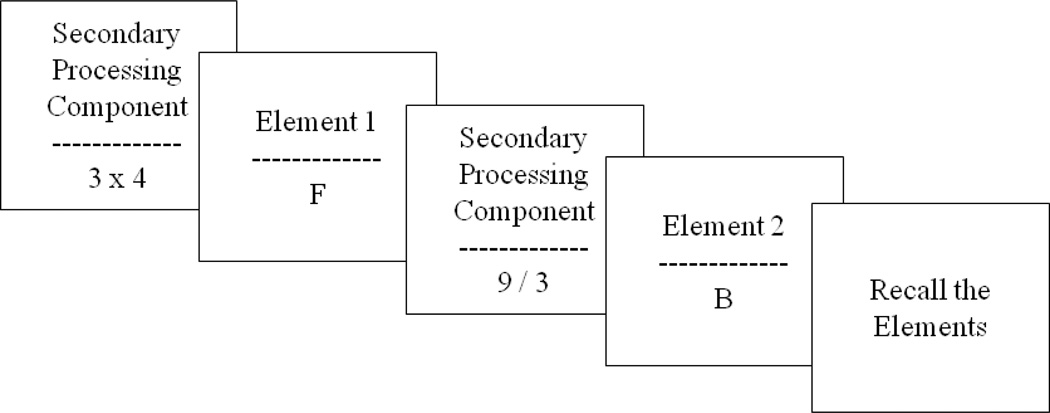
Researchers investigating the relationship between WM and human behavior outside the realm of aphasiology have commonly used tasks that are referred to as complex span tasks (CSTs). The first CST was designed by Daneman and Carpenter (1980) from the perspective of Baddeley and Hitch’s (1974) WM model. Since then, several variations have been created (see Conway, Kane, Bunting, Hambrick, Wilhelm, & Engle, 2005) and all of them are designed to combine a serial recall task with a concurrent processing load. Participants are instructed to remember a short list of stimuli (e.g., letters, numbers, words, shapes) for subsequent recall. They must simultaneously engage in a secondary processing task. Examples include solving mathematical equations, verifying the veracity of sentences, or making grammatically judgments. Specifically, each presentation of a to-be-remembered item is followed by the processing component. For each trial, participants listen to a randomly assigned number of items, typically varying from two to six; prior to recalling all the target items in order. Scoring is usually based on the number of items recalled in the correct serial position. As the number of the to-be-remembered items increases, participants have the opportunity to obtain better scores, assuming they have the WM capacity to recall the items correctly. See Figure 1 for CST example.
Figure 1.
Schematic of a single trail of a generic complex span task with example itmes. The presentation of each processing component (e.g., solving mathematical equations) is followed by to-be-remembered element (e.g., a letter). At the end of each trail, that usually consists of 2–6 elements (in this case 2), participants are asked to recall the elements presented in the correct serial position. Performance is estimated based on the total number of elements correctly recalled.
As with all tasks that are used to measure psychological constructs, performance on CSTs is multiply determined; that is, it includes several components to perform the task (e.g., processing, maintenance, and inhibition). The processing component is believed to challenge the primary task and increase the probability that the to-be-remembered items will be forgotten. Cowan (2005) has argued that introducing a demanding concurrent processing task results in displacement of stored information due to the limited capacity of the focus of attention. Others have suggested that the processing task disrupts the maintenance of the stored items; and, without maintenance the memory content is subject to decay (Camos, Lagner, & Barouillet, 2009). Attentional inhibitory processes are also thought to be involved in suppressing the representations of previously activated items thus keeping WM clutter-free (Hasher & Zacks, 1988; May, Hasher, & Kane, 1999). Failure to remove representations that are no longer relevant can result in buildup of proactive interference with detrimental consequences for the primary task.
Even though there is a lack of consensus on the exact processes that operate during CSTs, their psychometric properties have been investigated and established in numerous studies with neurologically intact adults. In terms of reliability Kane, Hambrick, Tuholski, Wilhelm, Payne, and Engle (2004) found that the coefficient alphas for three commonly used CST; operation span, reading span, and counting span ranged between .77 and .80. Related to predictive validity, performance on CST predicts performance on a great variety of higher order cognitive tasks, such as reading and listening comprehension (Daneman & Carpenter, 1983; Daneman & Merikle, 1996), language comprehension (King & Just, 1991), reading and mathematics (Hitch, Towse, & Hutton, 2001; Leather & Henry, 1994) and general fluid intelligence (Ackerman, Beier, & Boyle, 2002; Conway, Cowan, Bunting, Therriault, & Minkoff, 2002; Engle, Tuholski, Laughlin, & Conway, 1999). Despite researcher’s agreement that CSTs are valid measures of WM in neurologically intact adults, their construct validity invariance is open to empirical investigation when they are used with language impaired populations (see next section and conclusions).
N-back Task
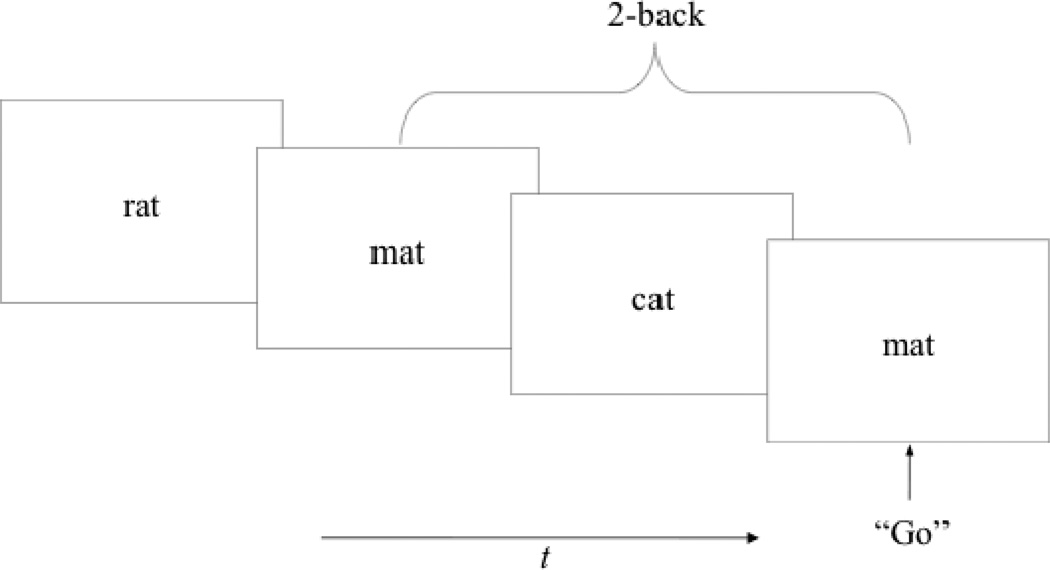
Kirchner (1958) first developed the n-back task to assess general retrieval processing. Since its inception, the n-back task has been used in numerous investigations as a measure of WM. On the surface, the use of the n-back task appears ideal for measuring WM; it requires participants to decide whether each stimulus in a sequence matches the one that appeared n items ago, where n is a pre-specified integer, usually 1, 2, or 3. Therefore, it requires temporary storage and manipulation of information while at the same time constantly updating the contents in WM. See Figure 2 for n-back example.
Figure 2.
Schematic of a 2-back as it unfolds in time. The participants are presented with a continuous stream of words and they have to respond to any token that was identical to the item appearing two tokents prior (e.g., rat… mat….cat… mat…) by pressing the spacebar on a keyboard to indicate a hit. Performance is usually determined using signal detection theory.
Even though the exact nature of the cognitive processes that are activated during the n-back is still not very clear, several components have been proposed to contribute to performance during an n-back task (Jonides, Schumacher, Smith, Lauber, Awh, Minoshima, & Koeppe, 1997; Oberauer, 2005). First, elements (e.g., words or letters) have to be encoded and interpreted. Then, a number of to-be-remembered elements, that is equal to the value of n in the task, have to be retained and remain available for intentional processing. Also, performance depends on the ability to suppress activation of elements that are irrelevant (in this case, elements further back than n items). Finally, successful performance depends on some mechanism that allows representations to be bound in a temporal context. That is, for every new item presented, elements have to be freed and the temporal order has to be re-established using only the necessary items.
Because of its simple and elegant structure that parallels the definition of WM, n-back is considered to have strong face validity that likely contributes to its extensive use in cognitive neuroscience and neuroimaging studies. However, despite its strong face validity, it appears that the available data in the literature make a mixed case for n-back’s construct validity. In a number of studies, researchers have tried to capture the essence of n-back by attempting to place it in a nomological net of interconnected constructs (McDonald, 1999). That is, they have used external criterion measures to investigate its convergent and predictive validity. With respect to intelligence, demanding levels of n-back (n>2) have been found to predict IQ (Hockey & Geffen, 2004), performance on complex cognitive tasks (Hull, Martin, Beier, Lane, & Hamilton, 2008), and brain activity in areas associated with WM-control functions. Further, a single common factor has been found to determine performance on both the n-back and complex span tasks (Schmiedek. Li, & Lindenberger, 2009). The latter finding is in agreement with the strong correlations among these tasks that have been reported previously in the literature (e.g., Shamosh et al., 2008). On the other hand, Kane, Conway, Miura, and Colflesh, (2007) found weak correlations between the n-back and general fluid intelligence (Raven’s Advanced Progressive Matrices Test; Raven, Raven, & Court, 1998). Also, a number of researchers have found that n-back and complex span tasks do not share the same variance (Jaeggi, Studer-Luethi, Buschkuehl, Su, Jonides, & Perig, 2010; Jaeggi, Buschkuehl, Perrig, & Meier, 2010; Kane et al., 2007; Oberauer, 2005; Roberts & Gibson, 2002).
To explain the conflicting data in the literature, it has been suggested that performance on the n-back could depend on processes that go beyond the traditional WM-related processes. Kane et al. (2007) attributed the n-back’s failure to measure WM to the fact that the n-back demands speeded recognition as opposed to serial recall, i.e., n-backs require participants to discriminate target elements from foils whereas tasks such as CSTs require participants to retrieve elements (e.g., words or letters) in a specific order without providing any external cues. Therefore, it is possible that different remembering processes are activated under conditions of interference depending on the nature of the task. Kane et al. hypothesized that the underlying mechanisms are so distinct that the two tasks may measure different aspects of the same construct or, possibly, even different constructs.
Considering the conflicting findings across n-back studies further research is warranted. Furthermore, WM measures developed and validated with cognitively healthy participants are often used with clinical populations. Reliability and validity of these WM measures with clinical populations cannot be assumed and should be empirically investigated.
Working Memory in Aphasia
General agreement exists in the literature that individuals with aphasia (IWA) present with impaired memory systems in conjunction with deficits in language processes (e.g., Burgio & Basso, 1997; Erickson, Goldinger, & LaPointe, 1996; LaPointe & Erickson, 1991; Martin & Saffran, 1997; Meier, Cohen, & Koemeda-Lutz, 1990; Ronnberg et al., 1996; Warrington & Shallice, 1969). Further, there is also general agreement that adults with aphasia present with a WM deficit that contributes to their language processing impairments (e.g., Caspari, Parkinson, LaPointe, & Katz, 1998, Friedmann & Gvion, 2003; Laures-Gore, Marshall, & Verner, 2010; Martin, 2008; Ronnberg et al., 1996; Sung, McNeil, Pratt, Dickey, Hula, Szuminsky, & Doyle, 2009; Ween, Verfaellie, & Alexander, 1996; Wright, Downey, Gravier, Love, & Shapiro, 2007; Wright & Shisler, 2005; Yasuda & Nakamura, 2000). Though conceptualized within different WM frameworks, researchers have suggested that IWA have limited WM capacity, impaired attention-control processes as well as impaired inhibitory mechanisms (e.g., Caspari et al., 1998; Hula & McNeil, 2008; Murray, 1999). As discussed earlier in regards to WM frameworks and measures, it is necessary to note that when investigating WM ability (in aphasia and other populations) attention-control processes should be considered. Several theories of WM acknowledge the inextricable nature of WM and attention (e.g., Baddeley’s inclusion of the CES). Further, terms such as ‘resources’ and ‘resource allocation’ are often used indiscriminately; that is, regardless of whether the discussion centers on attention ability or WM ability in IWA. As discussed in this section, many researchers have hypothesized that IWA present with an impaired CES, suggesting that PWA may present with impaired WM and attention-control processes. Consequently, such conclusions highlight the inextricable nature of WM and attention. Across studies investigating WM ability in IWA, different measures have been used to (1) quantify their WM ability, (2) identify the relationship between WM and language performance that is typically comprehension ability, and (3) to a lesser extent, specify the WM components that are impaired.
Span tasks have been used in several studies with IWA to investigate their WM abilities (e.g., Downey, Wright, Schwartz, Newhoff, Love, & Shapiro, 2004; Laures-Gore et al., 2010; Rönnberg et al., 1996; Ween et al., 1996). Span tasks typically include serial recall of digits or words, either in the order presented (i.e., forward span) or reverse order (i.e., backward span). Participants with and without aphasia perform more poorly on backward span tasks compared to forward span tasks (Downey et al., 2004; Laures-Gore et al., 2010; Wechsler, 2003), presumably because of the additional WM requirements. To complete the tasks, forward span tasks require storage and maintenance; whereas, backward span tasks require storage, maintenance, and mental manipulation of information (Baddeley, 2007; Wilde, Strauss, & Tulsky, 2004). The phonological loop is presumably active during span tasks, as are attention-control processes (i.e., central executive system), to maintain activation of the information.
Rönnberg et al. (1996) investigated memory ability in a small group of adults with mild aphasia due to subarachnoid hemorrhage. Study participants completed several tasks including forward digit and word span (rhyming and nonrhyming) tasks and a reading span task (Baddeley, Logie, Nimmosmith, & Brereton, 1985). Rönnberg et al. found that the IWA recalled significantly fewer items on the digit span, word span-rhyming, and reading span tasks compared to their control group. Further, they reported significant, positive correlations between digit span and word span-nonrhyming with the reading span task. Rönnberg et al. suggested that the IWA presented with an impaired phonological loop based on their reduced performance on the digit and word span tasks. Further, they suggested that the poor performance on the reading span task demonstrates a more general working memory deficit - an impaired CES. Rönnberg et al., discuss their findings using Baddeley’s (1986) WM model, however, the findings can be interpreted within Cowan’s Embedded Processes Model as well.
In a related study, Ween et al. (1996) also investigated memory ability in adults with mild aphasia. Study participants completed the auditory digit span subtest from the Wechsler Adult Intelligence Scale – Revised (WAIS-R; Wechsler, 1981). The aphasia participants also completed a nonword repetition (NWR) task to identify subgroups based on phonological processing ability. Results indicated that the aphasia group recalled significantly fewer items on the digit span task compared to the control group. Further, the aphasia subgroup that performed better on the NWR task performed significantly better on the digit span task compared to the aphasia subgroup that performed poorly on the NWR task. Ween et al. concluded that adults with mild aphasia may present with verbal memory deficits. Though Ween et al. do not discuss their results within any WM theoretical framework, their results replicate Rönnberg et al.’s findings, suggesting that IWA may present with an impaired phonological loop.
Laures-Gore et al. (2010) also explored digit span performance in IWA. Study participants included IWA and participants with right hemisphere brain damage (RBD). Participants completed forward and backward digit span tasks. Results indicated that the IWA recalled significantly fewer items on both span tasks compared to the RBD group. Both groups performed significantly better on the forward digit span task compared to the backward digit span task. Laures-Gore et al. (2010) also investigated the relationship between performance on the digit span tasks and aphasia severity (as measured by the Western Aphasia Battery-Revised [WAB-R; Kertesz, 2007]) in the IWA group. Participants’ performance on both the forward digit span and backward digit span significantly correlated with WAB-R aphasia quotients (AQ) leading Laures-Gore et al. (2010) to suggest that performance on the digit span tasks was related to aphasia severity. Results of the study are in line with others (i.e., Rönnberg et al., 1996; Ween et al., 1996); that is, IWA perform poorly on digit span tasks compared to control-matched peers.
Though the researchers did not consistently discuss their results within a theoretical framework, the results from these studies may be interpreted within Baddeley’s WM Model suggesting that adults with aphasia present with an impaired phonological loop. However, lacking from discussion is how an impaired phonological loop negatively impacts general language abilities; such as word retrieval ability, constructing and comprehending syntactically complex utterances, or producing and comprehending discourse. Finally, the significant relationship that Laures-Gore et al., (2010) found between digit span performance and WAB-R AQ is not entirely clear. It may simply indicate a general impairment in cognitive-linguistic ability rather than implicate a specific component of WM as contributing to severity of aphasia. Alternatively, findings could be interpreted as indicating a general impairment in attention-control processes (i.e., CES). As suggested by Baddeley (2003, 2007), the ability to store representations while concurrently performing a task (as required during digit span tasks and WAB tasks) reflects the demands imposed on the CES.
More complex, ‘language-heavy’ span tasks have also been administered to IWA to determine the extent of their WM deficit and further examine how WM impairments impact language processes; most commonly language comprehension. These types of span tasks are typically variations of Daneman and Carpenter’s (1980) reading span test (CSTs are described in detail in the previous section). Daneman and Carpenter developed the test to measure WM and test their hypothesis that variations in WM capacity can partly account for variations in reading efficiency in cognitively, healthy adults. Accordingly, a greater WM capacity reflects a more efficient CES, and subsequently, a more efficient reader. To complete the reading span task, participants read aloud sentences presented in sets and maintain the final word for later recall. The number of sentences within each set increases. The greatest number of final words correctly recalled indicates the individual’s reading span and serves as an estimate of their WM capacity. Variations of the reading span task have been created for use with IWA. Modifications have included reducing sentence length and complexity, including high frequency words as the final word for recall, and changing presentation modality (listening span v. reading span) and response type (recognition v. recall).
Just, Carpenter, and colleagues (Just & Carpenter, 1992; King & Just, 1991; MacDonald, Just, & Carpenter, 1992; Miyake et al., 1994) argued for a single resource model and hypothesized that individuals with limited WM capacity (such as IWA) would have impaired comprehension for syntactically complex sentences if concurrent memory load is required. Caplan and Waters (1999b) argued that such a model cannot explain syntactic comprehension performance by IWA. For example, Waters, Caplan, and Hildebrandt (1991) found that IWA performed poorly on Daneman and Carpenter’s (1980) reading span task; however, they were able to use syntactic structure to resolve sentence meaning. Caplan and Waters (1999a, 1999b) hypothesized that the WM system is specialized to include different components and proposed the ‘separate language resource theory’. This argument has been supported by others (e.g., Friedmann & Gvion, 2003; Wright et al., 2007).
Tompkins, Bloise, Timko, and Baumgaertner (1994) investigated WM ability in adults with right (RHD) and left hemisphere (LHD) brain damage. Of the 25 participants in the LHD group, 16 had been previously diagnosed with aphasia. They developed a listening span task, similar to the reading span task but more appropriate for use with individuals with brain damage; and, as a result their task has been used in numerous subsequent studies to estimate WM capacity in clinical populations (e.g., Monetta, Grindrod, & Pell, 2008; Sung et al., 2009; Wright, Newhoff, Downey, & Austermann, 2003). Tompkins et al. divided participants in the LHD group into two subgroups based on comprehension ability (i.e., high & low comprehension groups). The low comprehension group made significantly more errors on the WM measure compared to the high comprehension group suggesting a link between WM capacity and comprehension ability. Tompkins et al. suggested that performance on the WM measure may be a useful predictor of performance on tasks that maximize capacity limits. However, they also cautioned against using the task with individuals who have significant language comprehension and/or verbal production deficits (e.g., individuals with severe aphasia, apraxia of speech, etc…), as their performance on the measure may be more reflective of their linguistic and speech deficits, rather than a reflection of WM capacity limits.
Caspari et al. (1998) created two modified versions of the reading span task (listening span & reading span with recognition as the response type) and administered the tasks to 22 IWA. They found significant, positive correlations between listening span scores and WAB AQs as well as reading span scores and Reading Comprehension Battery for Aphasia (RCBA; LaPointe & Horner, 1979) scores. Caspari et al. interpreted these findings to suggest that WM capacity predicts language ability. Alternatively, the strong correlations may reflect that the same linguistic construct(s) are required to perform the tasks (i.e., language comprehension).
Sung et al. (2009) investigated the relationships among WM, sentence comprehension, and aphasia severity in 20 IWA. Measures included Tompkins et al.’s (1994) listening span task, listening and reading versions of the Revised Token Test (CRTT; McNeil et al., 2008), the Porch Index of Communicative Ability (PICA; Porch, 2001), and the Reading Comprehension Battery for Aphasia – 2 (RCBA-2; LaPointe & Horner, 1998). Sung et al. reported that performance on the WM measure predicted performance on both sentence comprehension measures. They also argued that these findings support Tompkins et al.’s (1994) hypothesis. That is, the significant correlations between the WM and sentence comprehension measures demonstrate that the sentence comprehension task taxed the IWA’s WM capacity limits. Further, they found strong correlations between performance on the WM measure and the aphasia severity measures (i.e., PICA & RCBA-2). They hypothesized that the strong correlations between the measures indicates that the same cognitive mechanisms underlie the processes required to perform them. Alternatively, it could be that the tasks are measuring similar processes; that is, language comprehension ability.
Recently, the n-back task (described in detail in the previous section) has been used in studies investigating WM ability in adults with aphasia (e.g., Christensen & Wright, 2010; Friedmann & Gvion, 2003; Wright et al., 2007). Friedmann and Gvion (2003) explored the relationship between verbal WM and sentence comprehension in adults with aphasia. Participants included three adults with conduction aphasia and three adults with agrammatic aphasia. Measures of WM included one level of an n-back task: a 2-back, and also several span measures (e.g., digit, word, and nonword span) and a listening span task similar to that of Tompkins et al. (1994). Results indicated that both aphasia groups presented with limited WM abilities; but they performed differently on the sentence comprehension task. The participants with agrammatic aphasia performed poorly in comprehending object-relative sentences (e.g., The boy that the girl chases is wearing a green shirt [Love & Oster, 2002]), whereas the participants with conduction aphasia did well comprehending these sentences. Statistical analysis was not possible due to the small N, so it is not known if a statistically significant relationship was present between WM and language comprehension. However, Friedmann and Gvion suggested that the effect of a verbal WM deficit on sentence comprehension is dependent on the type of processing (i.e., semantic, syntactic, phonological) required in the sentence.
Wright et al. (2007) employed the n-back task to examine the relationship between IWA’s performance on WM and auditory comprehension measures. Study participants included nine IWA and they completed three n-back tasks, each tapping different types of linguistic information (i.e., phonological, semantic, and syntactic), and the Subject-relative, Object-relative, Active, Passive Test of Syntactic Complexity (SOAP; Love & Oster, 2002) to assess syntactic sentence comprehension. The PhonoBack stimuli consisted of 25 CVC words, five ending in each of five frames: -at, -it, -in, -ill, and -ig. The SemBack stimuli consisted of five words from each of five different semantic categories: fruits, tools, furniture, animals, and clothing. Stimuli were controlled across categories for length and frequency of occurrence. The SynBack stimuli included five-word sentences with either active (“The doctor kissed the banker”) or passive (“The banker was kissed by the doctor”) sentence structures. Ten nouns and ten verbs were used; length, frequency of occurrence, and role (object/subject) were controlled. Participants’ performance declined as n-back task difficulty increased from 1-back to 2-back. Further, participants performed better on the semantic and phonological n-back tasks compared to the syntactic n-back task. Finally, a significant correlation was found between participants’ performances on the syntactic 2-back task and the SOAP non-canonical sentences. Based on the results of the study, Wright et al. concluded that WM ability for distinct types of linguistic information can be measured; and, findings support the growing literature that suggests separate WM systems for different types of linguistic information (e.g., Caplan & Waters, 1999).
Christensen and Wright (2010) used the n-back task to investigate the effect of varying linguistic processing demands in participants with and without aphasia. Stimuli for the n-back tasks varied in terms of “linguistic load” as determined by how rapidly the object could elicit a consistent name in a confrontation naming task. All participants performed significantly better when the stimuli carried a high linguistic load (e.g., fruits) compared to a low linguistic load (e.g., blocks in different arrays). Christensen and Wright suggested that poorer performance on the low linguistic load task reflects participants’ decreased ability to use linguistic strategies to perform the tasks. Further, as expected the IWA performed more poorly than the control group on the n-back tasks supporting previous results that IWA present with impaired WM abilities. Unlike findings with other WM measures (i.e., span tasks), Christensen and Wright did not find a significant correlation between aphasia severity (WAB-R AQ) and performance on the n-back tasks.
The n-back task has been used with IWA in relatively few studies. As such, cautious interpretation of findings with the n-back in the aphasia literature is warranted until future investigations reveal the underlying processes that determine performance on n-back tasks by IWA. Researchers who have used the n-back in investigations of WM in neurologically intact adults have found similar degraded performance as task difficulty (i.e., n back level) increases. However, as stated earlier the n-back has strong face validity, but its construct validity has not been well established. Future investigations using the n-back task with clinical and cognitively healthy populations should consider the task constructs and possible modifications to it to establish its construct validity; which in turn, will increase its usefulness in studies investigating WM ability in IWA.
Conclusions
In summary, several theoretical frameworks have been proposed and each conceptualizes WM differently. The differences include different architectures and forgetting mechanisms. Further, they differ in explaining what limits capacity and the source of individual differences; and, how information is encoded, maintained, and retrieved. When test methods are considered within a theoretical framework, specific hypotheses can be tested and stronger conclusions that are less susceptible to different interpretations can be made.
Though findings have been consistent across studies investigating WM ability in IWA; discussion of how WM is conceptualized and defined is often missing, as is discussion of results within a theoretical framework of WM. For example, when considering CSTs, IWA consistently perform more poorly on the measures compared to neurologically intact participants. Also, significant correlations between performance on CSTs and language measures have been reported. These findings have led researchers to conclude that IWA present with WM capacity deficits that contribute to their language processing impairments. However, task requirements must be considered and poor performance on the complex span measures may be due to comprehension and/or verbal production demands.
This example highlights limitations within the WM and aphasia literature. Experimental tasks are designed to measure specific cognitive functions; however, tasks are also designed for use with a specific population. When tasks are used with a different population than designed (i.e., IWA v. neurologically intact adults), measurement invariance and construct validity cannot be assumed and must be empirically evaluated. Further, when findings are considered within a theoretical framework; Baddeley’s original multicomponent WM Model (i.e., Baddeley & Hitch, 1986) is often applied. However, as Baddeley, Hitch, and Allen (2009) point out, the original model was better suited for investigating single-word level processes and could not easily account for performance on CSTs which is one of the main reasons they added the episodic buffer.
Finally, future investigations are warranted to better understand the interaction between WM and language processing abilities in aphasia. Most studies investigating the relationship between WM and language processing abilities in aphasia have focused on comprehension ability only. How WM ability contributes to verbal production ability in IWA should also be considered. Additionally, in future investigations, researchers should detail how they conceptualize and define WM and how the methods employed align within a theoretical framework. Further, precisely formulated hypotheses regarding WM and language processing should be tested using direct assessment of causal relationships rather than relying so heavily on correlational analyses. Finally, lesion information obtained from high resolution brain scans along with behavioral performance results should be considered to further investigate WM constructs that may contribute to the observed patterns exhibited in individuals with aphasia and, subsequently, contribute to the broader scientific community.
Acknowledgements
This research was partially supported by the National Institute on Aging Grant R01AG029476. We thank the two anonymous reviewers and Jamie Reilly for their thoughtful comments on a previous version of this article.
References
- Ackerman PL, Beier ME, Boyle MO. Individual differences in working memory within a nomological network of cognitive and perceptual speed abilities. Journal of Experimental Psychology-General. 2002;131(4):567–589. ER. [PubMed] [Google Scholar]
- Adams JA, Dijkstra S. Short-term memory for motor responses. Journal of Experimental Psychology. 1966;71(2):314–318. doi: 10.1037/h0022846. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Bower GH. Human associative memory. Oxford, England: V. H. Winston & Sons; 1973. [Google Scholar]
- Anderson MC, Neely JH. Interference and inhibition in memory retrieval. In: Bjork EL, Bjork RA, editors. Handbook of Perception and Memory. Vol. 10: Memory. San Diego: Academic Press; 1996. pp. 237–313. [Google Scholar]
- Atkinson R, Shiffrin R. Human memory: A proposed system and its control processes. In: Spence K, Spence J, editors. The psychology of learning and motivation: Advances in research and theory. New York: Academic Press; 1968. pp. 89–195. [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower GA, editor. The psychology of learning and motivation (vol. 8, pp. 47-89) New York, NY, USA: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baddeley AD. Short-term memory for word sequences as a function of acoustic, semantic and formal similarity. The Quarterly Journal of Experimental Psychology. 1966;18(4):362–365. doi: 10.1080/14640746608400055. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford, England: Oxford University Press; 1986. [Google Scholar]
- Baddeley A. Exploring the central executive. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology.Special Issue: Working Memory. 1996;49A(1):5–28. [Google Scholar]
- Baddeley A. The episodic buffer: A new component of working memory? Trends in Cognitive Sciences. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory, thought, and action. New York, NY, US: Oxford University Press; 2007. [Google Scholar]
- Baddeley A, Hitch GJ, Allen RJ. Working memory and binding in sentence recall. Journal of Memory and Language. 2009;61:438–456. [Google Scholar]
- Baddeley A, Chincotta D, Adlam A. Working memory and the control of action: Evidence from task switching. Journal of Experimental Psychology: General. 2001;130(4):641–657. [PubMed] [Google Scholar]
- Baddeley A, Logie R, Nimmosmith I, Brereton N. Components of fluent reading. Journal of Memory and Language. 1985;24(1):119–131. [Google Scholar]
- Baddeley AD, Thompson N, Buchanan M. Word length and the structure of short-term memory. Journal of Verbal Learning and Verbal Behavior. 1975 [Google Scholar]
- Balota DA. Automatic semantic activation and episodic memory encoding. Journal of Verbal Learning and Verbal Behavior. 1983;22(1):88–104. [Google Scholar]
- Bower GH, Hilgard ER. Theories of learning. 5th ed. Englewood Cliffs, NJ, US: Prentice-Hall; 1981. [Google Scholar]
- Broadbent DE. The magic number seven after fifteen years. In: Kennedy A, Wilkes A, editors. Studies in long-term memory. London, England: Wiley; 1975. pp. 3–18. [Google Scholar]
- Broadbent DE. Perception and communication. New York: Pergamon Press; 1958. [Google Scholar]
- Brown J. Some tests of the decay theory of immediate memory. Quarterly Journal of Experimental Psychology. 1958;10:12–21. [Google Scholar]
- Burgio F, Basso A. Memory and aphasia. Neuropsychologia. 1997;35(6):759–766. doi: 10.1016/s0028-3932(97)00014-6. [DOI] [PubMed] [Google Scholar]
- Butler KM, Zacks RT, Henderson JM. Suppression of reflexive saccades in younger and older adults: Age comparisons on an antisaccade task. Memory & Cognition. 1999;27(4):584–591. doi: 10.3758/bf03211552. [DOI] [PubMed] [Google Scholar]
- Camos V, Lagner P, Barrouillet P. Two maintenance mechanisms of verbal information in working memory. Journal of Memory and Language. 2009;61(3):457–469. ER. [Google Scholar]
- Campbell KL, Al-Aidroos N, Fatt R, Pratt J, Hasher L. The effects of multisensory targets on saccadic trajectory deviations: Eliminating age differences. Experimental Brain Research. 2010;201(3):385–392. doi: 10.1007/s00221-009-2045-5. ER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Waters G. Issues regarding general and domain specific resources. Behavioral Brain Sciences. 1999a;22:77–94. doi: 10.1017/s0140525x99001788. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters G. Verbal working memory capacity and language comprehension. Behavioral Brain Sciences. 1999b;22:114–126. doi: 10.1017/s0140525x99001788. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Hasher L, Connelly SL, Zacks RT. Aging, distraction, and the benefits of predictable location. Psychology and Aging. 1995;10(3):427–436. doi: 10.1037//0882-7974.10.3.427. [DOI] [PubMed] [Google Scholar]
- Caspari I, Parkinson SR, LaPointe LL, Katz RC. Working memory and aphasia. Brain and Cognition. 1998;37(2):205–223. doi: 10.1006/brcg.1997.0970. [DOI] [PubMed] [Google Scholar]
- Christensen SC, Wright HH. Verbal and non-verbal working memory in aphasia: What three n-back tasks reveal. Aphasiology. 2010;24(6–8):752–762. ER. [Google Scholar]
- Connelly SL, Hasher L, Zacks RT. Age and reading: The impact of distraction. Psychology and Aging. 1991;6(4):533–541. doi: 10.1037//0882-7974.6.4.533. [DOI] [PubMed] [Google Scholar]
- Conrad R. Acoustic confusions in immediate memory. British Journal of Psychology. 1964;55(1):75–84. [Google Scholar]
- Conrad R, Hull AJ. Information, acoustic confusion and memory span. British Journal of Psychology. 1964;55(4):429–432. doi: 10.1111/j.2044-8295.1964.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Cowan N, Bunting MF. The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin & Review. 2001;8(2):331–335. doi: 10.3758/bf03196169. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Cowan N, Bunting MF, Therriault DJ, Minkoff SRB. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30(2):163–183. [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin & Review.Special Issue: Memory Strength and Recency Judgments. 2005;12(5):769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychological Bulletin. 1988;104(2):163–191. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- Cowan N. Attention and memory: An integrated framework. New York, NY, US: Oxford University Press; 1995. [Google Scholar]
- Cowan N. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. New York, NY, US: Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24(1) doi: 10.1017/s0140525x01003922. 87-+ [DOI] [PubMed] [Google Scholar]
- Cowan N. Working memory capacity. New York, NY, US: Psychology Press; 2005. [Google Scholar]
- Cowan N. What are the differences between long-term, short-term, and working memory? Essence of Memory. 2008;169:323–338. doi: 10.1016/S0079-6123(07)00020-9. ER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder RG, Morton J. Precategorical acoustic storage (PAS) Perception & Psychophysics. 1969;5(6):365–373. [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning & Verbal Behavior. 1980;19(4):450–466. [Google Scholar]
- Daneman M, Carpenter PA. Individual-differences in integrating information between and within sentences. Journal of Experimental Psychology-Learning Memory and Cognition. 1983;9(4):561–584. [Google Scholar]
- Daneman M, Merikle PM. Working memory and language comprehension: A meta-analysis. Psychonomic Bulletin & Review. 1996;3(4):422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- Downey RA, Wright HH, Schwartz RG, Newhoff M, Love T, Shapiro LP. Toward a measure of working memory in aphasia. Poster presented at Clinical Aphasiology Conference; Park City, UT. 2004. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology-General. 1999;128(3):309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Erickson RJ, Goldinger SD, LaPointe LL. Auditory vigilance in aphasic individuals: Detecting nonlinguistic stimuli with full or divided attention. Brain and Cognition. 1996;30(2):244–253. doi: 10.1006/brcg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Erickson TD, Mattson ME. From words to meaning: A semantic illusion. Journal of Verbal Learning & Verbal Behavior. 1981;20(5):540–551. [Google Scholar]
- Friedmann N, Gvion A. Sentence comprehension and working memory limitation in aphasia: A dissociation between semantic-syntactic and phonological reactivation. Brain and Language. 2003;86(1):23–39. doi: 10.1016/s0093-934x(02)00530-8. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8(10):1298. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Hamm VP, Hasher L. Age and the availability of inferences. Psychology and Aging. 1992;7(1):56–64. doi: 10.1037//0882-7974.7.1.56. [DOI] [PubMed] [Google Scholar]
- Hasher L, Tonev ST, Lustig C, Zacks R. Inhibitory control, environmental support, and self initiated processing in aging. In: Naveh-Benjamin M, Moscovitch M, Roediger RL III, editors. Perspectives on Human Memory and Cognitive Aging: Essays in Honour of Fergus Craik. East Sussex, UK: Psychology Press; 2001. pp. 286–297. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory, vol. 22. San Diego, CA, US: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance XVII: Cognitive regulation of performance: Interaction of theory and application. Cambridge, MA, US: The MIT Press; 1999. pp. 653–675. [Google Scholar]
- Hebb DO. The organization of behavior; a neuropsychological theory. Oxford, England: Wiley; 1949. [Google Scholar]
- Hitch GJ, Towse JN, Hutton U. What limits children's working memory span? theoretical accounts and applications for scholastic development. Journal of Experimental Psychology-General. 2001;130(2):184–198. doi: 10.1037//0096-3445.130.2.184. ER. [DOI] [PubMed] [Google Scholar]
- Hockey A, Geffen G. The concurrent validity and test-retest reliability of a visuospatial working memory task. Intelligence. 2004;32(6):591–605. [Google Scholar]
- Hula WD, McNeil MR. Models of attention and dual-task performance as explanatory constructs in aphasia. Seminars in Speech and Language. 2008;29(03):169–187. doi: 10.1055/s-0028-1082882. [DOI] [PubMed] [Google Scholar]
- Hull R, Martin RC, Beier ME, Lane D, Hamilton AC. Executive function in older adults: A structural equation modeling approach. Neuropsychology. 2008;22(4):508–522. doi: 10.1037/0894-4105.22.4.508. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory (Hove, England) 2010;18(4):394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Studer-Luethi B, Buschkuehl M, Su Y, Jonides J, Perrig WJ. The relationship between n-back performance and matrix reasoning—Implications for training and transfer. Intelligence. 2010;38(6):625–635. [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9(4):462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: Individual differences in working memory. Psychological Review. 1992;99(1):122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent-variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology-General. 2004;133(2):189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Miura TK, Colflesh GJH. Working memory, attention control, and the n-back task: A question of construct validity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33(3):615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery- revised. New York: Grune and Stratton; 2007. [Google Scholar]
- Kim S, Hasher L, Zacks RT. Aging and benefit of distractibility. Psychonomic Bulletin & Review. 2007;14(2):301–305. doi: 10.3758/bf03194068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J, Just MA. Individual differences in syntactic processing: The role of working memory. Journal of Memory and Language. 1991;30(5):580. [Google Scholar]
- Kirchner WK. Age differences in short-term retention of rapidly changing information. Journal of Experimental Psychology. 1958;55(4):352–358. doi: 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed. New York, New York: Psychology Press; 2008. pp. 189–250. [Google Scholar]
- Lapointe LL, Erickson RJ. Auditory vigilance during divided task attention in aphasic individuals. Aphasiology. 1991;5(6):511–520. [Google Scholar]
- LaPointe LL, Horner J. Reading comprehension battery for aphasia. Austin, TX: Pro-Ed; 1979. [Google Scholar]
- LaPointe LL, Horner J. Reading comprehension battery for aphasia - 2. Austin, TX: Pro-Ed; 1998. [Google Scholar]
- Laures-Gore J, Marshall RM, Verner E. Digit span differences in aphasia and right brain damage. Aphasiology. 2010;25(1):43–56. doi: 10.1080/02687031003714426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leather CV, Henry LA. Working-memory span and phonological awareness tasks as predictors of early reading-ability. Journal of Experimental Child Psychology. 1994;58(1):88–111. doi: 10.1006/jecp.1994.1027. [DOI] [PubMed] [Google Scholar]
- Love T, Oster E. On the categorization of aphasic typologies: The SOAP (A test of syntactic complexity) Journal of Psycholinguistic Research. 2002;31(5):503–529. doi: 10.1023/a:1021208903394. [DOI] [PubMed] [Google Scholar]
- MacDonald MC, Just MA, Carpenter PA. Working memory constraints on the processing of syntactic ambiguity. Cognitive Psychology. 1992;24:56–98. doi: 10.1016/0010-0285(92)90003-k. [DOI] [PubMed] [Google Scholar]
- Martin N. The role of semantic processing in short-term memory and learning: Evidence from aphasia. Chapter 11. In: Thorn A, Page M, editors. Interactions between short-term and long-term memory in the verbal domain. Psychology Press; 2008. pp. 220–243. [Google Scholar]
- Martin N, Saffran EM. Language and auditory-verbal short-term memory impairments: Evidence for common underlying processes. Cognitive Neuropsychology. 1997;14(5):641–682. [Google Scholar]
- May CP, Hasher L, Bhatt A. Time of day affects susceptibility to misinformation in younger and older adults; Cognitive Aging Conference; Atlanta, GA. 1994. [Google Scholar]
- May C, Hasher L. Synchrony effects in inhibitory control over thought and action. Journal of Experimental Psychology: Human Perception and Performance. 1998;24(2):363–379. doi: 10.1037//0096-1523.24.2.363. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Kane MJ. The role of interference in memory span. Memory & Cognition. 1999;27(5):759–767. doi: 10.3758/bf03198529. [DOI] [PubMed] [Google Scholar]
- McDonald RP. Test theory: A unified treatment. Mahwah, New Jersey: Lawrence Erlbaum Associates Inc; 1999. [Google Scholar]
- McDowd JM, Shaw RJ. Attention and aging: A functional perspective. In: Craik FIM, Salthouse A T, editors. The handbook of aging and cognition. 2nd ed. Mahwah, New Jersey: Lawrence Erlbaum Associates Inc; 2000. pp. 221–292. [Google Scholar]
- McNeil MR, Sung JE, Pratt SR, Szuminsky N, Kim A, Ventura M, et al. Concurrent validation of the Computerised Revised Token Test (CRTT) and three experimental reading CRTT-R versions in normal elderly individuals and persons with aphasia. Presented at Clinical Aphasiology Conference; Teton Village, WY. 2008. [Google Scholar]
- Meier E, Cohen R, Koemeda-Lutz M. Short-term-memory of aphasics in comparing token stimuli. Brain and Cognition. 1990;12(2):161–181. doi: 10.1016/0278-2626(90)90013-e. [DOI] [PubMed] [Google Scholar]
- Miyake A, Carpenter PA, Just MA. A capacity approach to syntactic comprehension disorders: making normal adults perform like aphasic patients. Cognitive Neuropsychology. 1994;11(6):671–717. [Google Scholar]
- Monetta L, Grindrod CM, Pell MD. Effects of working memory capacity on inference generation during story comprehension in adults with parkinson's disease. Journal of Neurolinguistics. 2008;21(5):400–417. ER. [Google Scholar]
- Moray N. Attention in dichotic listening: Affective cues and the influence of instructions. The Quarterly Journal of Experimental Psychology. 1959;11:56–60. [Google Scholar]
- Murray LL. Review attention and aphasia: Theory, research and clinical implications. Aphasiology. 1999;13(2):91–111. [Google Scholar]
- Norman DA, Shallice T. Attention to action. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. New York: Plenum; 1986. pp. 1–18. [Google Scholar]
- Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior technical report no. 8006. 1980 No. CHIP-99)
- Oberauer K. Binding and inhibition in working memory: Individual and age differences in short-term recognition. Journal of Experimental Psychology: General. 2005;134(3):368–387. doi: 10.1037/0096-3445.134.3.368. [DOI] [PubMed] [Google Scholar]
- Peterson L, Peterson MJ. Short-term retention of individual verbal items. Journal of Experimental Psychology. 1959;58(3):193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Pillsbury WB, Sylvester A. Retroactive and proactive inhibition in immediate memory. Journal of Experimental Psychology. 1940;27(5):532–545. [Google Scholar]
- Plude DJ, Hoyer WJ. Age and the selectivity of visual information processing. Psychology and Aging. 1986;1(1):4. doi: 10.1037//0882-7974.1.1.4. [DOI] [PubMed] [Google Scholar]
- Porch BE. The Porch Index of Communicative Ability. 3rd ed. Palo Alto, CA: Consulting Psychologists Press; 2001. [Google Scholar]
- Rabbitt P. An age-decrement in the ability to ignore irrelevant information. Journal of Gerontology. 1965;20:233–238. doi: 10.1093/geronj/20.2.233. [DOI] [PubMed] [Google Scholar]
- Raven JC, Raven JE, Court JH. Progressive matrices. Oxford, England: Oxford Psychologists Press; 1998. [Google Scholar]
- Reder LM, Kusbit GW. Locus of the Moses illusion: Imperfect encoding, retrieval, or match? Journal of Memory and Language. 1991;30(4):385–406. [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139(1):5–21. doi: 10.1016/j.neuroscience.2005.12.061. ER. [DOI] [PubMed] [Google Scholar]
- Roberts R, Gibson E. Individual differences in sentence memory. Journal of Psycholinguistic Research. 2002;31(6):573–598. doi: 10.1023/a:1021213004302. [DOI] [PubMed] [Google Scholar]
- Ronnberg J, Larsson C, Fogelsjoo A, Nilsson LG, Lindberg M, Angquist KA. Memory dysfunction in mild aphasics. Scandinavian Journal of Psychology. 1996;37(1):46–61. doi: 10.1111/j.1467-9450.1996.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Rowe G, Valderrama S, Hasher L, Lenartowicz A. Attentional disregulation: A benefit for implicit memory. Psychology and Aging. 2006;21(4):826–830. doi: 10.1037/0882-7974.21.4.826. ER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedek F, Li S, Lindenberger U. Interference and facilitation in spatial working memory: Age-associated differences in lure effects in the n-back paradigm. Psychology and Aging. 2009;24(1):203–210. doi: 10.1037/a0014685. [DOI] [PubMed] [Google Scholar]
- Shallice T, Warrington EK. Independent functioning of verbal memory stores: A neuropsychological study. The Quarterly Journal of Experimental Psychology. 1970;22(2):261–273. doi: 10.1080/00335557043000203. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway ARA, et al. Individual differences in delay discounting: Relation to intelligence, working memory, and anterior prefrontal cortex. Psychological Science. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shuster LI. Resource theory and aphasia reconsidered: Why alternative theories can better guide our research. Aphasiology. 2004;18(9):811–830. ER. [Google Scholar]
- Simons DJ, Chabris CF. Gorillas in our midst: Sustained inattentional blindness for dynamic events. Perception (London) 1999;28:1059. doi: 10.1068/p281059. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. New York: Pergamon; 1963. [Google Scholar]
- Sperling G. The information available in brief visual presentations. Psychological Monographs: General and Applied. 1960;74(11):1–29. [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the alzheimer's type. Journal of Experimental Psychology-Human Perception and Performance. 1996;22(2):461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Sung JE, McNeil MR, Pratt SR, Dickey MW, Hula WD, Szuminsky NJ, Doyle PJ. Verbal working memory and its relationship to sentence-level reading and listening comprehension in persons with aphasia. Aphasiology. 2009;23(7–8):1040–1052. ER. [Google Scholar]
- Tompkins CA, Bloise CGR, Timko ML, Baumgaertner A. Working-memory and inference revision in brain-damaged and normally aging adults. Journal of Speech and Hearing Research. 1994;37(4):896–912. doi: 10.1044/jshr.3704.896. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Selective impairment of auditory verbal short-term memory. Brain. 1969;92:885–896. doi: 10.1093/brain/92.4.885. [DOI] [PubMed] [Google Scholar]
- Waters G, Caplan D, Hildebrandt N. On the structure of verbal short-term memory and its functional role in sentence comprehension: Evidence from neuropsychology. Cognitive Neuropsychology. 1991;8:81–126. [Google Scholar]
- Watkins MJ. Concept and measurement of primary memory. Psychological Bulletin. 1974;81:695–711. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale – revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler memory scale - 3rd edition. San Antonio, TX: Pyschological Corporation; 1997. Technical manual. [Google Scholar]
- Ween JE, Verfaellie M, Alexander MP. Verbal memory function in mild aphasia. Neurology. 1996;47(3):795–801. doi: 10.1212/wnl.47.3.795. [DOI] [PubMed] [Google Scholar]
- Wilde NJ, Strauss E, Tulsky DS. Memory span on the wechsler scales. Journal of Clinical and Experimental Neuropsychology. 2004;26(4):539–549. doi: 10.1080/13803390490496605. [DOI] [PubMed] [Google Scholar]
- Wright HH, Capilouto GJ, Srinivasan C, Fergadiotis G. Story processing ability in cognitively healthy younger and older adults. Journal of Speech, Language, and Hearing Research. 2011;54:900–917. doi: 10.1044/1092-4388(2010/09-0253). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HH, Downey RA, Gravier M, Love T, Shapiro LP. Processing distinct linguistic information types in working memory in aphasia. Aphasiology. 2007;21(6–8):802–813. doi: 10.1080/02687030701192414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HH, Newhoff M, Downey R, Austermann S. Additional data on working memory in aphasia. Journal of International Neuropsychological Society. 2003;9:302. [Google Scholar]
- Wright HH, Shisler RJ. Working memory in aphasia: Theory, measures, and clinical implications. American Journal of Speech-Language Pathology. 2005;14(2):107–118. doi: 10.1044/1058-0360(2005/012). [DOI] [PubMed] [Google Scholar]
- Yasuda K, Nakamura T. Comprehension and storage of four serially presented radio news stories by mild aphasic subjects. Brain and Language. 2000;75:399–415. doi: 10.1006/brln.2000.2377. [DOI] [PubMed] [Google Scholar]