Abstract
Mammalian cell-surface receptors typically display N- or O-linked glycans added post-translationally. Plant lectins such as PHA can activate the TCR and other cell surface receptors by binding to glycans and initiating receptor cross-linking. Pathogenic microorganisms such as Bordetella pertussis also express proteins with lectin-like activities. Similar to plant lectins, pertussis toxin (PTx) can activate the TCR and bind to a variety of glycans. However, whether the lectin-like activity of PTx is responsible for its ability to activate TCR signaling has not been formally proven. Here we examined the ability of PTx and a panel of lectins to activate the TCR or a CD8α/CD3ζ chimeric receptor (termed CD8ζ). We demonstrate that CD8ζ rescues PTx-induced signaling events lacking in TCR null cells. This result indicates that CD8ζ can substitute for TCR and supports the hypothesis that PTxB (functioning as a lectin) stimulates signaling via receptor cross-linking rather than by binding to a specific epitope on the TCR. Moreover, PTx is able to activate signaling by binding either N-linked or O-linked glycan modified receptors as the TCR displays N-Linked glycans while CD8ζ displays O-linked glycans. Finally, studies with a diverse panel of lectins indicate that the signaling activity of the lectins does not always correlate with the biochemical reports of ligand preferences. Comparison of lectin signaling through TCR or CD8ζ, allows us to better define the structural and functional properties of lectin-glycan interactions using a biological based signaling readout.
Keywords: Lectins, T cell Receptors, Signal Transduction, Pertussis Toxin, Calcium Signaling
Glycans are among the most abundant molecules present on the surface of cells. Cells and tissues possess unique patterns of glycosylation, which provide an additional layer of control in a variety of biological processes including pathogen recognition, cell signaling, and leukocyte extravasation. The two main forms of protein glycosylation are termed N-linked and O-linked. In the case of N-linked glycosylation, long oligosaccharide chains containing a mannose core are added to asparagine residues; for O-linked glycosylation, short oligosaccharide chains are attached to serine or threonine residues, and mannose is usually excluded. Proteins termed “lectins”, possess one or more glycan binding sites, and are produced by a wide variety of organisms, including animals, plants, and bacteria.
Several lectins derived from plants, including concanavalin A (ConA), phytohemagglutinin (PHA), and wheat-germ agglutinin (WGA), have been central to T cell research due to their ability to activate the T cell receptor (TCR) independently of antigen. These lectins can bind to multiple components of the TCR complex and induce clustering of intracellular receptor chains. This leads to phosphorylation of the ITAM motifs and activation of downstream signaling molecules (Figure 1). Most plant lectins contain multiple glycan binding sites, but they vary widely in structure and glycan recognition preferences. Table 1 summarizes the preferred binding ligands for a variety of lectins based on previously published data and glycan array data made publically available by the Consortium for Functional Glycomics (http://functionalglycomics.org). While ConA, PHA and WGA can cluster and activate the TCR, they do so through interactions with different glycans. ConA is a homotetramer, and each subumit contains a binding site for α-D-mannosyl oligomers found on N-linked glycans(1, 2). PHA is a tetramer, formed from two different types of subunits, PHA leucoagglutinin (PHA-L) and PHA erythroagglutinin (PHA-E), which can associate in any combination(3). PHA-L binds to the Galβ1–4GlcNAcβ1–2Man sequence commonly found in N-linked glycans, while PHA-E binds to terminal Gal and GlcNAc groups on branched N-linked glycans(5, 6). The PHA-L homotetramer is a strong activator of the TCR, while the PHA-E homotetramer primarily mediates red cell agglutination(4). WGA is a homodimer, and each subunit has two glycan binding sites(7). WGA can bind either GlcNAc or Neu5Ac present on N- or O-linked glycans(8, 9).
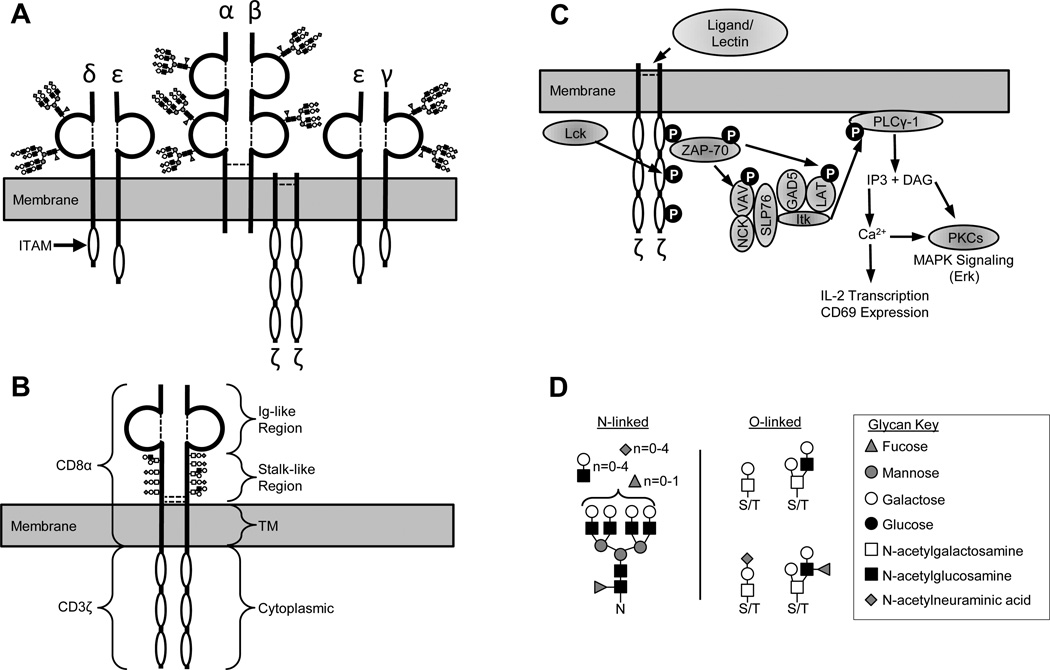
Figure 1. Schematic representation of TCR and CD8ζ structure, glycosylation patterns, and signaling ability.
(A) Schematic representation of the TCR complex showing the α, β, γ, δ, ε, and ζ chains. Disulfide bonds are represented by dashed lines, immunoglobulin-like domains are represented by ¾ circles, cytoplasmic ITAM motifs are represented by white ovals. N-linked glycans representative of those typically found on the TCR are shown in CFG symbol nomenclature (key in panel D). (B) Schematic representation of the CD8ζ chimeric protein (314 amino acids). Residues 1–203 are from CD8α, and contain an Ig-like fold, a stalk-like region and a transmembrane domain. Residues 203–314 are from the cytoplasmic domain of CD3ζ which contains 3 ITAM motifs. The chimera is expressed as a disulfide-linked homodimer, with extensive O-linked glycosylation on the stalk-like region. (C) Schematic representation of the signaling pathway downstream of ζ chain phosphorylation that can be activated by clustering either TCR α/β chains or CD8ζ chimeric molecules. (D) Representative examples of N-linked and O-linked glycans typically found on human T cells according to the CFG glycan profiling database. Glycans are shown in CFG symbol nomenclature (key on right). N, asparagine; S/T, serine/threonine. “n” indicates the number of modified branches.
Table 1.
Signaling and binding activity of bacterial and plant lectins.
| Ca2+ Signaling | ERK Signaling | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lectin | Abrv. | Preferred Ligands1 | Protein Glycan Targets |
WT | CD8ζ | TCR− | WT | CD8ζ | TCR− |
| Erythrina cristagalli lectin | ECL | Galβ1–4GlcNAc | N-glycans, O-glycans | + | + | − | + | ND | + |
| Jacalin (Artocarpus integliforia) | JAC | Galβ1–3GalNAc (T antigen) | O-glycans | + | + | − | + | + | + |
| Vicia villosa agglutinin | VVA | GalNAcα1-Ser/Thr (Tn antigen) | O-glycans | + | + | − | + | + | + |
| Phaseolus vulgaris erythroagglutinin | PHA-E | GlcNAcβ1–2Manα1–3(GlcNAcβ1–4)(Galβ1–4GlcNAcβ1–2Manα1–6)Man | N-glycans | + | + | − | + | + | − |
| Bordetella pertussis toxin | PTx | NeuAc, Complex-type N-glycans | N-glycans, O-glycans | + | + | − | + | + | − |
| Wheat germ agglutinin (Triticum aestivum) | WGA | GlcNAc, NeuAc | N-glycans, O-glycans | + | + | − | + | + | − |
| Succinylated wheat germ agglutinin (T aestivum) | sWGA | GlcNAc | N-glycans, O-glycans | + | + | − | + | + | − |
| Soybean agglutinin (Glycine max) | SBA | Terminal GalNAc | N-glycans, O-glycans | + | + | − | + | + | − |
| Ricinus communis agglutinin | RCA | Terminal Gal | N-glycans, O-glycans | + | + | − | ND | ND | ND |
| Phaseolus vulgaris leucoagglutinin | PHA-L | Galβ1–4GlcNAcβ1–2Man | N-glycans | + | − | − | + | + | − |
| Datura stramonium lectin | DSL | GlcNAcb1–4, Galb1–4GlcNAc | N-glycans, O-glycans | + | − | − | + | + | − |
| Lens culinaris agglutinin | LCA | Branched (Manα)n with Fucα1–6GlcNAc | N-glycans | + | − | − | + | − | − |
| Pisum sativum agglutinin | PSA | Branched (Manα)n with Fucα1–6GlcNAc | N-glycans | + | − | − | + | − | − |
| Concanavalin A (Canavalia ensiformis) | ConA | Branched (Manα)n | N-glycans | + | − | − | + | − | − |
| Griffonia simplicifolia lectin II | GSL II | Terminal GlcNAc | N-glycans, O-glycans | + | − | − | − | − | − |
| Lycopersicon esculentum lectin | LEL | GlcNAc | N-glycans, O-glycans | + | − | − | − | − | − |
| Dolichos bitflorus Agglutinin | DBA | GalNAcα1–3GalNAc, GalNAcα1–3(Fucα1–2)Gal (A antigen) | N-glycans, O-glycans | − | − | − | − | − | − |
| Peanut Agglutinin (Arachis hypogaea) | PNA | Terminal Galβ1–3GalNAc (T antigen) | O-glycans | − | − | − | − | − | − |
| Sophora japonica agglutinin | SJA | Terminal GalNAc/Gal | N-glycans, O-glycans | − | − | − | − | − | − |
| Ulex europaetus agglutinin | UEA-I | Fucα1–2Gal (H antigen) | N-glycans, O-glycans | − | − | − | − | − | − |
The stated ligand preferences are based on information available at public databases [Consortium for Functional Glycomics (http://www.functionalglycomics.org) and Lectin frontier Database (http://riodb.ibase.aist.go.jp/rcmg/glycodb/LectinSearch)] and published reports of lectin/glycan binding (1–9, 23, 62–64). ND, Not Determined
In addition to the plant lectins, it is now clear that pathogenic microorganisms express proteins with lectin-like activities. One example is the Bordetella pertussis encoded pertussis toxin (PTx). PTx is an AB5 toxin comprised of a hexameric polypeptide complex with five binding (B) subunits arranged in a ring structure and a single active (A) subunit with enzymatic properties sitting on top of the pore of the ring structure. The A subunit of PTx, S1, is an ADP-ribosyltransferase that targets the α-subunit of some GTP-binding proteins(10). The five B subunits of PTx (collectively referred to as B-pentamer or PTxB) are required for binding and cytosolic entry of S1 into mammalian cells. Unlike other AB5 toxins, which have five identical B subunits, PTxB is comprised of four different subunits S2, S3, S4, and S5 in the ratio 1:1:2:1. All the binding activities have thus far been mapped to the S2 and S3 subunits of PTxB(11–22). Analogous to WGA, each S2 and S3 subunit contains multiple glycan binding sites. Interestingly, although the S2 and S3 share 71% amino acid identity, each has distinct binding preferences. PTx has been shown to bind a broad array of glycans, including sialylated and non-sialylated N-glycans, sialylated O-glycans, and sialylated gangliosides(23).
The glycan binding activity of PTxB mediates activities independent of its role in delivering the S1 catalytic subunit to cellular targets. Via the B-subunits, PTx can bind to a variety of cellular receptors and activate their associated signaling pathways(24). Receptor targets for PTxB include the TCR in T cells, Toll like receptor 4 (TLR4) in dendritic cells, and CD14 in myelomonocytic cells(25–33). In T-cells the binding of PTxB to the TCR leads to T-cell activation and mitogenesis(24). The various receptors that PTxB bind share little structural similarity but are heavily glycosylated(34–36). The broad binding specificity of PTx likely allows it to act as a super-lectin, able to trigger signaling by clustering a wide variety of glycosylated receptors(37–42). This hypothesis, however, has yet to be formally proven.
Studies to understand the molecular basis of lectin activity in cellular systems have been hampered by several factors. One problem is the incomplete understanding of the repertoire of binding sites for each particular lectin. Glycans also typically interact with their cognate binding sites with very low affinity, and tight binding is often achieved by engaging multiple binding sites. Additionally, since cell-surface glycans are built by sequential enzymatic processing, they can be in various stages of “completion”, resulting in considerable heterogeneity. Furthermore, a single cell-surface protein may display both N-linked and O-linked glycosylation. Some of these problems have been overcome by the development of glycan arrays similar to that established by the Consortium for Functional Glycomics. These arrays can be very useful for identifying binding sites for lectins by identifying similar motifs within different glycans. However, technical issues can complicate the analysis, necessitating additional confirmatory studies. For example, if the glycans on the array are not spaced to allow for engagement of multiple binding sites, or if the lectin in question binds multiple different ligands by distinct mechanisms, array-type experiments are less useful. A cellular system in which some of these variables could be experimentally controlled would contribute valuable information to our understanding of PTxB and lectin action.
PTxB activates a canonical TCR/CD3 signaling pathway, including events such as phosphatidyl inositol (PI) hydrolysis, Ca2+ flux, and MAP kinase activity(24, 43, 44). Furthermore, while wildtype Jurkat cells robustly respond to PTxB, Jurkat derivatives lacking the TCR are devoid of any detectable TCR-related signaling responses to PTxB(24). The lack of PTx-induced signaling in cells missing the TCR provides the opportunity to engineer TCR minus mutant cell lines to express exogenously added “receptor” components to rescue PTx-induced signaling. In this report, we used the system previously described by Irving et. al. to engineer a TCR minus Jurkat T cell line to express a chimeric protein containing the extracellular CD8α domain and the intracellular CD3ζ domain (CD8ζ chimera)(45, 46). Comparing the responses of the CD8ζ chimera to the wild type TCR is a useful model system to study the mechanism of action of molecules such as PTx, ConA, PHA, etc. Additionally, this model system can provide important insights into the structural and functional properties of TCR activation.
Materials and Methods
Cell lines and reagents
The human Jurkat T cell lymphoma line (clone E6.1) was obtained from the ATCC, while the J.EMS-T3.3(47, 48) Jurkat derivative lacking TCR expression was obtained from Dr. A. Weiss (University of California, San Francisco). All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100µg/mL). PTx and PTxB were purchased from List Biologicals. The presence of contaminating PTx holotoxin in PTxB preparations was assessed using the CHO cell-clustering assay(49). The concentration of residual PTx holotoxin in PTxB preparations was found to be less than 2%. No effect was observed in experiments using the PTx holotoxin at these concentrations in short term assays, therefore we conclude that the observed effects are due to PTxB and not contaminating catalytic subunit activity. PTx was inactivated by boiling for 30 minutes. Stimulating antibodies, αCD8 monoclonal antibody (mAb) clone OKT8 and αCD3 mAb clone HIT3a was purchased from eBioscience and BD Pharmingen respectively. Antibodies used in immunoprecipitation assays, anti-ZAP-70 (clone 2F3.2) and the anti-phosphotyrosine antibody (clone 4G10), were obtained from Upstate Biotechnology(50, 51). Western blotting antibodies for phosphorylated and total p44/42 ERK MAP kinases were obtained from Cell Signaling Technologies. The CD8ζ chimera construct (comprised of the extracellular and transmembrane domains of CD8α fused to the cytoplasmic domain of CD3ζ) was a kind gift of Dr. A. Weiss (University of California, San Francisco).
Construction of a Jurkat derivative cell line expressing the CD8ζ chimera
The CD8ζ chimera was sub-cloned into the HpaI site of the Mig-R1 retroviral vector (Mig-R1/CD8ζ). Mig-R1 is a bicistronic retroviral vector containing a multiple cloning site upstream of an internal ribosome entry site followed by the cDNA encoding enhanced green fluorescent protein (GFP)(52). The Mig-R1 and Mig-R1/CD8ζ constructs were co-transfected with the envelope construct encoding pantropic VSVg into GP-293 cells (HEK cells stably expressing retroviral gag and pol) (Clontech). Jurkat cells were then transduced with the HEK supernatant by spinfection at 1800 rpm for 45 minutes and gene expression from Mig-R1 was monitored by FACS analysis for GFP. After several weeks in culture the top 10% of GFP expressing cells from each population were selected using a FACSAria cell sorter and expanded. Expression of the CD8ζ chimera was analyzed by flow cytometric analyses of live cells stained with anti-CD8 mAb (OKT8) primary antibody and anti-mouse PE secondary antibody.
Immunoprecipitations and western blotting assays
2.5×107 Jurkat cells were washed once with PBS and suspended in serum-free RPMI. The samples were slowly warmed to 37°C, and then stimulated with 10µg/mL anti-CD3 mAb, anti-CD8 mAB, or PTxB for 5 minutes. Samples were washed once with ice cold PBS and lysed in lysis buffer (1% NP-40, 0.1% SDS, 0.1% deoxycholate, 150 mM NaCl, 50 mM Tris, 20mM Na3V04, and Complete™ protease inhibitor tablets (Roche)). Lysates were clarified by centrifugation and supernatants containing 1 µg of immunoprecipitating antibody per 100 µg total protein were incubated overnight at 4°C with 30 µL of 60% protein A/G bead slurry (Calbiochem) or combined with 3X Laemmli Sample Buffer for whole cell extracts. Beads were washed four times with 1 mL lysis buffer, suspended in 3× Laemmli Sample Buffer, boiled for 10 minutes, and electrophoresed in 8% SDS PAGE gels(24). The gels were transferred to nitrocellulose, probed with the appropriate primary and secondary antibodies and visualized via chemiluminescence (Super Signal, Pierce).
Measurement of ERK activity
1×106 cells/treatment were serum starved when indicated and stimulated for various times with 2 or 5µg/mL PTxB, 2µg/mL α-CD3, or 2µg/mL anti-CD8 mAb. After the indicated treatment, cells were lysed directly in Laemmli sample buffer and analyzed for phosphorylated ERK 1 and 2 (phospho-ERK) and total ERK by western blotting(24).
Inositol phosphate assays
5×105 Jurkat cells/mL were labeled with 1.5µCi/mL [3H]myo-inositol (Perkin Elmer) for 18 hours in RMPI containing 10% serum. The cells were washed with cold PBS and suspended at 107 cells/mL in serum-free RMPI containing 20 mM LiCl. 106 cells/treatment were stimulated with designated amounts of 5 or 10µg/mL PTxB, 2µg/mL anti-CD3 mAb, 2µg/mL anti-CD8 mAb, or 10µg/mL PHA for 2 hours at 37°C. After stimulation, the cells were lysed with 0.4 M perchloric acid at 4°C for 15 minutes and neutralized with 0.72 M KOH and 0.6 M KHCO3. Total inositol phosphates were isolated with Dowex resin (BioRad), washed with water and eluted with 1 M ammonium formate/0.1 M formic acid. The eluted samples were counted with a liquid scintillation counter and the percent conversion of inositol phosphates from total incorporated [3H] was calculated(24). Data was graphed and analyzed using GraphPad Prism 4 software.
Ca2+ flux assays
Cells (2 × 105/sample) were washed with serum free RPMI and plated. Cells were loaded with the Fluo-4 NW Calcium Assay Kit (Invitrogen) according to the manufacture’s protocol and treated with PBS (without Ca2+/ Mg2+), PTxB, αCD8 mAb, αCD3 mAb, or lectin (2 µg/mL). Ca2+ flux was analyzed(24) by the increase in fluorescence reported by the Flex Station II (Molecular Probes) using SoftMax Pro software. Data was imported into Graphpad Prism software for analysis and production of graphs.
Aggregation assays
Cells were washed with serum free HBSS (Gibco) and plated in 96-well microtiter plates at 1 × 106/mL. Cells were treated with HBSS, PTxB, or lectin (2 µg/mL). The microtiter plates were then incubated at 37°C and observed for agglutination after 1–2 h.
Results
Generation and characterization of TCR minus Jurkat cells stably expressing the CD8ζ Chimera
To gain mechanistic insight into bacterial and plant lectin signaling through Ig superfamily receptors such as TCR/CD3, we sought to investigate lectin-mediated activation of a chimeric “receptor” termed CD8ζ, which contains the extracellular and transmembrane portions of human CD8α chain fused to the intracellular cytoplasmic domain of CD3ζ (Figure 1B)(45, 46, 53, 54). Previous studies indicated that this chimera activates a classical signaling pathway similar to that activated by TCR/CD3 (Figure 1C)(45, 46). Additional studies with the CD8ζ chimera demonstrated that signaling through CD3 can be propagated by clustering or aggregating the three cytoplasmic ITAM motifs found in each CD3ζ molecule, and that signaling through CD3 does not necessarily require ligand-induced conformational changes in the α/β chains of the TCR(45, 46, 55, 56). Interestingly, the extracellular ligand binding domains of the TCR and CD8α differ in their glycosylation status; the TCR α/β chains contain predominantly N-linked glycans while CD8α contains only O-linked glycans (35, 53, 57). Computational profiling of the TCR and CD8 polypeptides supports these data as the TCR α/β chains contain eight predicted sites of N-linked glycosylation while the CD8 α chain contains seven predicted sites of O-linked glycosylation (Table S1 of the Supporting Information) (58, 59). By comparing PTx and lectin stimulated signaling through the TCR or the CD8ζ chimera, we can ask specific questions regarding the potential role of the peptide backbone structure and glycosylation status in receiving and propagating the PTx/lectin signal.
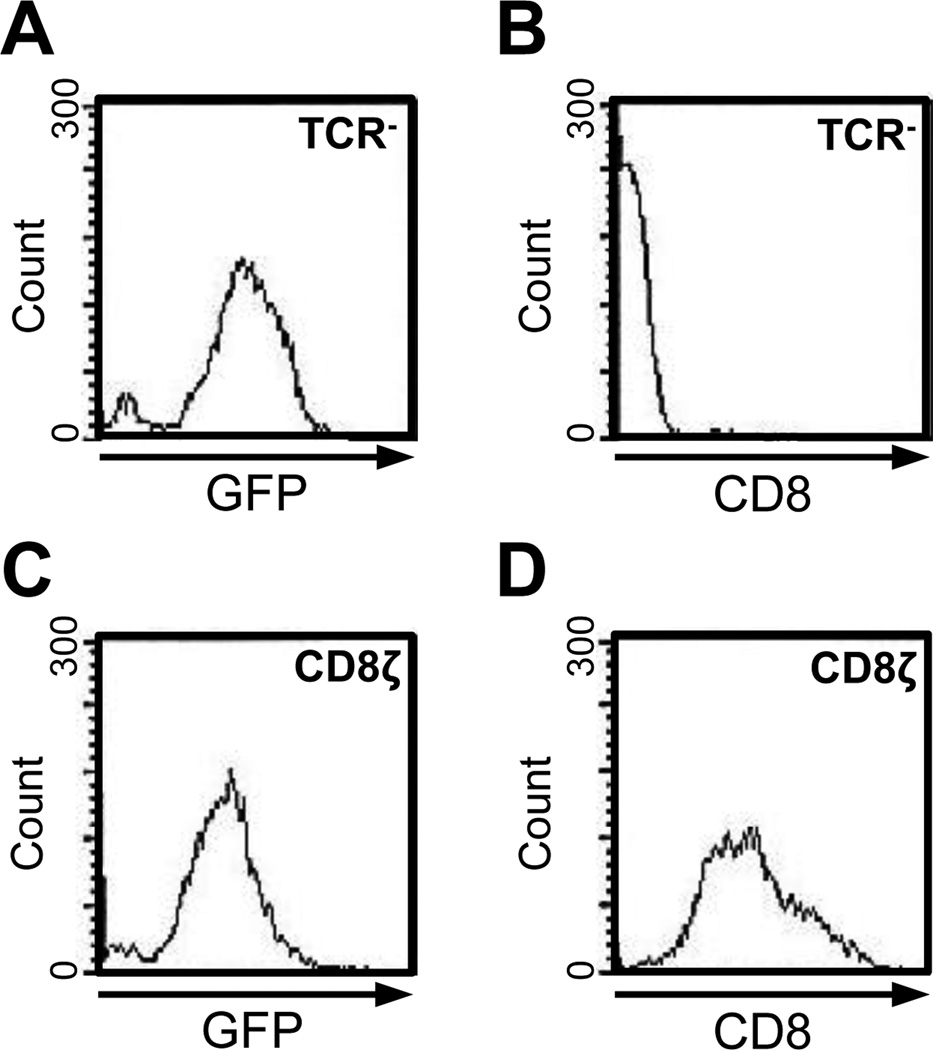
The CD8ζ chimeric gene was transduced into the TCR negative Jurkat T cell line (clone J.EMS-T3.3(47, 48)) using a Mig-R1/CD8ζ retrovirus. Since Mig-R1/CD8ζ drives gene expression with a bicistronic mRNA simultaneously expressing GFP and CD8ζ(52), we were able to use cell sorting based on high level GFP expression to generate a stable CD8ζ positive cell line. Cells transduced with the empty Mig-R1 retrovirus vector (designated TCR−) expressed high levels of GFP (Figure 2A) compared to untransduced controls (data not shown), but did not express any detectable CD8 or CD8ζ (Figure 2B). In contrast, cells transduced with the Mig-R1/CD8ζ retroviral vector (designated CD8ζ) expressed high levels of GFP (Figure 2C) and also expressed high levels of CD8ζ (Figure 2D). These cells, which express CD8ζ on a TCR null background, serve as the basis for the following studies.
Figure 2. Retroviral Expression of CD8ζ in TCR minus Jurkat T cells.
TCR minus Jurkat T cells (clone J.EMS-T3.3) were transduced with the empty Mig-R1 retrovirus (A and B) or with a Mig-R1/CD8ζ retrovirus (C and D). Cells expressing high GFP were sorted and re-analyzed by FACS for GFP expression (A and C) or for CD8 expression (B and D).
CD8ζ substitutes for the TCR and rescues PTx signaling in TCR− Jurkat cells
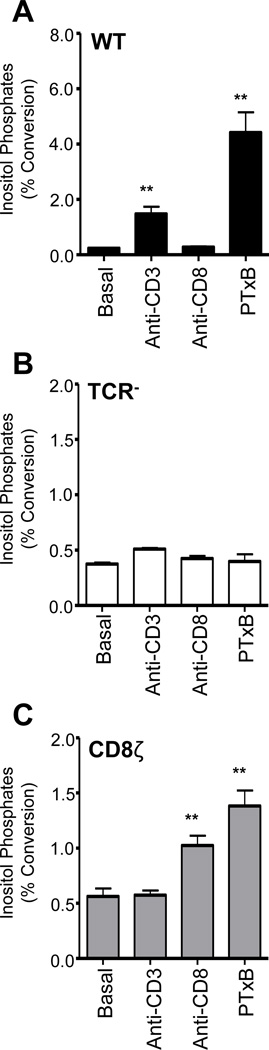
Next, we determined if expression of the CD8ζ chimera could rescue PTxB stimulated signaling events, which occur in WT Jurkat, but not in TCR negative Jurkat cells(24). WT, TCR−, or CD8ζ cells were stimulated for two hours with PTxB, anti-CD3, or anti-CD8 antibodies and analyzed for the production of inositol phosphates (InsP) (Figure 3). Consistent with previous reports(24, 43, 45), WT Jurkats exhibited increased InsP levels when treated with anti-CD3 antibody (Figure 3A), CD8ζ cells had increased InsP levels when treated with anti-CD8 antibody (Figure 3C), and TCR− cells had no detectable response to either antibody (Figure 3B). PTxB strongly induced InsP accumulation in WT cells (Figure 3A), but not in TCR− cells (Figure 3B), and expression of CD8ζ rescued the ability of PTxB to induce InsP accumulation (Figure 3C). This result indicates that CD8ζ can substitute for the TCR and supports the hypothesis that PTxB (functioning as a lectin) stimulates signaling via receptor crosslinking/clustering rather than by binding to a specific epitope in the TCR α/β chains and initiating signaling via a conformational change in the α/β chains themselves.
Figure 3. PTx Utilizes CD8ζ to Induce Inositol Phosphate Accumulation.
WT (A), TCR− (B), or CD8ζ (C) Jurkats cells were left unstimulated or stimulated with 2 µg/mL anti-CD3, 2 µg/mL anti-CD8, or 10 µg/mL PTxB for 2 hours, and inositol phosphate levels were determined. Statistical analysis using a student’s T-test was performed comparing each treatment to the basal sample for each cell type, **p<0.01.
PTxB mediated signaling through the CD8z chimera involves canonical TCR signaling components
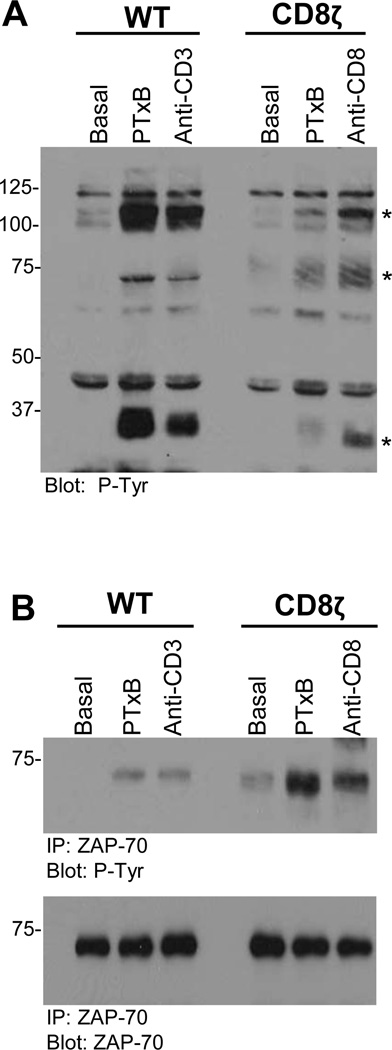
Based on the observation that PTxB signaling was rescued by expression of the CD8ζ chimera, we next determined whether PTxB-activated CD8ζ signaling events were similar to those typically initiated by the TCR(45, 46, 55). WT Jurkats stimulated with PTxB or anti-CD3 antibodies exhibit increased tyrosine phosphorylation of proteins at molecular weights of 100kDa, 70kDa, and 35kDa, consistent with that reported for canonical TCR signaling and observed in earlier studies from our laboratory (Figure 4A)(24). Interestingly, CD8ζ cells stimulated with PTxB or anti-CD8 antibodies demonstrated increases in tyrosine phosphorylation similar to that observed in WT cells stimulated with PTxB or anti-CD3 antibodies indicating that the CD8ζ chimera activates a signaling pathway very similar to that of TCR/CD3(45, 46, 55, 60–62). Increases in tyrosine phosphorylation were not seen in TCR− Jurkats following treatment with PTxB, anti-CD3, or anti-CD8 antibodies (data not shown)(24).
Figure 4. PTx Utilizes CD8ζ to Activate Key TCR Signaling Proteins.
WT or CD8ζ Jurkat cells were left untreated or stimulated with 10 µg/mL PTxB, 5 µg/mL anti-CD8 (CD8ζ cells only), or 5 µg/mL anti-CD3 (WT cells only) for 5 minutes. (A)Tyrosine phosphorylated proteins were analyzed in the whole cell extracts. Proteins phosphorylated in response to PTxB are indicated with asterisks. (B) ZAP-70 was immunoprecipitated from lysates using an anti-ZAP-70 mAb. Anti-phosphotyrosine antibody (clone 4G10) was used to detect tyrosine phosphorylation. Representative blots are shown for each analysis.
Phosphorylation of ZAP-70 is thought to be the most critical event in TCR activation, as it is responsible for the phosphorylation of downstream proteins including SLP-76 and PLCγ-1(50, 60, 62). To determine if ZAP-70 was tyrosine phosphorylated and therefore active in CD8ζ cells stimulated with PTxB, we stimulated WT or CD8ζ Jurkats with PTxB or the appropriate control antibody, isolated ZAP- 70 by immunoprecipitation, and probed western blots with anti-phosphotyrosine or anti-ZAP-70 antibodies. WT Jurkat cells treated with an anti-CD3 antibody or PTxB exhibited increased tyrosine phosphorylation of ZAP-70 (Figure 4B). Similarly CD8ζ cells treated with anti-CD8 antibody or PTxB displayed increased tyrosine phosphorylation of ZAP-70. Thus, these results indicate that CD8ζ can substitute for TCR/CD3 to activate CD3 dependent signaling, and that PTxB can utilize either the native TCR/CD3 complex or CD8ζ to initiate the activation of canonical TCR signaling.
PTx utilizes CD8ζ to activate the MAP kinase pathway
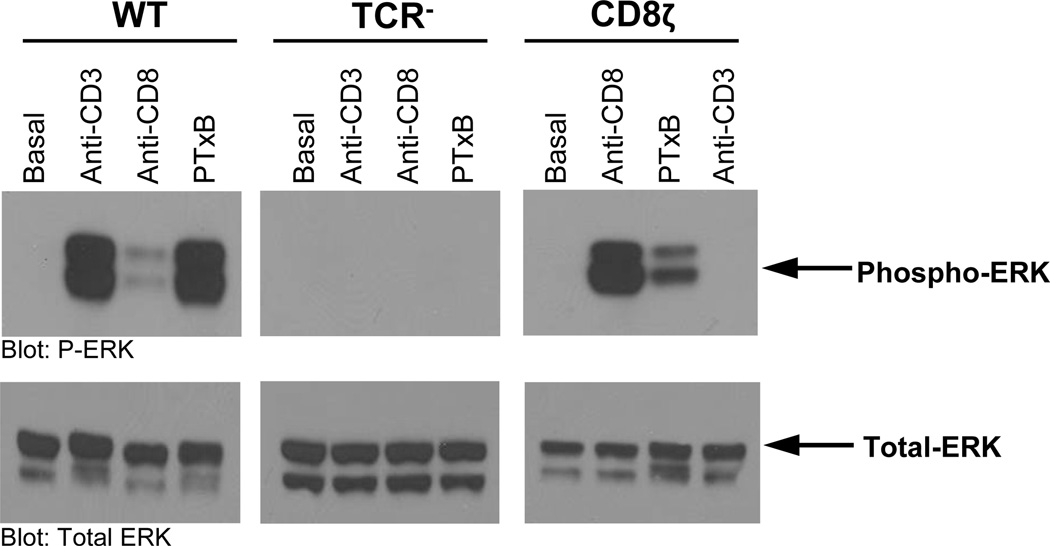
PTxB can activate the ERK MAP kinase in endothelial cells, monocyte-like cells, and T-cells(24, 26, 29, 31). In T cells, this activity has been attributed to the expression and function of the TCR(24). To determine if PTxB can also use the CD8ζ chimera to drive ERK signaling, we assayed ERK phosphorylation in WT, TCR− and CD8ζ Jurkat cells. Consistent with previous findings, WT Jurkat cells treated with either anti-CD3 or PTxB displayed increased phosphorylation of ERK 1/2 (Figure 5, left panel), and TCR− Jurkat cells failed to respond to any treatment (Figure 5, middle panel). CD8ζ cells treated with either anti-CD8 or PTxB displayed increased phosphorylation of ERK 1/2 (Figure 5, right panel). CD8ζ cells treated with anti-CD3 antibodies did not show an increase in phosphorylated ERK 1/2, confirming that the PTxB-induced InsP accumulation observed was a result of CD8ζ signaling and not low level TCR expression (Figure 5C). Thus, similar to InsP accumulation experiments described earlier, PTxB can also activate the ERK MAP kinase pathway in T cells using either the TCR or CD8ζ.
Figure 5. PTx Utilizes CD8ζ to Activate the MAP Kinase Pathway.
WT (left), TCR− (middle), or CD8ζ (right) Jurkat T cells were left unstimulated or stimulated with 1 µg/mL anti-CD3, or 1 µg/mL anti-CD8 mAb, or 5 µg/mL PTxB for 15 minutes. Whole cell extracts were analyzed by western blot for activated ERK with phospho-specific antibodies, or total ERK with anti-ERK antibodies. Representative blots are shown.
PTx utilizes CD8ζ to induce calcium signaling
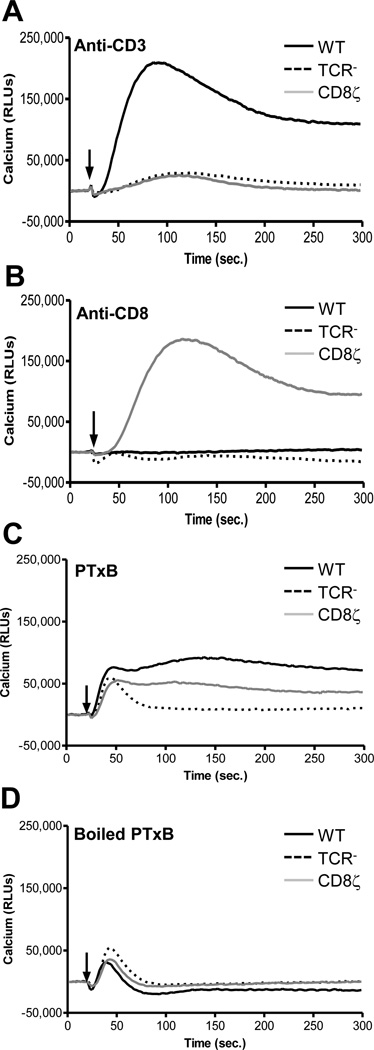
In addition to InsP accumulation and MAP kinase activity, PTxB has been shown to promote a rapid and prolonged elevation of intracellular Ca2+ in T-cells in a TCR dependent manner, and therefore provides a platform to confirm the effects of PTxB signaling through the CD8ζ chimera(24, 43). Ca2+ flux in response to anti-CD3, anti-CD8, or PTxB was examined in all three Jurkat cell derivatives (Figure 6). Anti-CD3 antibody stimulated Ca2+ flux only in WT cells (Figure 6A) and the anti-CD8 antibody stimulated Ca2+ in only the CD8ζ expressing cells (Figure 6B). WT and CD8ζ expressing cells stimulated with PTxB displayed a rapid and sustained calcium response that remains elevated for more than 5 minutes post-PTxB addition (Figure 6C), although the magnitude of the PTxB response was less than that observed for anti-CD3 or anti-CD8. The TCR− cells exhibited a transient spike in intracellular Ca2+ in response to PTxB (Figure 6C). This response was non-specific and unrelated to the biological activity of the toxin itself, as cells stimulated with 5 µg/mL boiled PTxB (Figure 6D) or 5 µg/mL BSA (data not shown) also demonstrated this rapid transient spike. All together these Ca2+ results support our earlier studies indicating that PTxB can utilize either the TCR or the CD8ζ chimera to induce rapid signaling in T cells.
Figure 6. PTx Induces Calcium Flux in CD8ζ Jurkat Cells.
WT, TCR−, or CD8ζ Jurkat cells were loaded with the Fluo-4 NW calcium indicator dye and stimulated with 1 µg/mL anti-CD3 (A), 1 µg/mL anti-CD8 (B), 5 µg/mL PTxB (C), or 5 µg/mL boiled PTxB (B). The time at which ligand is added is indicated by black arrow. Data were collected on a Molecular Dynamics FlexStation. Representative data is shown for each treatment.
Comparison of the ability of plant lectins to signal through the TCR or CD8ζ
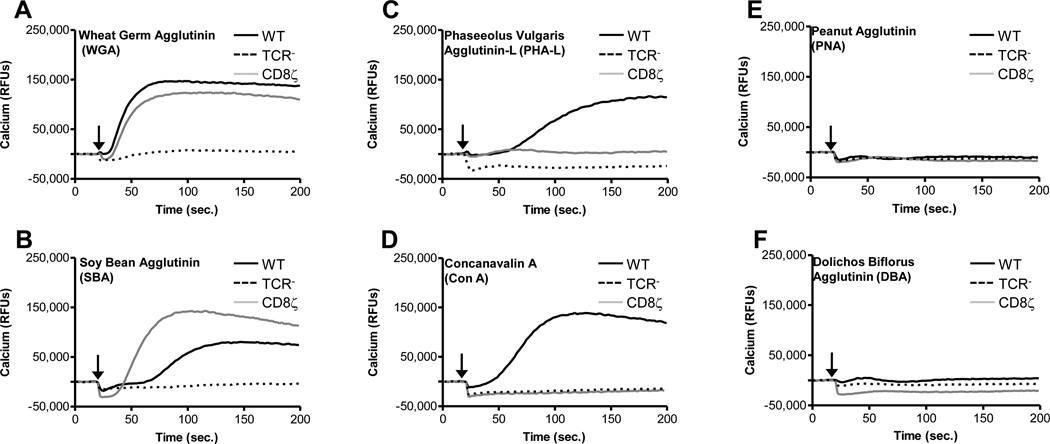
Many of the plant lectins are known to activate TCR signaling via the binding of glycan moieties typically found on the TCR α and β chains (63). However, it remains unknown if these lectins would function similarly to PTxB and exhibit biological activity towards other Ig superfamily members, or more specifically, would be capable of activating the CD8ζ chimera. Therefore, we compared the ability of various lectins to activate calcium signaling through TCR (in WT Jurkats) or the CD8ζ chimera (in the CD8ζ stables). Both wheat germ agglutinin (WGA) and soy bean agglutinin (SBA) function similarly to PTx in that they stimulated Ca2+ signaling via either TCR or CD8ζ (Figure 7, panels A and B). This is consistent with their ability to bind either N-linked (TCR) or O-linked (CD8ζ) glycans (Table 1). Phytohemagluttinin leucoagglutinin (PHA-L) and Concanavalin A (ConA) are also capable of activating Ca2+ signaling via TCR, but not CD8ζ (Figure 7, panels C and D), which is consistent with their ability to primarily bind N-linked glycans (Table 1). In contrast, peanut agglutinin (PNA) and Dolichos bilforous agglutinin (DBA) are unable to activate either receptor type (Figure 7, panels E and F).
Figure 7. Lectin-mediated Ca2+ Signaling.
WT, TCR−, or CD8ζ Jurkat cells were loaded with the Fluo-4 NW calcium indicator dye and stimulated with 2ug/ml WGA (A), SBA (B), PHA-L (C), ConA (D), PNA (E), or DBA (F). The time at which ligand is added is indicated by black arrow. Data were collected on a Molecular Dynamics FlexStation. Representative data is shown for each treatment.
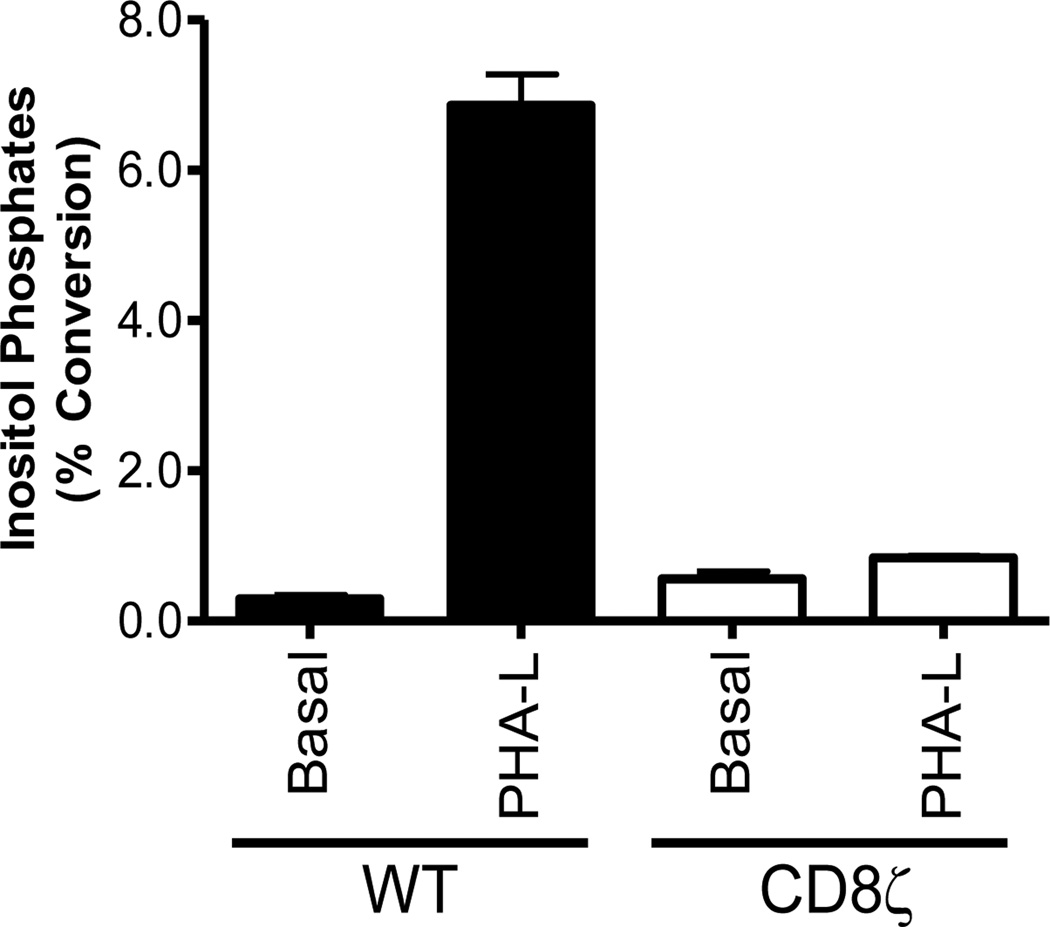
To validate our assay using the CD8ζ chimera as a biological tool to explore aspects of lectin binding and signaling, we examined PHA-L mediated InsP accumulation. PHA-L is able to induce Ca2+ signaling through the TCR, but not through CD8ζ (Figure 7C), and therefore we would expect PHA-L to behave similarly in InsP assays. As predicted, PHA-L is indeed capable of inducing InsP accumulation in WT Jurkats, but is unable to stimulate InsP accumulation in CD8ζ cells (Figure 8). Since PHA-L is known to bind predominantly to complex N-glycans on the TCR (63) (Table 1 and Figure 1) but not to O-glycans on CD8, our results demonstrate that comparing lectin signaling via TCR versus CD8ζ is a useful approach to explore lectin/glycan interactions on a biological level. Specifically, we can differentiate between lectin responses mediated by receptors decorated with N- or O-linked glycans.
Figure 8. PHA-L Utilizes the TCR but not CD8ζ to Activate Signaling Events.
WT or CD8ζ Jurkat cells were untreated or stimulated with 10 µg/mL PHA-L for 2 hours and inositol phosphates were analyzed. n=2.
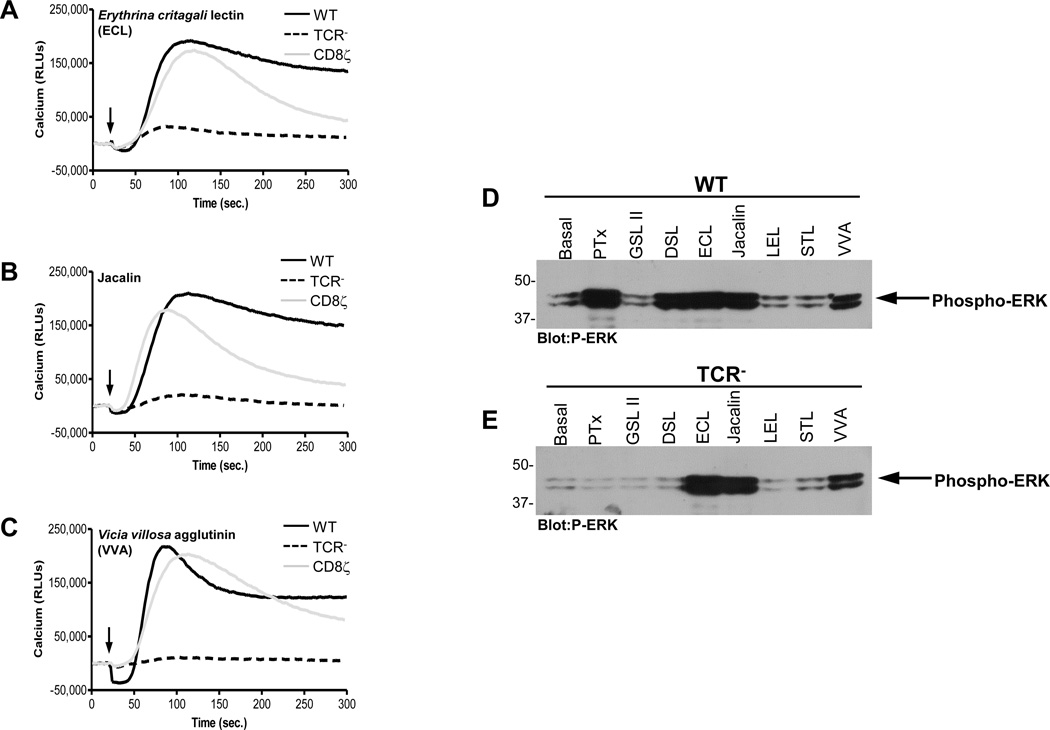
Next, we broadened our study to include a large panel of plant lectins (Table 1) that were selected to represent a diverse cross section of lectins that have the capability of binding to an equally diverse set of ligands. The preferred binding ligand denoted for each lectin in Table 1 is based on a consensus of published reports and glycoarray results, although from analyses of glycoarray results, it is clear that lectin/glycan binding cannot be simplified to state that a particular lectin can interact with only one particular ligand (Table S2 of the Supporting Information). The abilities of these lectins to activate downstream signaling events in WT, CD8ζ, or TCR− cells were assessed by monitoring Ca2+ responses (Table 1). The signaling responses for many lectins (WGA, sWGA, SBA, RCA, LCA, PSA, and ConA) were entirely consistent with their predicted abilities to bind N-linked or O-linked glycans. We also examined ERK activation and the results are largely consistent with the observed Ca2+ responses (Table 1). However, some lectins exhibited biological responses not predicted by their binding preferences (Table 1 and Figure 9). For example, while ECL, JAC, and VVA require either TCR or CD8ζ to induce Ca2+ signaling (Figures 9A, 9B, and 9C), these lectins were able to activate ERK signaling in cells lacking TCR or CD8ζ (Figures 9D and 9E). This suggests that these lectins activate a receptor(s) linked to ERK, but distinct from one that uses the canonical TCR signaling pathway involving ZAP-70, PLCγ1, etc.
Figure 9. Calcium and ERK MAPK signaling induced by the Lectins ECL, VVA, and Jacalin.
WT, TCR−, or CD8ζ Jurkat cells were loaded with the Fluo-4 NW calcium indicator dye and stimulated with 2ug/ml ECL (A), VVA (B), or Jacalin (C). The time at which ligand is added is indicated by black arrow. Data were collected on a Molecular Dynamics FlexStation. WT (A), or TCR− (B) Jurkat T cells were left unstimulated or stimulated with 2 µg/mL of the indicated lectins for 15 minutes. Whole cell extracts were analyzed by western blot for activated ERK with phospho-specific antibodies. Representative data is shown for each treatment.
Several lectins (DBA, PNA, SJA, and UEA-1) did not display any signaling activity in our assays, suggesting that neither TCR nor CD8ζ displays the appropriate glycans for these lectins, or they were unable to crosslink the receptors in a way that promotes activation. Since lectin activity is commonly associated with the ability to aggregate cells, each of the three cell types (WT, CD8ζ, and TCR−) was incubated with the lectins in Table 1, and cellular aggregation was assessed (data not shown). Lectin-mediated cellular aggregation was seen in all cases where cellular signaling (Ca2+ and/or ERK activation) was observed. In contrast, cells treated with DBA, PNA, SJA, and UEA-1 (which failed to induce Ca2+ or ERK signaling) did not display any aggregation, suggesting that Jurkat cells fail to display the terminal glycan required for responsiveness to these lectins.
Discussion
A CD8/CD3ζ chimeric receptor engineered to artificially link the extracellular domain of CD8α to the intracellular domain of CD3ζ has been shown to transduce signaling events typically associated with the TCR/CD3 complex when stimulated with α-CD8 antibody (45, 46). Unlike the TCR α/β chains, which are modified predominantly by N-linked glycans, the CD8ζ chimera contains O-linked glycans (35, 53, 57–59). This difference in glycan modification on an otherwise common signaling background allowed us to examine the ability of PTxB and a diverse panel of lectins to activate canonical CD3 signaling via receptors modified with N-linked or O-linked glycans. PTxB has at least four glycan binding sites, and can bind sialylated and non-sialylated N-glycans, sialylated O-glycans, and sialylated gangliosides (23). In previous studies (25–32), PTxB has been shown to activate a variety of receptors, including the TCR, TLR4, and CD14. In this study we have shown that PTxB also activates the CD8ζ chimera. Specifically, the CD8ζ chimera rescued PTxB-activated signaling events in TCR− Jurkat cells, including increased tyrosine phosphorylation of TCR signaling molecules (e.g. ZAP-70), accumulation of inositol phosphates, activation of ERK, and mobilization of intracellular Ca2+ (Figures 3–6). Together, these results strongly support the hypothesis that PTxB does not recognize amino acid sequences present on receptor molecules, but rather acts like a super-lectin to promote signaling by clustering a wide variety of glycosylated receptors.
We did observe some differences in the magnitude of signaling when comparing TCR versus CD8ζ activity. While the anti-CD8 antibodies induced signaling via the CD8ζ chimera to levels comparable to that of anti-CD3 stimulation of the TCR (Figures 3–6), PTxB and lectin mediated activation of signaling via these two receptors were not always equivalent. For example, PTxB activated ERK (Figure 5) and mobilized Ca2+ (Figure 6C) more efficiently on cells expressing wild type TCR than on cells expressing the CD8ζ chimera, suggesting there is an inherit difference between the TCR and CD8ζ complexes that makes the TCR a better receptor for PTxB. Whether this is attributed to binding efficiency or some other factor remains unclear at this point.
We also examined the ability of various plant lectins to initiate activation of signaling activities downstream of wild type TCR or CD8ζ chimera, including mobilization of intracellular Ca2+ and activation of ERK (Figures 7–9, Table 1). The activation patterns for many of these lectins, either via the TCR (containing N-linked glycans) or the CD8ζ chimera (containing O-linked glycans), could be predicted based on their preference for binding to N- or O-linked glycans. However, some of the lectins displayed activities in the biological assays that would not have been predicted based on their glycan binding profiles in cell free systems. For example, while PHA-E has not been previously reported to bind to O-linked glycans, it does activate signaling via the CD8ζ chimera (Table 1). The preferred ligand for PHA-E is particularly complex, requiring three different binding elements; a bisecting GlcNAc group, a Gal group at the non-reducing terminus of the Man(α1–6) branch, and a GlcNAc group on the Man(α1–3) branch(64). In this case, the preferred ligand represents the highest affinity ligand discovered through careful biochemical analyses and while each of the binding elements is required for high affinity binding, some ligands lacking an individual element still bound albeit with reduced affinity. A branched N-linked glycan can display different sugars on each branch, and can satisfy this requirement for different adjacent glycans. In contrast, O-linked glycans display minimal branching and a single glycan cannot provide the three binding elements for PHA-E. However, as seen in Figure 1B, each CD8 monomer possesses five closely spaced sites for O-linked modification. Activation by PHA-E could be mediated by the simultaneous engagement of closely-spaced heterogeneous O-linked glycans. Thus, biochemical binding assays, such as glycan arrays using a single purified O-glycan, are unlikely to reveal the diversity allowable in biological systems such as the one described in this report.
Another deviation from predicted results is the failure of PNA to activate the CD8ζ chimera. PNA is reported to recognize Galβ1–3GalNAc, also called Thomsen-Friedenreich antigen (T-antigen)(65), which is a major O-glycan structure found on human T cells (Table S3 of the Supporting Information). However, the inability of PNA to mediate cellular aggregation suggests Jurkat cells lack PNA-receptor expression. In contrast, another lectin, Jacalin, which also binds the T-antigen, can promote signaling (Table 1), suggesting that T-antigen is expressed on Jurkat cells. An explanation for these seemingly contradictory results is that binding of PNA but not Jacalin, is inhibited when the T-antigen is further modified by sialylation(66), suggesting that the majority of T-antigen present on Jurkat cells is sialylated(67). This is further supported by the observation that tumor cells, like the Jurkat cell line, express increased sialylation compared to normal cells(67, 68).
It is also intriguing that some lectin-receptor complexes promoted either Ca2+ or ERK signaling, but not both (Table 1). PHA-L and DSL activated CD8ζ leading to ERK, but not Ca2+ signaling. In contrast, GSL II and LEL activated TCR leading to Ca2+, but not ERK signaling. The ability to activate either ERK or Ca2+ but not both could be due to quantitative or qualitative differences in receptor activation, although it is difficult to imagine how quantitative differences could lead to diminished Ca2+ signaling in one case and diminished ERK signaling in another. Activation of the TCR promotes receptor translocation and clustering into signaling regions called immunological synapses or supramolecular activation clusters (SMACs)(69, 70). It is likely that the spatial orientation of the receptors within the SMAC can drastically affect the ability to support and sustain signaling activity. The complex and dynamic structure of the immunologic synapse makes it very difficult to study. Lectins capable of promoting the assembly of TCR or CD8ζ mediated signaling complexes with incomplete functional capacity (e.g. Ca2+ but not ERK) are very useful tools to study the dynamic assembly of intermediates involved in the TCR signaling process.
Finally, we found that three lectins, ECL, JAC, and VVA, promoted ERK signaling, but not Ca2+ signaling in cells lacking the TCR or the CD8ζ chimera (Table 1 and Figure 9). These results suggest that these lectins are capable of activating T cells by a TCR-independent signaling pathway, which has yet to be identified.
In summary, we have developed a model system for examining the ability of PTxB and other lectins to activate canonical CD3 signaling via either N-linked or O-linked glycans. Our results confirm the hypothesis that biological responses to lectins cannot be predicted based simply on binding preferences determined from glycan array binding studies. Furthermore, the ability to crosslink a given receptor is not the sole prerequisite for driving signaling, since different lectins elicit quantitative and qualitative differences in downstream responses. This model system will be useful to probe the specific details involved in receptor activation, including defining the importance of microdomains and membrane substructures critical for recruitment of various signaling molecules to activated receptors.
Supplementary Material
Acknowledgments
We thank Rodney Dekoter (University of Western Ontario) for providing the Mig-R1 retroviral vector, Arthur Weiss (University of California, San Francisco) for providing the CD8/CD3ζ chimera, the Cincinnati Children’s Hospital Flow Cytometry Core Facility for cell sorting, and Dr. Jeanette L.C. Miller for critical reading of the manuscript. We would like to acknowledge the Consortium for Functional Glycomics for their maintenance of public databases containing information on lectin/glycan binding.
This work was supported by National Institutes of Health Grant R01 AI023695 (to A.A.W and W.E.M.) and by unrestricted funds from the University of Cincinnati (to W.E.M.). A.A.W. acknowledges support from the Epidemiology and Surveillance Division in the National Immunization Program at the Centers for Disease Control and Prevention. O.D.S. and S.H.M. was supported in part by a National Institutes of Health Training Grant T32 AI055406. AAW is a member of the Consortium for Functional Glycomics, and acknowledges the use of their resources in this study.
Abbreviations
- ConA
Concanavalin A
- DBA
Dolichos bitflorus Agglutinin
- DSL
Datura stramonium lectin
- ECL
Erythrina cristagalli lectin
- Gal
Galactose
- GalNAc
N-Acetylgalactosamine
- Glc
Glucose
- GlcNAc
N-Acetylglucosamine
- GSL II
Griffonia simplicifolia lectin II
- ITAM
Immunoreceptor tyrosine-based activation motif
- JAC
Jacalin
- LCA
Lens culinaris agglutinin
- LEL
Lycopersicon esculentum lectin
- Man
Mannose
- Neu5Ac
N-Acetylneuraminic Acid
- PHA
Phytohemagluttinin
- PHA-L
Phytohemagluttinin leucoagglutinin
- PHA-E
Phytohemagluttinin erythroagglutinin
- PNA
Peanut Agglutinin
- PSA
Pisum sativum agglutinin
- PTx
Pertussis Toxin
- PTxB
Pertussis Toxin B Subunit
- RCA
Ricinus communis agglutinin
- SBA
Soybean agglutinin
- SJA
Sophora japonica agglutinin
- sWGA
Succinylated wheat-germ agglutinin
- TCR
T Cell Receptor
- UEA-I
Ulex europaetus agglutinin
- VVA
Vicia villosa agglutinin
- WGA
Wheat-germ agglutinin
- WT
Wild-type
Footnotes
Disclosures The authors have no financial conflicts of interest.
Supporting Information
Glycosylation profiles for TCR and CD8 and links to Center for Glycomics lectin/glycan binding results are provided in Tables S1, S2, and S3 of the Supporting Information. The supporting information may be accessed free of charge online at http://pubs.acs.org.
References
- 1.Edelman GM, Cunningham BA, Reeke GN, Becker JW, Waxdal MJ, Wang JL. The covalent and three-dimensional structure of concanavalin A. Proc. Natl. Acad. Sci. U.S.A. 1972;69:2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardman KD, Ainsworth CF. Structure of concanavalin A at 2.4-A resolution. Biochemistry. 1972;11:4910–4919. doi: 10.1021/bi00776a006. [DOI] [PubMed] [Google Scholar]
- 3.Hamelryck TW, Dao-Thi M-H, Poortmans F, Chrispeels MJ, Wyns L, Loris R. The crystallographic structure of phytohemagglutinin-L. Journal of Biological Chemistry. 1996;271:20479–20485. doi: 10.1074/jbc.271.34.20479. [DOI] [PubMed] [Google Scholar]
- 4.Nowell P. Phytohemagglutinin: an initiator of mitosis in culture of animal and human leukocytes. Cancer Res. 1960;20:462–466. [PubMed] [Google Scholar]
- 5.Hammarström S, Hammarström ML, Sundblad G, Arnarp J, Lönngren J. Mitogenic leukoagglutinin from Phaseolus vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc. Natl. Acad. Sci. U.S.A. 1982;79:1611–1615. doi: 10.1073/pnas.79.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irimura T, Kawaguchi T, Terao T, Osawa T. Carbohydrate-binding specificity of the so-called galactose-specific phytohemagglutinins. Carbohydr. Res. 1975;39:317–327. doi: 10.1016/s0008-6215(00)86141-8. [DOI] [PubMed] [Google Scholar]
- 7.Wright CS. The crystal structure of wheat germ agglutinin at 2-2 A resolution. J. Mol. Biol. 1977;111:439–457. doi: 10.1016/s0022-2836(77)80063-6. [DOI] [PubMed] [Google Scholar]
- 8.Monsigny M, Sene C, Obrenovitch A, Roche AC, Delmotte F, Boschetti E. Properties of succinylated wheat-germ agglutinin. Eur. J. Biochem. 1979;98:39–45. doi: 10.1111/j.1432-1033.1979.tb13157.x. [DOI] [PubMed] [Google Scholar]
- 9.Monsigny M, Roche AC, Sene C, Maget-Dana R, Delmotte F. Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur. J. Biochem. 1980;104:147–153. doi: 10.1111/j.1432-1033.1980.tb04410.x. [DOI] [PubMed] [Google Scholar]
- 10.Katada T, Tamura M, Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983;224:290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- 11.Stein PE, Boodhoo A, Armstrong GD, Heerze LD, Cockle SA, Klein MH, Read RJ. Structure of a pertussis toxin-sugar complex as a model for receptor binding. Nat Struct Biol. 1994;1:591–596. doi: 10.1038/nsb0994-591. [DOI] [PubMed] [Google Scholar]
- 12.Rozdzinski E, Burnette WN, Jones T, Mar V, Tuomanen E. Prokaryotic peptides that block leukocyte adherence to selectins. J Exp Med. 1993;178:917–924. doi: 10.1084/jem.178.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capiau C, Petre J, Van Damme J, Puype M, Vandekerckhove J. Protein-chemical analysis of pertussis toxin reveals homology between the subunits S2 and S3, between S1 and the A chains of enterotoxins of Vibrio cholerae and Escherichia coli and identifies S2 as the haptoglobin-binding subunit. FEBS Lett. 1986;204:336–340. doi: 10.1016/0014-5793(86)80839-0. [DOI] [PubMed] [Google Scholar]
- 14.Sekura RD, Zhang Y. Pertussis toxin: structural elements involved in the interaction with cells. In: Sekura RD, Moss J, Vaughan M, editors. Pertussis Toxin. Orlando, FL: Academic Press, Inc.; 1985. pp. 45–64. [Google Scholar]
- 15.Francotte M, Locht C, Feron C, Capiau C, de Wilde M. Monoclonal antibodies specific for pertussis toxin subunits and identification of the haptoglobin-binding site. In: Lerner RA, Ginsberg H, Chanock RM, Brown F, editors. Vaccines. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. pp. 243–247. [Google Scholar]
- 16.Loosmore S, Zealey G, Cockle S, Boux H, Chong P, Yacoob R, Klein M. Characterization of pertussis toxin analogs containing mutations in B-oligomer subunits. Infect Immun. 1993;61:2316–2324. doi: 10.1128/iai.61.6.2316-2324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt W, Schmidt MA. Mapping of linear B-cell epitopes of the S2 subunit of pertussis toxin. Infect Immun. 1989;57:438–445. doi: 10.1128/iai.57.2.438-445.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt MA, Schmidt W. Inhibition of pertussis toxin binding to model receptors by antipeptide antibodies directed at an antigenic domain of the S2 subunit. Infect Immun. 1989;57:3828–3833. doi: 10.1128/iai.57.12.3828-3833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt MA, Raupach B, Szulczynski M, Marzillier J. Identification of linear B-cell determinants of pertussis toxin associated with the receptor recognition site of the S3 subunit. Infect Immun. 1991;59:1402–1408. doi: 10.1128/iai.59.4.1402-1408.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobet Y, Feron C, Dequesne G, Simoen E, Hauser P, Locht C. Site-specific alterations in the B oligomer that affect receptor-binding activities and mitogenicity of pertussis toxin. J Exp Med. 1993;177:79–87. doi: 10.1084/jem.177.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandros J, Rozdzinski E, Zheng J, Cowburn D, Tuomanen E. Lectin domains in the toxin of Bordetella pertussis: selectin mimicry linked to microbial pathogenesis. Glycoconj J. 1994;11:501–506. doi: 10.1007/BF00731300. [DOI] [PubMed] [Google Scholar]
- 22.Saukkonen K, Burnette WN, Mar VL, Masure HR, Tuomanen EI. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc Natl Acad Sci U S A. 1992;89:118–122. doi: 10.1073/pnas.89.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millen SH, Lewallen DM, Herr AB, Iyer SS, Weiss AA. Identification and characterization of the carbohydrate ligands recognized by pertussis toxin via a glycan microarray and surface plasmon resonance. Biochemistry. 2010;49:5954–5967. doi: 10.1021/bi100474z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider OD, Weiss AA, Miller WE. Pertussis toxin utilizes proximal components of the T-cell receptor complex to initiate signal transduction events in T cells. Infect. Immun. 2007;75:4040–4049. doi: 10.1128/IAI.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denkinger CM, Denkinger MD, Forsthuber TG. Pertussis toxin-induced cytokine differentiation and clonal expansion of T cells is mediated predominantly via costimulation. Cell. Immunol. 2007;246:46–54. doi: 10.1016/j.cellimm.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia JG, Wang P, Liu F, Hershenson MB, Borbiev T, Verin AD. Pertussis toxin directly activates endothelial cell p42/p44 MAP kinases via a novel signaling pathway. AmJ. Physiol., Cell Physiol. 2001;280:C1233–C1241. doi: 10.1152/ajpcell.2001.280.5.C1233. [DOI] [PubMed] [Google Scholar]
- 27.Gray LS, Huber KS, Gray MC, Hewlett EL, Engelhard VH. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989;142:1631–1638. [PubMed] [Google Scholar]
- 28.Hou W, Wu Y, Sun S, Shi M, Sun Y, Yang C, Pei G, Gu Y, Zhong C, Sun B. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. J. Immunol. 2003;170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Wong WS. Pertussis toxin activates tyrosine kinase signaling cascade in myelomonocytic cells: a mechanism for cell adhesion. Biochem Biophys Res Commun. 2001;283:1077–1082. doi: 10.1006/bbrc.2001.4910. [DOI] [PubMed] [Google Scholar]
- 30.Morse JH, Kong AS, Lindenbaum J, Morse SI. The mitogenic effect of the lymphocytosis promoting factor from Bordetella pertussis on human lymphocytes. J Clin Invest. 1977;60:683–692. doi: 10.1172/JCI108820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racke MK, Hu W, Lovett-Racke AE. PTX cruiser: driving autoimmunity via TLR4. Trends Immunol. 2005;26:289–291. doi: 10.1016/j.it.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Rosoff PM, Mohan C. Unidirectional, heterologous desensitization of the pertussis toxin receptor by the CD3/TCR complex. J Immunol. 1992;149:3191–3199. [PubMed] [Google Scholar]
- 33.Sindt KA, Hewlett EL, Redpath GT, Rappuoli R, Gray LS, Vandenberg SR. Pertussis toxin activates platelets through an interaction with platelet glycoprotein Ib. Infect Immun. 1994;62:3108–3114. doi: 10.1128/iai.62.8.3108-3114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng J, Parroche P, Golenbock DT, McKnight CJ. The differential impact of disulfide bonds and N-linked glycosylation on the stability and function of CD14. J. Biol. Chem. 2008;283:3376–3384. doi: 10.1074/jbc.M707640200. [DOI] [PubMed] [Google Scholar]
- 35.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 36.Saitoh S-I, Miyake K. Mechanism regulating cell surface expression and activation of Toll-like receptor 4. Chem Rec. 2006;6:311–319. doi: 10.1002/tcr.20093. [DOI] [PubMed] [Google Scholar]
- 37.Hausman SZ, Burns DL. Binding of pertussis toxin to lipid vesicles containing glycolipids. Infect Immun. 1993;61:335–337. doi: 10.1128/iai.61.1.335-337.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hausman SZ, Burns DL. Interaction of pertussis toxin with cells and model membranes. J. Biol. Chem. 1992;267:13735–13739. [PubMed] [Google Scholar]
- 39.Hausman SZ, Burns DL, Sickler VC, Manclark CR. Immune response to dimeric subunits of the pertussis toxin B oligomer. Infect. Immun. 1989;57:1760–1764. doi: 10.1128/iai.57.6.1760-1764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heerze LD, Chong PC, Armstrong GD. Investigation of the lectin-like binding domains in pertussis toxin using synthetic peptide sequences. Identification of a sialic acid binding site in the S2 subunit of the toxin. J Biol Chem. 1992;267:25810–25815. [PubMed] [Google Scholar]
- 41.Spangler BD, Heerze LD, Clark CG, Armstrong GD. Hydrophobic binding of pertussis toxin is enhanced by oligosaccharide receptors. Arch. Biochem. Biophys. 1993;305:153–158. doi: 10.1006/abbi.1993.1405. [DOI] [PubMed] [Google Scholar]
- 42.Witvliet MH, Burns DL, Brennan MJ, Poolman JT, Manclark CR. Binding of pertussis toxin to eucaryotic cells and glycoproteins. Infect Immun. 1989;57:3324–3330. doi: 10.1128/iai.57.11.3324-3330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosoff PM, Walker R, Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987;139:2419–2423. [PubMed] [Google Scholar]
- 44.Tonon S, Badran B, Benghiat FS, Goriely S, Flamand V, Willard-Gallo K, Willems F, Goldman M, De Wit D. Pertussis toxin activates adult and neonatal naive human CD4+ T lymphocytes. Eur J Immunol. 2006;36:1794–1804. doi: 10.1002/eji.200535697. [DOI] [PubMed] [Google Scholar]
- 45.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 46.Irving BA, Chan AC, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J. Exp. Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss A, Stobo JD. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J. Exp. Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juang Y-T, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J. Clin. Invest. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hewlett EL, Sauer KT, Myers GA, Cowell JL, Guerrant RL. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983;40:1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 51.Nunes J, Klasen S, Franco MD, Lipcey C, Mawas C, Bagnasco M, Olive D. Signalling through CD28 T-cell activation pathway involves an inositol phospholipid-specific phospholipase C activity. Biochem. J. 1993;293(Pt 3):835–842. doi: 10.1042/bj2930835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, Baltimore D. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 53.Merry AH, Gilbert RJC, Shore DA, Royle L, Miroshnychenko O, Vuong M, Wormald MR, Harvey DJ, Dwek RA, Classon BJ, Rudd PM, Davis SJ. O-glycan sialylation and the structure of the stalk-like region of the T cell co-receptor CD8. J. Biol. Chem. 2003;278:27119–27128. doi: 10.1074/jbc.M213056200. [DOI] [PubMed] [Google Scholar]
- 54.Julius M, Maroun CR, Haughn L. Distinct roles for CD4 and CD8 as co-receptors in antigen receptor signalling. Immunol. Today. 1993;14:177–183. doi: 10.1016/0167-5699(93)90282-p. [DOI] [PubMed] [Google Scholar]
- 55.Chan AC, Irving BA, Fraser JD, Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc. Natl. Acad. Sci. U.S.A. 1991;88:9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan AC, Irving BA, Weiss A. New insights into T-cell antigen receptor structure and signal transduction. Curr. Opin. Immunol. 1992;4:246–251. doi: 10.1016/0952-7915(92)90072-m. [DOI] [PubMed] [Google Scholar]
- 57.Rudd PM, Wormald MR, Stanfield RL, Huang M, Mattsson N, Speir JA, DiGennaro JA, Fetrow JS, Dwek RA, Wilson IA. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J. Mol. Biol. 1999;293:351–366. doi: 10.1006/jmbi.1999.3104. [DOI] [PubMed] [Google Scholar]
- 58.Gupta R, Brunak S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput. 2002:310–322. [PubMed] [Google Scholar]
- 59.Julenius K, Mølgaard A, Gupta R, Brunak S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology. 2005;15:153–164. doi: 10.1093/glycob/cwh151. [DOI] [PubMed] [Google Scholar]
- 60.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 61.Straus DB, Chan AC, Patai B, Weiss A. SH2 domain function is essential for the role of the Lck tyrosine kinase in T cell receptor signal transduction. J. Biol. Chem. 1996;271:9976–9981. doi: 10.1074/jbc.271.17.9976. [DOI] [PubMed] [Google Scholar]
- 62.Straus DB, Weiss A. The CD3 chains of the T cell antigen receptor associate with the ZAP-70 tyrosine kinase and are tyrosine phosphorylated after receptor stimulation. J. Exp. Med. 1993;178:1523–1530. doi: 10.1084/jem.178.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chilson OP, Boylston AW, Crumpton MJ. Phaseolus vulgaris phytohaemagglutinin (PHA) binds to the human T lymphocyte antigen receptor. EMBO J. 1984;3:3239–3245. doi: 10.1002/j.1460-2075.1984.tb02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narasimhan S, Freed JC, Schachter H. The effect of a “bisecting” N-acetylglucosaminyl group on the binding of biantennary, complex oligosaccharides to concanavalin A, Phaseolus vulgaris erythroagglutinin (E-PHA), and Ricinus communis agglutinin (RCA-120) immobilized on agarose. Carbohydr. Res. 1986;149:65–83. doi: 10.1016/s0008-6215(00)90370-7. [DOI] [PubMed] [Google Scholar]
- 65.Skutelsky E, Lotan R, Sharon N, Danon D. Distribution of the T-antigen on erythroid cell surfaces. Studies with peanut agglutinin, an anti-T specific lectin. Biochim. Biophys. Acta. 1977;467:165–174. doi: 10.1016/0005-2736(77)90193-6. [DOI] [PubMed] [Google Scholar]
- 66.Novogrodsky A, Lotan R, Ravid A, Sharon N. Peanut agglutinin, a new mitogen that binds to galactosyl sites exposed after neuraminidase treatment. J. Immunol. 1975;115:1243–1248. [PubMed] [Google Scholar]
- 67.Yogeeswaran G, Salk PL. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981;212:1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]
- 68.Miyagi T, Wada T, Yamaguchi K, Hata K. Sialidase and malignancy: a minireview. Glycoconj. J. 2004;20:189–198. doi: 10.1023/B:GLYC.0000024250.48506.bf. [DOI] [PubMed] [Google Scholar]
- 69.Schamel WWA, Reth M. Clustering models. Adv. Exp. Med. Biol. 2008;640:64–73. doi: 10.1007/978-0-387-09789-3_6. [DOI] [PubMed] [Google Scholar]
- 70.Jacobelli J, Andres PG, Boisvert J, Krummel MF. New views of the immunological synapse: variations in assembly and function. Curr. Opin. Immunol. 2004;16:345–352. doi: 10.1016/j.coi.2004.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.