SUMMARY
Neutralizing antibodies have been thought to be required for protection against acutely cytopathic viruses, such as the neurotropic vesicular stomatitis virus (VSV). Utilizing mice that possess B cells but lack antibodies, we show here that survival upon subcutaneous (s.c.) VSV challenge was independent of neutralizing antibody production or cell-mediated adaptive immunity. However, B cells were absolutely required to provide lymphotoxin (LT) α1β2, which maintained a protective subcapsular sinus (SCS) macrophage phenotype within virus draining lymph nodes (LNs). Macrophages within the SCS of B cell-deficient LNs, or of mice that lack LTα1β2 selectively in B cells, displayed an aberrant phenotype, failed to replicate VSV, and therefore did not produce type I interferons, which were required to prevent fatal VSV invasion of intranodal nerves. Thus, although B cells are essential for survival during VSV infection, their contribution involves the provision of innate differentiation and maintenance signals to macrophages, rather than adaptive immune mechanisms.
INTRODUCTION
Adaptive immunity, especially neutralizing antibody production, is thought to play a critical role in controlling cytopathic viral infections in mammals (Hangartner et al., 2006). However, external barrier breach by rapidly replicating viruses can place a host at risk long before adaptive immune components can be mobilized. Indeed, mice infected with VSV, an acutely cytopathic neurotropic rhabdovirus, can suffer fatal neuroinvasion despite high neutralizing antibody titers (Iannacone et al., 2010). This observation led us to revisit the contribution of humoral immune responses to survival after VSV infection.
Intravenous (i.v.) infection of mice with VSV elicits neutralizing T cell-independent IgM and T cell-dependent IgG responses that become detectable by days 4 and 7 postinfection, respectively (Bachmann et al., 1994, 1996; Charan and Zinkernagel, 1986; Karrer et al., 1997; Thomsen et al., 1997). Because B cell-deficient or CD4+ T cell-deficient mice die after i.v. VSV infection, it had been thought that neutralizing T cell-dependent antibodies were absolutely required for survival (Bründler et al., 1996). In addition to adaptive immune mechanisms, the naive host response to VSV infection is characterized by type I interferon (IFN-I) production, which precedes high-affinity antibodies and is also required for survival (Müller et al., 1994; Steinhoff et al., 1995). IFN-I can contribute to humoral immunity by directly enhancing B cell responses (Bach et al., 2007; Le Bon et al., 2006; Swanson et al., 2010), and it also triggers cell-intrinsic anti-viral resistance in somatic cells, including neurons (Detje et al., 2009; Trottier et al., 2005).
Most studies exploring the role of antibodies during VSV infection have challenged mice via the i.v. route (Bachmann et al., 1997; Bründler et al., 1996). However, VSV is usually transmitted in the wild by bites of infected insects (Smith et al., 2009), so subcutaneous (s.c.) infection arguably represents a more physiological route. We have recently characterized the fate of VSV and the resulting immune response after s.c. deposition of a small VSV inoculum in the footpad of mice (Iannacone et al., 2010; Junt et al., 2007). Intact virions are rapidly transported from the injection site via lymphatics to the draining popliteal lymph node (LN). LNs play a critical role in host defense by providing a specialized environment to stage adaptive immune responses and by acting as filter stations to prevent systemic dissemination of lymph-borne pathogens (Junt et al., 2007; von Andrian and Mempel, 2003). The cellular constituents of this LN filter are CD169+ macrophages that line the principal lymph conduits: the SCS and the medullary sinuses (Carrasco and Batista, 2007; Junt et al., 2007; Phan et al., 2007). Although macrophages capture VSV in both the SCS and the medulla, viral replication is anatomically restricted to CD169hi SCS macrophages, whereas CD169+/lo medullary macrophages are refractory to VSV infection (Iannacone et al., 2010). Their unique permissiveness to productive VSV infection allows SCS macrophages to sense viral presence and rapidly commence IFN-I production, which in turn protects intranodal nerves from VSV replication and ultimately precludes viral ascension to the CNS (Iannacone et al., 2010). Consequently, in macrophage-depleted LNs, the intranodal nerves are vulnerable to VSV infection.
We have shown recently that the susceptibility to VSV neuro-invasion upon LN macrophage depletion has a fatal outcome in ~60% of infected mice, with both dying and surviving animals producing similar neutralizing antibody titers (Iannacone et al., 2010). Thus, humoral immunity was apparently not sufficient for most individuals’ survival of s.c. VSV infection, although it remained possible that antibodies afforded viral clearance in the surviving ~40% of mice. To clarify the role of B cells and antibodies and to re-examine the requirements for protection against VSV, we undertook the present study. By utilizing animals that selectively lack antibodies but retain B cells, we found that neither humoral nor cell-mediated adaptive immunity were required for protection against VSV. However, B cells were essential because they provided a critical source of LTα1β2 required for proper SCS macrophage differentiation. In the absence of B cells, SCS macrophages lost their permissiveness to VSV replication and failed to produce IFN-I, which enabled lymph-borne VSV to access intranodal nerves as a conduit for fatal CNS invasion.
RESULTS
Antibodies, but Not B Cells, Are Dispensable for Protection against Subcutaneous VSV Infection
Previous studies have shown that B cell-deficient mice are highly susceptible to acutely cytopathic viruses, including VSV (Bachmann et al., 1995; Bründler et al., 1996; Gobet et al., 1988; Hangartner et al., 2006). Although this finding was interpreted as evidence that antibodies are absolutely required, it must be considered that B cell-deficient mice not only lack antibodies but also display abnormal lymphoid architecture (Kitamura et al., 1991). Therefore, we sought to re-evaluate the relative contribution of antibody-dependent and -independent functions of B cells to protective immunity against VSV. We took advantage of a recently generated mouse strain, DHLMP2A, in which the JH segment of the IgH locus was replaced by the Epstein-Barr virus-derived LMP2A protein (Casola et al., 2004). Because LMP2A provides tonic survival signals, B cells develop without a B cell receptor; therefore, DHLMP2A mice retain B cells and normal lymphoid tissue architecture, yet are devoid of surface-expressed and secreted antibodies.
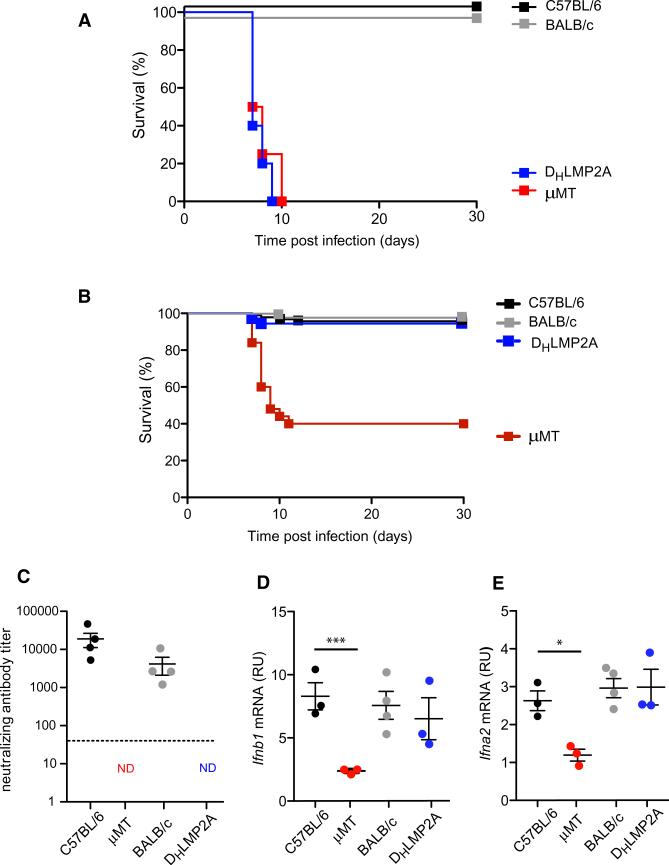
Consistent with previous studies (Bründler et al., 1996), B cell-deficient (μMT) mice died within 10 days of i.v. infection, whereas WT mice survived the viral challenge (Figure 1A). DHLMP2A mice were also susceptible to death after i.v. VSV infection, with a clinical course and mortality rate that were indistinguishable from those of μMT mice (Figure 1A). However, when mice were challenged s.c. (the natural transmission route for arboviruses, such as VSV [Mead et al., 1999]), ~60% of μMT mice died after developing ascending paralysis. In contrast, DHLMP2A mice, like WT mice, were protected (Figure 1B), even though (as expected) they were incapable of mounting a neutralizing antibody response to VSV (Figure 1C). Susceptibility to death in μMT mice after s.c. infection was a consequence of neuroinvasion by VSV, as indicated by the fact that virus became detectable in the CNS in symptomatic μMT mice. By contrast, VSV was never detectable in the systemic circulation (Figure S1 available online), indicating that the site of VSV entry to the CNS was the peripheral nervous system in the infected hindleg. Indeed, previous studies on the route of viral neuroinvasion after footpad infection have shown that VSV invades peripheral nerve fibers in the draining popliteal LN to gain access into the CNS (Iannacone et al., 2010).
Figure 1. Antibodies, but Not B Cells, Are Dispensable for Protection against Subcutaneous VSV Infection.
(A) Kaplan-Meier survival curves of C57BL/6 (n = 9), BALB/c (n = 10), μMT (C57BL/6 background; n = 5), and DHLMP2A (BALB/c background; n = 6) mice infected with 106 pfu of VSV-IND i.v. DHLMP2A versus μMT: p = 0.51, Log-rank (Mantel-Cox) test.
(B) Kaplan-Meier survival curves of C57BL/6 (n = 93), BALB/c (n = 30), μMT (n = 50), and DHLMP2A (n = 36) mice infected with 104 pfu of VSV-IND i.fp. DHLMP2A versus μMT: p < 0.0001, Log-rank (Mantel-Cox) test.
(C) VSV neutralizing antibody titers in the sera of the mice described in (B), 15 days after infection. Graph is representative of two experiments (n = 4 per experiment); ND = not detected.
(D and E) Ifnb1 (D) and Ifna2 (E) mRNA levels in total RNA isolated from LNs of the animals described in (B) and sacrificed 8 hr p.i. Analysis was performed by quantitative RT-PCR and results are expressed as relative units (RU) after normalization to the mRNA content of the housekeeping gene GAPDH. Graphs are representative of two experiments (n = 3 per experiment). **p < 0.001, *p < 0.05.
Although our results after i.v. viral challenge support an antibody requirement for survival of acutely cytopathic viral infection (Bachmann et al., 1997; Bründler et al., 1996), our findings in the s.c. infection model are not compatible with this antibody-centric paradigm. Rather, our findings in DHLMP2A mice imply that B cells may have an additional innate role in antiviral immunity that must be antibody independent.
B Cells Are Required for SCS Macrophage-Dependent IFN-I Production upon VSV Infection
With the footpad VSV infection model, we have previously shown that IFN-I production by macrophages in the draining popliteal LN is essential for survival of the viral challenge (Iannacone et al., 2010). Therefore, we examined the production of IFN-I family members in virus-draining LNs of μMT and DHLMP2A mice. When compared to WT animals, IFN-I production in VSV-draining LNs was reduced in μMT mice, but not in DHLMP2A mice, indicating that B cells, and not antibodies, are required for optimal IFN-I production upon VSV infection (Figures 1D and 1E). In VSV-challenged WT animals, IFN-I is largely produced by infected SCS macrophages and exerts its protective function by acting on intranodal nerves to prevent viral neuroinvasion (Iannacone et al., 2010). Indeed, LN macrophage depletion in WT mice results in a clinical course, mortality rate, and suppressed IFN-I response after s.c. VSV infection (Iannacone et al., 2010) that are reminiscent of those in μMT animals. Thus, we reasoned that the difference in viral susceptibility between μMT mice and DHLMP2A animals might reflect a divergence in LN macrophage composition and/or function.
As lymph drains from peripheral tissues and enters LN sinuses, it flows past two discrete macrophage populations: first the CD169hi subset in the SCS and, subsequently, CD169+/lo macrophages in the medulla. In WT mice, these two macrophage subsets can be distinguished not only by virtue of their anatomical location and differential expression of CD169, but also by several other surface markers. One such marker is the C-type lectin SIGN-R1, which in WT mice is highly expressed by medullary but not SCS macrophages (Figures 2A and 2B; Kang et al., 2004). By contrast, μMT mice not only expressed CD169 more uniformly, but also SIGN-R1 was expressed homogeneously by macrophages within both the SCS and medulla (Figures 2A and 2B). Lymphatic endothelial cells in the medullary sinuses express high levels of Lyve-1, so staining for Lyve-1 is useful to localize medullary and SCS macrophages in LN sections, especially in μMT mice where the absence of B cell follicles and homogeneous SIGN-R1 expression make distinction of SCS and medullary regions less apparent (Figure S2). Aberrant expression of SIGN-R1 was seen in μMT mice, but not in DHLMP2A mice, whose LNs contained somewhat fewer macrophages than strain-matched WT LNs (not shown), but their SCS macrophage population was phenotypically indistinguishable from both C57BL/6 and BALB/c WT controls (Figures 2A and 2B). Consequently, in single-cell suspensions of LNs from the latter three strains, only ~40% of macrophages displayed a medullary phenotype, whereas ~90% of macrophages were SIGN-R1+ in μMT LNs (Figure 2C). These findings are consistent with recent work indicating that B cell-deficient mice have a reduced number of LN macrophages and show aberrant expression of F4/80 (another surface marker restricted to medullary macrophages in WT mice) on SCS macrophages (Phan et al., 2009).
Figure 2. Surface Phenotype of SCS Macrophages in WT, μMT, and DHLMP2A Mice.
(A) Representative confocal micrographs of frozen popliteal LN sections stained with MAbs against SIGN-R1 (red), and CD169 (green). Scale bars represent 100 μm.
(B) FACS histograms of SIGN-R1 expression on CD11b+CD169+ LN cells of the indicated mouse strains. Histograms are representative of three experiments (n = 3–4 mice/experiment).
(C) Percentage of SIGN-R1+ cells within the CD11b+CD169+ compartment in LNs of the indicated mouse strains. ***p < 0.001.
Previous work has shown that both SCS and medullary macrophages rapidly capture virions that access LNs via afferent lymphatics (Junt et al., 2007). Other studies employing footpad infection with eGFP transgenic VSV (VSV-eGFP), which reports viral protein expression in infected cells (Chandran et al., 2005), demonstrated that medullary macrophages captured but did not replicate VSV (Iannacone et al., 2010). By contrast, SCS macrophages uniquely expressed GFP, indicating that they became preferentially infected (Hickman et al., 2008; Iannacone et al., 2010). When whole mounts of popliteal LNs were examined by confocal or multi-photon microscopy 8 hr after footpad infection with VSV-eGFP, GFP was not uniformly distributed but appeared most prominent above cortical bulges indicative of underlying B follicles (Movie S1). Indeed, experiments in LNs that had been populated by adoptively transferred differentially labeled T and B cells revealed that the majority of VSV-eGFP+ macrophages were in direct physical contact with follicular B cells, whereas GFP expression was absent in the medulla and sparse among SCS macrophages in the interfollicular T cell area (Movies S2 and S3).
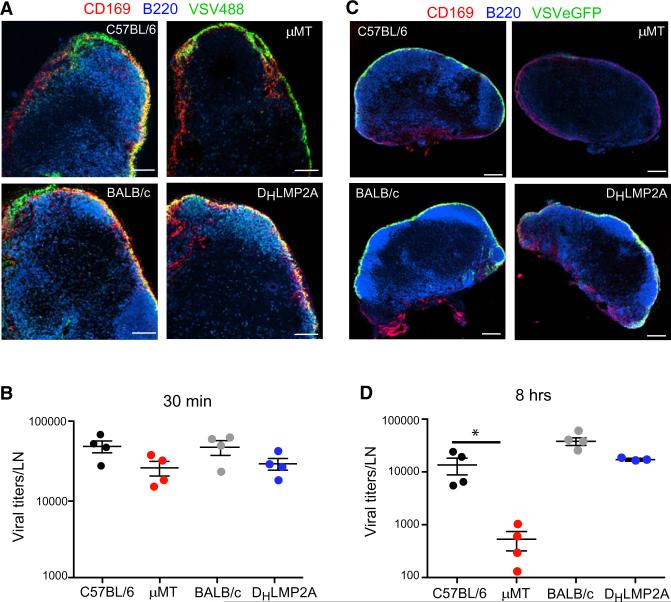
The apparent correlation between B cell-macrophage interactions and VSV replication capacity led us to test whether antibodies or B cell deficiency altered viral capture or infectivity of LN macrophages after s.c. VSV challenge. Thirty minutes after footpad injection, both μMT and DHLMP2A LNs efficiently captured and retained lymph-borne VSV at levels similar to WT controls (Figures 3A and 3B). Thus, the defect in macrophage number and surface phenotype in μMT mice did not compromise LN filter function. However, μMT LN macrophages failed to replicate VSV-eGFP, whereas WT and DHLMP2A mice showed robust VSV-eGFP replication that was restricted to SCS macrophages (Figure 3C). Accordingly, 8 hr after VSV challenge, μMT LNs contained nearly undetectable levels of replicating VSV, whereas substantial viral titers were apparent in WT and DHLMP2A LNs (Figure 3D). SCS macrophages are thought to produce IFN-I only when they are actively infected (Iannacone et al., 2010), so the failure of μMT macrophages to replicate virus in the SCS was probably the cause of their impaired IFN-I production (Figures 1D and 1E). Together these data imply that follicular B cells in LNs instruct a macrophage phenotype that is conducive to VSV replication, which appears necessary for subsequent local IFN-I production and ultimately the prevention of fatal CNS neuroinvasion after s.c. VSV infection.
Figure 3. B Cells Are Required for VSV Replication in LN Macrophages.
(A) Representative confocal micrographs of popliteal LN sections 30 min after footpad infection with Alexa Fluor 488-labeled VSV (VSV488, green). Sections were stained with MAbs against CD169 (red) and B220 (blue). Scale bars represent 100 μm.
(B) Viral titers in popliteal LNs of mice infected 30 min earlier with VSV-IND into the footpad. Results are representative of two experiments (n = 4 per experiment).
(C) Representative confocal micrographs of popliteal LN sections 8 hr after footpad infection with VSV-eGFP (green). Sections were stained as in (A). Scale bars represent 150 μm.
(D) Viral titers in popliteal LNs 8 hr after footpad infection with VSV-IND. Results are representative of three experiments (n = 3–4 mice/experiment).
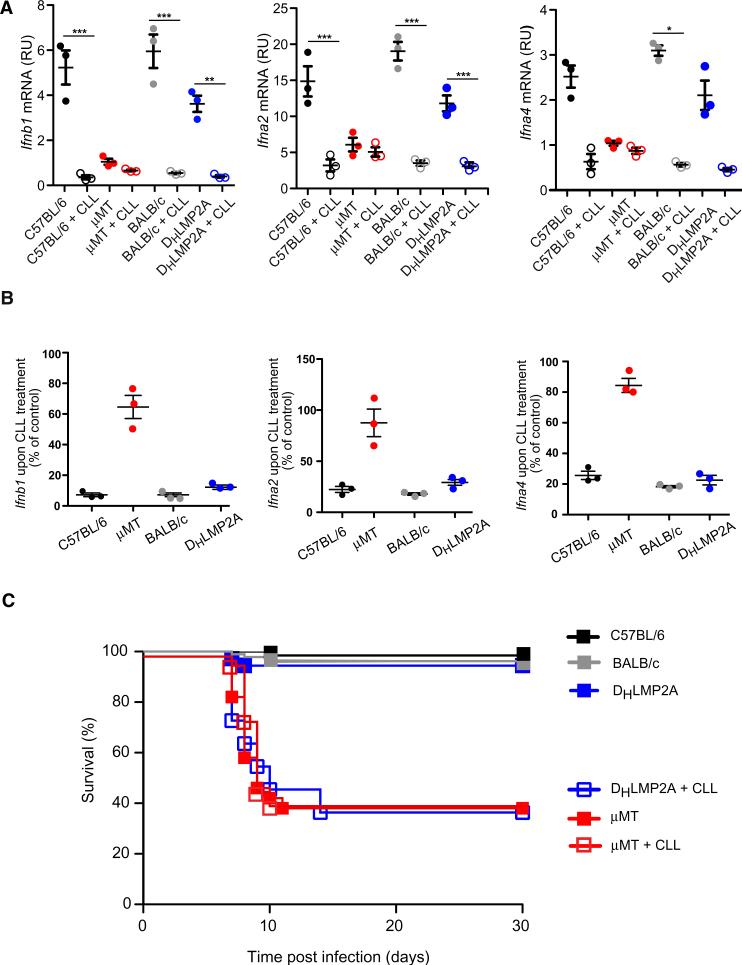
Consistent with this interpretation, depletion of LN macrophages by footpad CLL injection did not further decrease IFN-I production in μMT LNs (Figures 4A and 4B), nor did it enhance susceptibility to viral neuroinvasion (Figure 4C). However, when CLL was used to deplete LN macrophages from DHLMP2A or WT mice prior to infection, LN IFN-I production was greatly diminished (Figures 4A and 4B). Moreover, CLL-treated animals became susceptible to death after s.c. VSV infection with clinical course and mortality rate that were identical to those of μMT mice (Figure 4C). Thus, the antibody-independent survival of DHLMP2A animals is dependent upon the presence of functional SCS macrophages.
Figure 4. LN Macrophage Depletion Renders Antibody-Deficient Mice Susceptible to Fatal VSV Neuroinvasion.
(A) Ifnb1 (left), Ifna2 (middle), and Ifna4 (right) mRNA levels in total RNA isolated from LNs of the indicated mouse strains, with or without clodronate liposomes (CLL) treatment, 8 hr after footpad infection with VSV-IND. Analysis was performed as indicated in Figures 1D and 1E. Graphs are representative of two experiments (n = 3–4 per experiment). ***p < 0.001, *p < 0.05.
(B) The same set of data as in (A) are expressed as: (mRNA levels in CLL-treated LNs)/(mRNA levels in control LN) × 100.
(C) Kaplan-Meier survival curves of C57BL/6 (n = 93), BALB/c (n = 30), and DHLMP2A with (n = 12) or without (n = 36) CLL treatment, and μMT with (n = 15) or without (n = 50) CLL treatment prior to footpad infection with VSV-IND. DHLMP2A versus DHLMP2A + CLL: p < 0.0001; μMT versus μMT + CLL: not significant; μMT versus DHLMP2A + CLL: not significant; Log-rank (Mantel-Cox) test.
B Cell-Derived LTα1β2 Maintains the Protective Antiviral Phenotype of SCS Macrophages
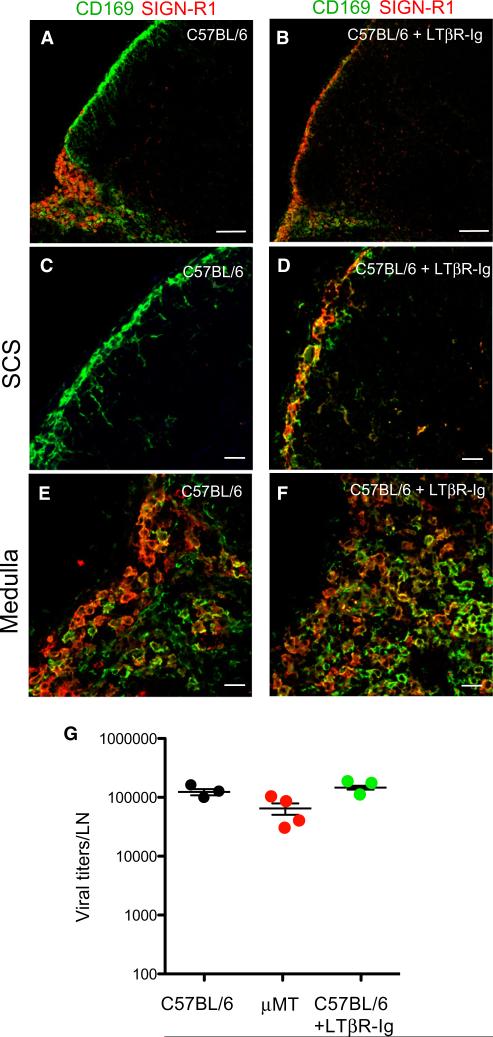
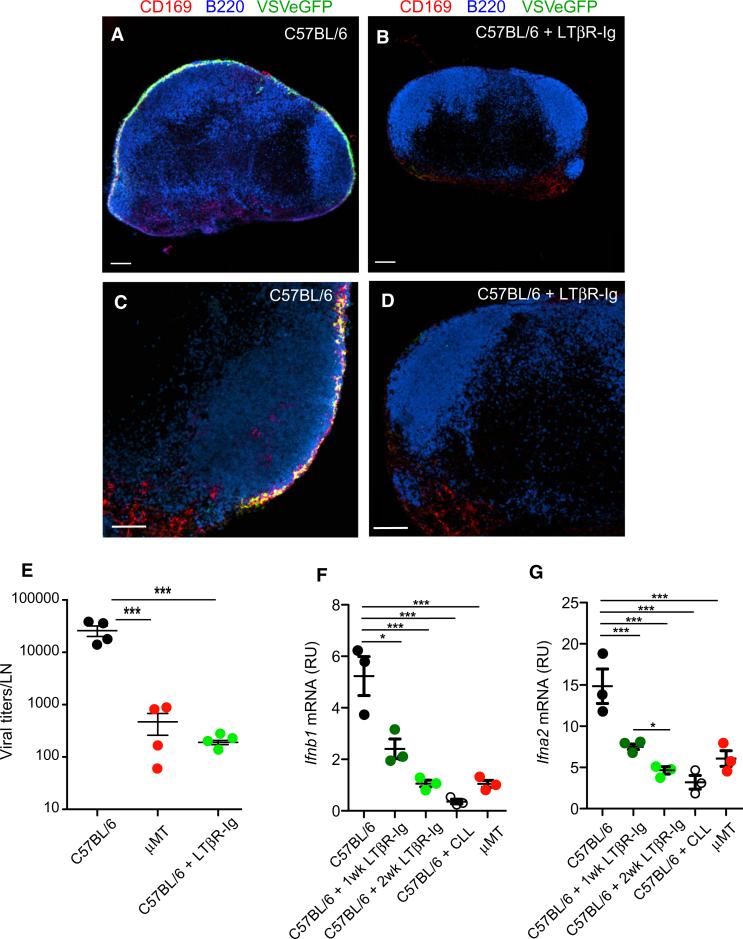
B cell contributions to the development of secondary lymphoid organs (Mebius, 2003) and the homeostasis of their stromal architecture and function (Schneider et al., 2008) are well known. A key homeostatic signal provided by B cells is LTα1β2, a TNF family member. B cell-expressed LTα1β2 controls splenic macrophage phenotype (Tumanov et al., 2002; Yu et al., 2002) and maintains the number and phenotype of SCS macrophages in LNs (Phan et al., 2009), but the role of this pathway in antiviral immunity is less understood. To explore this question, we treated WT mice for 2–3 weeks with LTβR-Ig, a decoy receptor that blocks lymphotoxin signaling (Fava et al., 2003). SCS macrophages in LTβR-Ig-treated LNs acquired SIGN-R1 (Figures 5A–5D) and lost expression of ligands for the cysteine-rich domain of the mannose receptor (CR-Fc), which are normally abundant on SCS macrophages but absent from the medulla (Figure S3). SIGN-R1 and CD169 expression by medullary macrophages was grossly unaffected by LTβR-Ig (Figures 5E and 5F). Thus, lymphotoxin blockade induced phenotypic changes in WT SCS macrophages that were strikingly similar to those in μMT LNs (Figures 2 and S3). Moreover, similar to B cell-deficient mice (Figure 3), the initial capture of lymph-borne VSV by LN macrophages was not affected by LTβR-Ig treatment (Figure 5G), but the capacity of treated LN macrophages to replicate VSV-eGFP (Figures 6A–6E) and to secrete IFN-I (Figures 6F and 6G) was compromised. We conclude that lymphotoxin signaling is dispensable for the lymph-filtering function of LN macrophages, but required to establish VSV replication competence and IFN-I production by SCS macrophages.
Figure 5. LT Signaling Is Required for SCS Macrophage Differentiation but Is Dispensable for LN Viral Capturing.
(A–F) Representative confocal micrographs of LN sections from control (A, C, E) and LTβR-Ig-treated (B, D, F) C57BL/6 mice, stained with MAbs against CD169 (green) and SIGN-R1 (red). Panels (C) and (D) are high-magnification images of SCS regions, and (E) and (F) are high-magnification images of medullary regions. Scale bars represent 100 μm (A, B) and 20 μm (C–F).
(G) Viral titers in popliteal LNs of C57BL/6, μMT, and LTβR-Ig-treated C57BL/6 mice, 30 min after footpad VSV infection. Results are representative of two experiments (n = 3–4 per experiment). Differences among means are not statistically significant.
Figure 6. LT Signaling Is Required for LN VSV Replication and IFN-I Production.
(A–D) Representative confocal micrographs of LN sections from control (A, C) and LTβR-Ig-treated (B, D) C57BL/6 mice at 8 hr after footpad infection with VSV-eGFP (green). Sections were stained with MAbs against CD169 (red) and B220 (blue). Scale bars represent 150 μm (A, B) and 100 μm (C, D).
(E) Viral titers in popliteal LNs of C57BL/6, μMT, and LTβR-Ig-treated C57BL/6 mice, 8 hr after footpad VSV infection. Results are representative of three experiments (n = 4 per experiment). ***p < 0.001.
(F and G) Ifnb1 (F) and Ifna2 (G) mRNA levels in total RNA isolated from LNs of μMT and C57BL/6 mice that were treated with CLL or with LTβR-Ig for 1 or 2 weeks prior to infection with VSV-IND. Mice were sacrificed 8 hr p.i. Analysis was performed as indicated in Figures 1D and 1E. Graphs are representative of two experiments (n = 3–4 per experiment). ***p < 0.001; **p < 0.01; *p < 0.05.
We have previously shown that SCS macrophage-derived IFN-I prevents VSV infection of peripheral nerves within draining LNs and ultimately protects against fatal neuroinvasion (Iannacone et al., 2010). Consistent with these data, and similar to our findings in μMT animals, the majority of LTβR-Ig-treated WT mice died within ~10 days of s.c. VSV infection (Figure 7A).
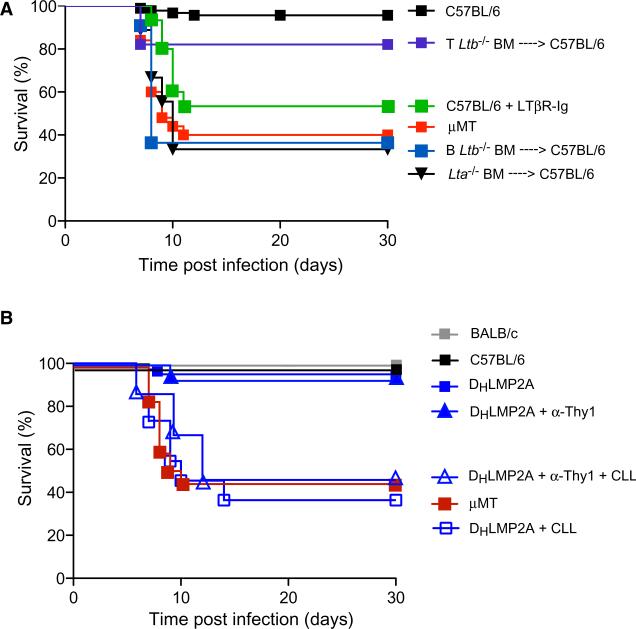
Figure 7. B Cell-Derived LTα1β2 Is Required for Survival upon Subcutaneous VSV Infection, although Adaptive Immunity Is Dispensable.
(A) Kaplan-Meier survival curves of footpad VSV-infected C57BL/6 (treated or not with LTβR-Ig), μMT, or irradiated C57BL/6 mice that were reconstituted with Lta–/–, B Ltb–/–, or T Ltb–/– bone marrow (BM). C57BL/6 versus B Ltb–/– BM → C57BL/6, p < 0.0001; C57BL/6 versus T Ltb–/– BM → C57BL/6, not significant; B Ltb–/– BM → C57BL/6 versus C57BL/6 + LTβR-Ig, not significant; B Ltb–/– BM → C57BL/6 versus μMT, not significant; B Ltb–/– BM → C57BL/6 versus Lta–/– BM → C57BL/6, not significant; Log-rank (Mantel-Cox) test.
(B) Kaplan-Meier survival curves of C57BL/6, (n = 10) BALB/c, (n = 10), and μMT (n = 13) DHLMP2A mice that were untreated (n = 11) or treated with CLL alone (n = 11), or Thy1 antibody alone (n = 15) or with CLL and Thy1 antibody (n = 15) prior to footpad VSV infection. BALB/c versus DHLMP2A + α-Thy1, not significant; BALB/c versus DHLMP2A + α-Thy1 + CLL, p < 0.0001; DHLMP2A + α-Thy1 + CLL versus μMT, not significant; DHLMP2A + α-Thy1 + CLL versus DHLMP2A + CLL, not significant; Log-rank (Mantel-Cox) test.
LTα1β2 expression is not restricted to B cells but is found on other cell types (Junt et al., 2006), particularly activated CD4+ T cells (Millet and Ruddle, 1994), and inflammatory signals can upregulate LTα1β2 on other LN cells (Agyekum et al., 2003). However, because of the spatial proximity between B cells and SCS macrophages within normal LNs, B cells seemed the most likely source of relevant LTα1β2 in this system. To formally test this prediction, we used B Ltb–/– mice that lacked LTα1β2 selectively in B cells (Tumanov et al., 2002). To avoid potential effects of compromised lymphoid organogenesis and disturbed lymphoid architecture in these animals, B Ltb–/– or WT bone marrow was used to reconstitute irradiated WT recipients. Bone marrow chimeric mice that lacked LTα1β2 specifically in T cells (T Ltb–/–) served as additional controls. Similar to observations in LTβR-Ig-treated animals, B Ltb–/– chimeric animals failed to replicate VSV within SCS macrophages after s.c. infection (Figures S4A and S4B), and ~60% of them died with signs of ascending paralysis, characteristic of peripheral VSV neuroinvasion (Figure 7A). By contrast, T Ltb–/– mice were protected against s.c. VSV challenge, confirming that B cells are the critical source of LTα1β2 that maintains protective SCS macrophages within peripheral LNs.
Adaptive Immunity Is Dispensable during Primary SubcutaneousVSV Infection
Most macrophage-depleted WT mice died when challenged s.c. with VSV even though they possessed neutralizing antibody titers that were much higher than in macrophage-sufficient animals (Iannacone et al., 2010). In fact, antibody titers were indistinguishable between macrophage-depleted WT mice that succumbed to VSV infection and those that remained asymptomatic (Figure S4C). Although the mechanism by which CLL-induced macrophage depletion leads to increased antibody titers in this model remains to be determined, these data, together with our findings in DHLMP2A mice, firmly establish that antibodies are neither required nor sufficient for survival of a primary s.c. infection with VSV.
This observation raised the question whether adaptive immunity mediated by other lymphocytes is required for protection against peripheral VSV infection. Indeed, both T cells (Kündig et al., 1996; Zinkernagel et al., 1978) and a subset of Thy1+ natural killer cells (Paust et al., 2010) can mount virus-specific effector and memory responses against VSV. To address this question, DHLMP2A mice were depleted of these adaptive lymphocytes by administration of Thy1 antibodies, which resulted in greater than 95% loss of circulating T cells (Figures S4D and S4E). Remarkably, despite the complete lack of both humoral and cellular adaptive immunity, all anti-Thy1-treated DHLMP2A mice survived VSV infection (Figure 7B). This indicates that the innate immune system, particularly the presence of fully differentiated SCS macrophages, provides sufficient protection to clear this acutely cytopathic viral infection without the need for adaptive immunity.
DISCUSSION
The results presented here contradict the current view that B cell-derived neutralizing antibodies are absolutely required to survive a primary cytopathic viral infection, such as that caused by VSV. This paradigm arose originally from experiments in B cell-deficient mice (Bachmann et al., 1994, 1997; Bründler et al., 1996; Gobet et al., 1988), which lack antibodies, but also have abnormal lymphoid tissue architecture and altered macrophage phenotype. Our experiments in mice that lack antibodies but possess B cells and normal lymphoid tissues confirm that both B cells and antibodies are critical to survive a systemic infection after i.v. bolus administration of VSV. However, only B cells are essential when VSV is encountered via the more “natural” s.c. route, whereas antibodies are neither needed nor sufficient for protection. Our data collectively indicate that immunity to s.c. VSV infection relies on B cell-derived LTα1β2, rendering SCS macrophages capable of replicating VSV and producing neuroprotective IFN-I.
Although VSV infections are typically self-limiting in mammals, rabies virus, a close relative, is responsible for >55,000 human deaths every year. Neutralizing antibodies are also believed to be required to survive rabies infections, as shown by the fact that passive antibody transfer and active vaccination to elicit humoral immunity are standard of care. Although neutralizing antibodies are undoubtedly effective prophylaxis against rhabdoviruses, our findings indicate that antibody therapy may be insufficient to treat existing rhabdoviral infections in nonimmune subjects, at least in the case of VSV. It is unclear whether this caveat applies also to rabies virus infection, but failures of both passive and active vaccination after exposure to rabies are known to occur (Anonymous, 1988). Thus, it will be important to further dissect the role of antibodies and interferon in this disease. In addition, recent years have seen the emergence and/or spread of other arthropod-borne neurotropic viral infections, such as West Nile virus, Japanese encephalitis virus, and Eastern and Western equine encephalitis virus, to name a few (Weaver and Barrett, 2004). It remains to be determined whether the cellular and molecular immunological events that occur upon inoculation of these pathogens in the skin are similar to the ones identified here.
Most viral pathogens, including rabies and VSV, must breach the body's external barriers to cause disease. Viruses deposited in the interstitial space of peripheral tissues are readily drained via lymph vessels to downstream LNs. Once a lymph-borne virus enters the intranodal lymph conduits, it runs a gauntlet across two phenotypically and functionally distinct LN macrophage populations within the lymphatic space. Initial encounter is with SCS macrophages, which populate the superficial cortex above B cell follicles, often with macrophages penetrating across the sinus floor such that they contact both follicular B cells and afferent lymph (Junt et al., 2007). Subsequently, lymph-borne viruses are exposed to macrophages within medullary sinuses where lymph is collected to be discharged into efferent lymphatics. Together, SCS and medullary macrophages filter the lymph and capture particulate matter, including viruses (Junt et al., 2007). The recognition mechanism(s) mediating this “flypaper” activity is not fully understood, but this process is extremely effective, as shown by the fact that >99% of infectious virions are retained in LNs, preventing systemic dissemination (Junt et al., 2007).
Once a lymph-borne virus has been captured by LN macrophages, at least three distinct fates await the surface-bound virion: (1) phagocytosis and destruction in lysosomal compartments, (2) presentation and handover to follicular B cells for the induction of humoral responses, or (3) productive infection of the capturing cells (Iannacone et al., 2010; Junt et al., 2007). Paradoxically, our findings indicate that the latter event is indispensable for host resistance to VSV; infected mice survived efficiently when SCS macrophages supported VSV replication. Although both SCS and medullary macrophages captured VSV, only SCS macrophages support VSV replication, which is required for IFN-I production (Iannacone et al., 2010). In settings where SCS macrophage differentiation was compromised, as in μMT mice, the cells failed to replicate VSV or to produce IFN-I, which is required to protect intranodal nerves from serving as viral conduits to the CNS.
Several phenotypic and functional features of SCS and medullary macrophages could regulate their difference in VSV replication capacity. For example, medullary macrophages are more phagocytic and express higher levels of endosomal degradative enzymes than their SCS counterparts (Delemarre et al., 1990b; Fossum, 1980; Phan et al., 2009; Sainte-Marie and Peng, 1985; Szakal et al., 1983). The two subsets differ also in their expression of antiviral sensing receptors. Compared to SCS macrophages, published microarray data (Phan et al., 2009) suggest that the medullary subset expresses higher mRNA levels for several TLRs, including TLR4 and TLR13, which both recognize VSV (Georgel et al., 2007; Shi et al., 2011). By contrast, SCS macrophages express more RIG-I, which enables intracellular VSV recognition (Gerlier and Lyles, 2011; Kato et al., 2006). Thus, medullary macrophages are well equipped to detect and destroy extracellular virus whereas SCS macrophages appear to be relatively inefficient at eliminating surface-bound virions, which may facilitate productive VSV infection. However, SCS macrophages may be preferentially responsive to viral patterns in their cytoplasm, inducing IFN-I production. Similar to differences between SCS and medullary macrophages in WT animals, our data would predict that SCS macrophages from DHLMP2A and μMT mice are similarly divergent in their viral sensing and phagocytic capacity.
LTβR is expressed on both LN macrophage subsets, but SCS macrophage maintenance and function appear to be more sensitive to LTβR signaling (Phan et al., 2009). Moreover, SCS but presumably not medullary macrophages are in intimate contact with LTα1β2-expressing follicular B cells. Consequently, in vivo LTα1β2 inhibition minimally affects the medullary compartment, but alters the phenotype of SCS macrophages and compromises their ability to capture lymph-borne immune complexes (Phan et al., 2009). The present findings indicate that LTα1β2 also regulates SCS macrophage susceptibility to VSV and the ensuing IFN-I response. In the absence of B cell-expressed LTα1β2, SCS macrophages assume a phenotype resembling medullary macrophages and no longer support VSV replication. One possible explanation for this observation is that tonic exposure to LTα1β2 on follicular B cells attenuates macrophage responsiveness to autocrine IFN-I. Without LTβR signaling, as is physiologically the case in the medulla or may be experimentally achieved in the SCS by deletion of B cells or treatment with LTβR-Ig, macrophages may gain IFN-I responsiveness and VSV resistance.
It remains to be determined whether there are additional mechanisms by which B cell-derived LTα1β2 renders SCS macrophages physiologically susceptible to VSV infection. Nonetheless, the net effect is that LTα1β2 transforms SCS macrophages into unique sentinels that execute a rapid antiviral cytokine response. It seems counterintuitive that this defense mechanism should depend upon the amplification of a potentially lethal virus, but beyond jump-starting the IFN-I response, increased viral antigen availability may facilitate adaptive memory in LNs (Hickman et al., 2008). Thus, SCS macrophage infection appears to be a “necessary evil” to ensure short-term survival and to promote long-term immunity of the host.
Defects in LTα1β2 signaling have been implicated previously in impaired antiviral immune responses (Berger et al., 1999; Spahn et al., 2005). Efficient IFN-I production upon cytomegalo-virus infection requires lymphotoxin signaling in splenic stromal cells (Benedict et al., 2001; Schneider et al., 2008), and Ltb–/– mice are vulnerable to death after i.v. VSV infection resulting from defective viral capture by splenic marginal zone macrophages (Junt et al., 2006). However, these earlier studies performed by systemic viral challenge were focused on the role of LTα1β2 in antiviral responses mediated by adaptive immune cells. Our findings highlight a previously underappreciated role for lymphotoxin in innate antiviral immunity in LNs draining a local infection site.
While LTα1β2 confers antiviral resistance to blood-borne or lymph-borne VSV in the spleen or peripheral LNs, respectively, our observations reveal the critical role that the route of infection plays in determining the molecular and cellular requirements for immune protection. Both i.v. and s.c. VSV infections elicit robust IFN-I and neutralizing antibody production, and both infection routes rely on macrophages for survival (Ciavarra et al., 2005; Iannacone et al., 2010). Consistent with earlier findings (Thomsen et al., 1997), our experiments confirm that antibodies are critical to survive i.v. VSV infection; however, a humoral response is neither required nor sufficient to prevent fatal neuroinvasion when VSV is given subcutaneously. In the s.c. setting, VSV gains access to the CNS via peripheral nerves in draining LNs (Iannacone et al., 2010). It is unclear how VSV accesses the CNS from the blood; however, it appears to do so rapidly, at least when given at a lethal dose, because subsequent treatment with neutralizing antibodies is protective only within 3 hr of i.v. infection (Steinhoff et al., 1995).
VSV infection of SCS macrophages, although required for production of neuroprotective IFN-I, results in death of the host cells within 12–18 hr. Through this act of self-sacrifice, SCS macrophages establish a local antiviral state that prevents further viral replication. However, once depleted, SCS and medullary macrophages are slow to return. When CLL is used to eliminate essentially all LN macrophages, LNs require as long as 6 months to re-establish normal cell numbers (Delemarre et al., 1990a). This lag time is markedly shorter after VSV infection, where SCS macrophages return to preinfection numbers within 1 month (data not shown). Nevertheless, the void of SCS macrophages for several weeks after VSV infection probably renders LNs transiently hypervulnerable to reinfection. In these cases the induction of protective memory responses by adaptive immune cells may be critical for preventing early reinfection.
In contrast to the likely benefit of adaptive immunity during reinfection, our results demonstrate that during a primary s.c. infection, recognition of viral epitopes by either antibody or TCR is neither necessary nor sufficient to prevent fatal VSV neuroinvasion. This observation runs counter to the commonly held view that during viral infections, innate immunity must orchestrate the induction of antiviral adaptive responses to achieve sterilizing immunity. Given the rapid replication of some viruses, a proliferating pathogen may overwhelm its host before adaptive immune countermeasures can be mobilized. Innate defenses like complement, type I interferon, and others are believed to provide stopgap measures, lowering pathogen burden and buying time for adaptive immune responses to develop. Although this concept may apply to other viral infections, our findings with VSV turn this view upside down, indicating that during a primary infection with this cytopathic virus, innate immunity can be sterilizing without adaptive immune contributions. Thus, the essential contribution to VSV immunity by adaptive immune cells appears to be 2-fold. (1) During primary infection, B cells are critical enablers of innate immune responses by inducing and maintaining SCS macrophage phenotype through LTα1β2 presentation. (2) During secondary infection in the presence of established immunological memory, antiviral antibodies neutralize infectious virions before host cells can be infected.
In summary, we demonstrate that naive mice can survive a s.c. VSV challenge without requiring antigen-specific adaptive immunity. Efficient protection against VSV is provided by SCS macrophages in the draining LNs that rely on contact with follicular B cells expressing LTα1β2 on their surface. The constant exposure to LTα1β2 induces and maintains the protective SCS macrophage phenotype. Consequently, SCS macrophages in B cell-deficient mice or in mice that lack B cell-expressed LTα1β2 display an altered phenotype that resembles that of medullary macrophages, which are not protective in VSV infection. Like medullary macrophages, SCS macrophages that are deprived of LTα1β2 capture lymph-borne VSV but fail to replicate it. Without replication, SCS macrophages do not produce IFN-I that is required to prevent VSV invasion of intranodal nerves. These findings establish a critical innate function for B cells in antiviral immunity. This setting requires B cells not as a source of antibodies, but as providers of an anatomically restricted maintenance signal and as the day-to-day custodians of macrophage differentiation.
EXPERIMENTAL PROCEDURES
Full details of experimental procedures can be found online with Supplemental Information.
Mice
C57BL/6 and BALB/c mice, 6–8 weeks old, were purchased from Charles River or The Jackson Laboratory. DHLMP2A mice were provided by K. Rajewsky (Harvard Medical School). Lta-deficient and μMT mice were purchased from The Jackson Laboratory. Radiation bone marrow chimeras were generated by transfer of C57BL/6, B Ltb–/–, T Ltb–/–, or Lta–/– bone marrow into C57BL/6 mice. Mice were housed under specific-pathogen-free conditions in accordance with National Institutes of Health guidelines. All experimental animal procedures were approved by the Institutional Animal Committees of Harvard Medical School and IDI.
Viruses and Viral Plaque and Neutralization Assays
VSV serotype Indiana and VSV-eGFP (Chandran et al., 2005) were propagated and purified as described (Iannacone et al., 2010; Junt et al., 2007). Some batches were fluorescently labeled as described (Junt et al., 2007). Infectivity of VSV preparations and VSV titers from organs of infected mice were quantified by plaque assay as described (Iannacone et al., 2010; Junt et al., 2007). VSV neutralization assay was performed as described (Iannacone et al., 2010; Junt et al., 2007). Mice were infected with 104 plaque-forming units (pfu) of VSV Indiana into the right hind footpad (i.fp.), 106 pfu of VSV-eGFP i.fp., 107 pfu of VSV488, or 106 pfu of VSV Indiana intravenously (i.v.). All infectious work was performed in designated BL2+ workspaces, in accordance with institutional guidelines, and approved by the Harvard Committee on Microbiological Safety.
Tissue Digestion and Flow Cytometry
Single-cell suspensions of LNs for flow cytometry were generated as described (Iannacone et al., 2010). All flow cytometric analyses were performed and analyzed as described (Iannacone et al., 2010).
Confocal Microscopy
Popliteal LNs were harvested, processed, sectioned, stained, and analyzed as described (Iannacone et al., 2010; Junt et al., 2007). Whole-mount immunofluorescence analysis was performed as described (Iannacone et al., 2010).
Measurement of Type I Interferon
Total RNA was extracted from popliteal LNs harvested 8 hr p.i. RNA was reverse transcribed and real-time quantitative PCR reactions were performed to detect Ifnb1, Ifna2, Ifna4, and Gapdh message.
In Vivo Depletions and Blockade
LN macrophages were depleted by subcutaneous injections of clodronate liposomes (CLL), and Thy1+ T cells and NK cells were depleted with an intraperitoneal injection of mThy1.2 depleting antibody 4 days prior to and throughout infection. For lymphotoxing signaling blockade, mice received weekly intraperitoneal injections of 100 μg of LTβR-mIgG1 for 1, 2, or 3 weeks prior to infection or tissue harvest.
Statistical Analyses
Results are expressed as mean ± SEM. All statistical analyses were performed in Prism (GraphPad Software). Means between two groups were compared with two-tailed t test. Means among three or more groups were compared with one-way or two-way analysis of variance with Bonferroni's post-test. Kaplan-Meier survival curves were compared with the Log-rank (Mantel-Cox) test.
Supplementary Material
ACKNOWLEDGMENTS
We thank G. Cheng and M. Flynn for technical support; M. Perdue for secretarial assistance; K. Rajewsky for providing DHLMP2A mice; L. Martinez-Pomares for CR-Fc; S. Whelan for VSV and VSVeGFP; N. van Rooijen for clodronate liposomes; J. Browning for LTβR-Ig; L.G. Guidotti for helpful advice; and the members of the U.H.v.A. laboratory for helpful discussion. This work was supported by the National Institutes of Health (NIH) grants AI069259, AI072252, and AI078897 (to U.H.v.A.), the Giovanni Armenise-Harvard Foundation (to M.I.), and a NIH T32 Training Grant in Hematology 5T32-HL07623-20 (to E.A.M.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, four figures, and three movies and can be found with this article online at doi:10.1016/j.immuni.2012.01.013.
REFERENCES
- Agyekum S, Church A, Sohail M, Krausz T, Van Noorden S, Polak J, Cohen J. Expression of lymphotoxin-beta (LT-beta) in chronic inflammatory conditions. J. Pathol. 2003;199:115–121. doi: 10.1002/path.1249. [DOI] [PubMed] [Google Scholar]
- Rabies vaccine failures. Lancet. 1988;1:917–918. Anonymous. [PubMed] [Google Scholar]
- Bach P, Kamphuis E, Odermatt B, Sutter G, Buchholz CJ, Kalinke U. Vesicular stomatitis virus glycoprotein displaying retrovirus-like particles induce a type I IFN receptor-dependent switch to neutralizing IgG antibodies. J. Immunol. 2007;178:5839–5847. doi: 10.4049/jimmunol.178.9.5839. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Kündig TM, Hengartner H, Zinkernagel RM. Regulation of IgG antibody titers by the amount persisting of immune-complexed antigen. Eur. J. Immunol. 1994;24:2567–2570. doi: 10.1002/eji.1830241046. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Hengartner H, Zinkernagel RM. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 1995;25:3445–3451. doi: 10.1002/eji.1830251236. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Odermatt B, Hengartner H, Zinkernagel RM. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J. Exp. Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, Aguet M, Hengartner H, Zinkernagel RM. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- Benedict CA, Banks TA, Senderowicz L, Ko M, Britt WJ, Angulo A, Ghazal P, Ware CF. Lymphotoxins and cytomegalovirus cooperatively induce interferon-beta, establishing host-virus détente. Immunity. 2001;15:617–626. doi: 10.1016/s1074-7613(01)00222-9. [DOI] [PubMed] [Google Scholar]
- Berger DP, Naniche D, Crowley MT, Koni PA, Flavell RA, Oldstone MB. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology. 1999;260:136–147. doi: 10.1006/viro.1999.9811. [DOI] [PubMed] [Google Scholar]
- Bründler MA, Aichele P, Bachmann M, Kitamura D, Rajewsky K, Zinkernagel RM. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur. J. Immunol. 1996;26:2257–2262. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan S, Zinkernagel RM. Antibody mediated suppression of secondary IgM response in nude mice against vesicular stomatitis virus. J. Immunol. 1986;136:3057–3061. [PubMed] [Google Scholar]
- Ciavarra RP, Taylor L, Greene AR, Yousefieh N, Horeth D, van Rooijen N, Steel C, Gregory B, Birkenbach M, Sekellick M. Impact of macrophage and dendritic cell subset elimination on antiviral immunity, viral clearance and production of type 1 interferon. Virology. 2005;342:177–189. doi: 10.1016/j.virol.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Delemarre FG, Kors N, Kraal G, van Rooijen N. Repopulation of macrophages in popliteal lymph nodes of mice after liposome-mediated depletion. J. Leukoc. Biol. 1990a;47:251–257. doi: 10.1002/jlb.47.3.251. [DOI] [PubMed] [Google Scholar]
- Delemarre FG, Kors N, van Rooijen N. Elimination of spleen and of lymph node macrophages and its difference in the effect on the immune response to particulate antigens. Immunobiology. 1990b;182:70–78. doi: 10.1016/S0171-2985(11)80584-X. [DOI] [PubMed] [Google Scholar]
- Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, Bechmann I, Prinz M, Kalinke U. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J. Immunol. 2009;182:2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- Fava RA, Notidis E, Hunt J, Szanya V, Ratcliffe N, Ngam-Ek A, De Fougerolles AR, Sprague A, Browning JL. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J. Immunol. 2003;171:115–126. doi: 10.4049/jimmunol.171.1.115. [DOI] [PubMed] [Google Scholar]
- Fossum S. The architecture of rat lymph nodes. IV. Distribution of ferritin and colloidal carbon in the draining lymph nodes after foot-pad injection. Scand. J. Immunol. 1980;12:433–441. doi: 10.1111/j.1365-3083.1980.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MB, Beutler B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Gerlier D, Lyles DS. Interplay between innate immunity and negative-strand RNA viruses: towards a rational model. Microbiol. Mol. Biol. Rev. 2011;75:468–490. doi: 10.1128/MMBR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobet R, Cerny A, Rüedi E, Hengartner H, Zinkernagel RM. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp. Cell Biol. 1988;56:175–180. doi: 10.1159/000163477. [DOI] [PubMed] [Google Scholar]
- Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat. Rev. Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- Hickman HD, Takeda K, Skon CN, Murray FR, Hensley SE, Loomis J, Barber GN, Bennink JR, Yewdell JW. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat. Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, Whelan SP, Guidotti LG, von Andrian UH. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Tumanov AV, Harris N, Heikenwalder M, Zeller N, Kuprash DV, Aguzzi A, Ludewig B, Nedospasov SA, Zinkernagel RM. Expression of lymphotoxin beta governs immunity at two distinct levels. Eur. J. Immunol. 2006;36:2061–2075. doi: 10.1002/eji.200626255. [DOI] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kang YS, Kim JY, Bruening SA, Pack M, Charalambous A, Pritsker A, Moran TM, Loeffler JM, Steinman RM, Park CG. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer U, Althage A, Odermatt B, Roberts CW, Korsmeyer SJ, Miyawaki S, Hengartner H, Zinkernagel RM. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(-)/-) mutant mice. J. Exp. Med. 1997;185:2157–2170. doi: 10.1084/jem.185.12.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kündig TM, Bachmann MF, Oehen S, Hoffmann UW, Simard JJ, Kalberer CP, Pircher H, Ohashi PS, Hengartner H, Zinkernagel RM. On the role of antigen in maintaining cytotoxic T-cell memory. Proc. Natl. Acad. Sci. USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- Mead DG, Maré CJ, Ramberg FB. Bite transmission of vesicular stomatitis virus (New Jersey serotype) to laboratory mice by Simulium vittatum (Diptera: Simuliidae). J. Med. Entomol. 1999;36:410–413. doi: 10.1093/jmedent/36.4.410. [DOI] [PubMed] [Google Scholar]
- Mebius RE. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- Millet I, Ruddle NH. Differential regulation of lymphotoxin (LT), lymphotoxin-β (LT-β), and TNF-α in murine T cell clones activated through the TCR. J. Immunol. 1994;152:4336–4346. [PubMed] [Google Scholar]
- Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainte-Marie G, Peng F-S. Distribution pattern of drained antigens and antibodies in the subcapsular sinus of the lymph node of the rat. Cell Tissue Res. 1985;239:31–35. doi: 10.1007/BF00214899. [DOI] [PubMed] [Google Scholar]
- Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Cai Z, Sanchez A, Zhang T, Wen S, Wang J, Yang J, Fu S, Zhang D. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J. Biol. Chem. 2011;286:4517–4524. doi: 10.1074/jbc.M110.159590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PF, Howerth EW, Carter D, Gray EW, Noblet R, Mead DG. Mechanical transmission of vesicular stomatitis New Jersey virus by Simulium vittatum (Diptera: Simuliidae) to domestic swine (Sus scrofa). J. Med. Entomol. 2009;46:1537–1540. doi: 10.1603/033.046.0643. [DOI] [PubMed] [Google Scholar]
- Spahn TW, Eugster HP, Fontana A, Domschke W, Kucharzik T. Role of lymphotoxin in experimental models of infectious diseases: potential benefits and risks of a therapeutic inhibition of the lymphotoxin-beta receptor pathway. Infect. Immun. 2005;73:7077–7088. doi: 10.1128/IAI.73.11.7077-7088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff U, Müller U, Schertler A, Hengartner H, Aguet M, Zinkernagel RM. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J. Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J. Exp. Med. 2010;207:1485–1500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakal AK, Holmes KL, Tew JG. Transport of immune complexes from the subcapsular sinus to lymph node follicles on the surface of nonphagocytic cells, including cells with dendritic morphology. J. Immunol. 1983;131:1714–1727. [PubMed] [Google Scholar]
- Thomsen AR, Nansen A, Andersen C, Johansen J, Marker O, Christensen JP. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus. Int. Immunol. 1997;9:1757–1766. doi: 10.1093/intimm/9.11.1757. [DOI] [PubMed] [Google Scholar]
- Trottier MD, Jr., Palian BM, Reiss CS. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology. 2005;333:215–225. doi: 10.1016/j.virol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, Drutskaya L, Stewart C, Chervonsky A, Nedospasov S. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004;2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Wang Y, Chin RK, Martinez-Pomares L, Gordon S, Kosco-Vibois MH, Cyster J, Fu YX. B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J. Immunol. 2002;168:5117–5123. doi: 10.4049/jimmunol.168.10.5117. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Adler B, Holland JJ. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp. Cell Biol. 1978;46:53–70. doi: 10.1159/000162882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.