Abstract
The structure of the GAG (glycosaminoglycan) chain of recombinantly expressed decorin proteoglycan was examined using a combination of intact-chain analysis and domain compositional analysis. The GAG had a number-average molecular mass of 22 kDa as determined by PAGE. NMR spectroscopic analysis using two-dimensional correlation spectroscopy indicated that the ratio of glucuronic acid to iduronic acid in decorin peptidoglycan was 5 to 1. GAG domains terminated with a specific disaccharide obtained by enzymatic degradation of decorin GAG with highly specific endolytic and exolytic lyases were analysed by PAGE and further depolymerized with the enzymes. The disaccharide compositional profiles of the resulting domains were obtained using LC with mass spectrometric and photometric detection and compared with that of the polysaccharide. The information obtained through the disaccharide compositional profiling was combined with the NMR and PAGE data to construct a map of the decorin GAG sequence motifs.
Keywords: decorin, dermatan sulfate (DS), LC-MS, proteoglycan, structural domain, structural motif
INTRODUCTION
PGs (proteoglycans) are composed of one or more GAG (glycosaminoglycan) chains with MMs (molecular masses) in the range 5–40 kDa, covalently attached to a protein core [1]. PGs make up a large proportion of the extracellular matrix, performing a variety of roles in the excretory system, respiratory system, circulatory system and skeletal system, and are involved in multisystem diseases of aging and cancer [2]. The GAG chains are responsible for many of the PG biological functions, and subtle variations in the GAG structure can have a pronounced effect on the organism physiology and pathophysiology [3].
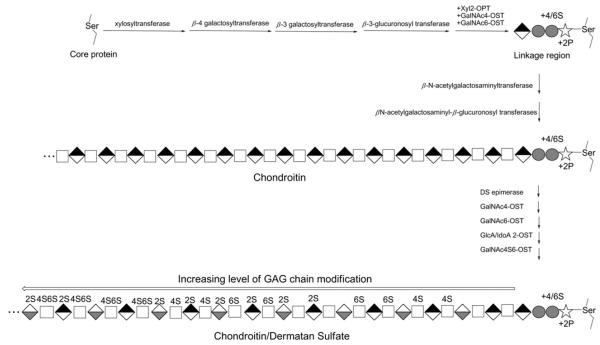
Decorin is one of the simplest cellular or pericellular matrix PGs belonging to the SLRP (small leucine-rich proteoglycan) family. Decorin consists of a protein core containing multiple leucine repeats with flanking cysteine-rich disulfide domains and a single CS (chondroitin sulfate) or DS (dermatan sulfate) GAG chain [4]. Decorin maps to human chromosome 12 and, under the influence of a responsive promoter, is biosynthesized in the endoplasmic reticulum as a nascent protein containing a signal peptide and propeptide [5]. The single GAG attachment site in decorin is located near its N-terminus (Ser34; Swiss-Prot accession number P07585), and several N-linked oligosaccharide attachment sites are located on Asn211, Asn262 and Asn303 [6]. Decorin GAG is predominantly a CS-type in bone and cartilage tissues, and DS-type in other tissues and is biosynthesized through the addition of xylose to the GAGylation site serine, DEASGIG [7], in the endoplasmic reticulum followed by the sequential addition of two galactose residues and a glucuronate residue in the cis-Golgi [5]. The resulting linker tetrasaccharide is further extended and modified through the action of glycosyltransferases, O-sulfotransferases and C5 epimerase (in tissues expressing this enzyme and synthesizing DS chains), which complete the decorin GAG biosynthesis (Figure 1). Site-directed mutagenesis studies have demonstrated the importance of decorin glycosylation for the process of its folding and secretion [6].
Figure 1. Biosynthesis of decorin.
Following the biosynthesis of the core protein, the linkage region is biosynthesized and the nascent GAG is extended with a simple→4)-β-d-GlcA (1→3)β-d-GalNAc(1→repeating disaccharide unit (chondroitin). Subsequent chainmodification can result in up to 16 different disaccharide units in chondroitin/DS. Symbols correspond to xylose (stars), galactose (grey circles), N-acetylgalactosamine (white squares), GlcA (black-and-white diamonds) and iduronic acid (white-and-grey diamonds).
Both components of decorin PG, the core protein and GAG, are important for its biological function. For example, the decorin core-protein interactions with EGF (epidermal growth factor) are important in the EGF signalling pathway, and proper assembly of collagen fibrils, fibrillogenesis, is regulated by decorin protein core and its GAG chain [8]. Decorin GAG plays a primary role in interactions with cytokines and growth factors, such as TNF (tumour necrosis factor) and FGF-2 (fibroblast growth factor 2), regulating cell growth, wound healing, axon regeneration and neural-stem-cell proliferation [9]. More highly sulfated GAG domains containing disulfated and trisulfated CS/DS disaccharides represent structural features important in protein– GAG interactions [2]. Decorin GAG may also inhibit blood coagulation through its interaction with heparin cofactor II [4].
Detailed knowledge of the decorin PG structure is necessary to fully understand its structure–function relationship. Although the sequence and the sites of post-translational modifications of decorin core protein have been determined [4] and its quaternary structure has been solved [10], GAG sequence analysis remains a challenge, in part due to the lack of GAG sequencing tools capable of handling the tremendous structural complexity of this polysaccharide. Structural characterization of large complex polysaccharides such as decorin GAG relies on enzymology combined with an array of analytical techniques. Using exhaustive depolymerization of recombinant decorin GAG with CS/DS lyases and LC analysis, several linkage-region hexasaccharide structures have been characterized by Kitagawa et al. [11] (Figure 1). The majority of the linkage-region hexasaccharides had the sequence ΔUA β1-3GalNAc β1-4GlcA β1-3Gal β1-3Gal β1-4Xyl-ol (where ΔUA is 4-deoxy-α-L-threo-hex-4-enopyranosyl uronic acid, GalNAc is 2-deoxy-2-acetamido galactopyranose, Gal is galactopyranose, GlcA is glucuronic acid and Xyl-o1 is xylitol), 12% of which were unsulfated and 60% contained a GalNAc4S residue. A minor component in the mixture (11%) contained an internal IdoA (iduronic acid) residue [11]. LC and MSn (multistage MS) characterization of CS/DS disaccharides and hexasaccharides obtained through enzymatic digestion of the decorin GAG demonstrated that it is composed of unsulfated, monosulfated and disulfated disaccharides, and up to 88% of decorin disaccharides are HexA-GalNAc4S [11–13].
Previously, we reported MM and composition analysis of intact bikunin GAG using a high-resolution MS technique, Fourier-transform ion cyclotron resonance MS [14]. However, this approach has limitations in handling the higher MM and greater structural complexity of the decorin GAG. Thus the MM analysis of the intact decorin GAG and high-MM products of its enzymatic degradation was performed using PAGE. A series of selective enzymatic degradation steps was used for the preparation and characterization of decorin GAG oligosaccharides in a bottom-up type sequencing approach. Information obtained in the process of oligosaccharide mapping was combined with the data obtained from NMR spectroscopy and LC-MS disaccharide compositional analyses and used for reconstructing the decorin GAG sequence motifs.
MATERIALS AND METHODS
Materials
Decorin was generously provided by Life Cell Corporation. Actinase E [EC (Enzyme Commission) 3.4.24.4] was from Kaken Biochemicals. CS-A was purchased from Celsus Laboratories. Endolytic chondroitinase ABC from Proteus vulgaris (EC 4.2.2.4), endolytic chondroitinase ACI from Flavobacterium heparinum (EC 4.2.2.5), exolytic chondroitinase ACII from Arthrobacter aurescens (EC 4.2.2.5), endolytic chondroitinase B from F. heparinum (EC 4.2.2.x), and 4,5-unsaturated CS/DS disaccharide standards [ΔUA-GalNAc (0S, where S is sulfo), ΔUA-GalNAc4S (4S), ΔUA-GalNAc6S (6S), ΔUA2S-GalNAc (2S), ΔUA2S-GalNAc4S (2S4S), ΔUA2S-GalNAc6S (2S6S), ΔUA-GalNAc4S6S (4S6S), ΔUA2SGalNAc4S6S (2S4S6S)] were from Seikagaku (Associates of Cape Cod). CS-C, urea, CHAPS, Alcian Blue and tributylamine were from Sigma. Electrophoresis-grade acrylamide, N,N’ -methylenebis(acrylamide), glycine, TEMED (N,N,N’ ,N’ -tetramethylethylenediamine), APS (ammonium persulfate), Tris and sodium EDTA were from Bio-Rad Laboratories. All solvents were HPLC grade.
Preparation and purification of decorin PG
Recombinant human decorin PG was expressed as a polyhistidine fusion protein in a stably transfected HEK (human embryonic kidney)-293-EBNA cell line using a Celligen Plus bioreactor (New Brunswick Scientific) as previously described [15]. Initial purification of recombinant decorin was performed by passing HEK-293-cell conditioned medium over a nickel-charged HiTrap chelating column (GE Healthcare) followed by elution with a gradient of 0–250 mM imidazole in 20 mM Tris/HCl, pH 8.0, 500 mM NaCl and 0.2% CHAPS. Decorin PG was further purified by applying to a HiTrap Q anion-exchange column (GE Healthcare) and eluting with a gradient of 0–2 M NaCl in 20 mM Tris/HCl, pH 8.0, and 0.2% CHAPS.
Preparation of decorin pG (peptidoglycan)
A 25% (w/v) aqueous solution of actinase E (100 μl) was added to 1 ml of 5 mg/ml decorin PG aqueous solution. The proteolysis reaction was allowed to proceed at 50 °C overnight, after which 800 mg of urea and 36 mg of CHAPS were added to the reaction mixture to give a 1.7 ml solution [2% (w/v) CHAPS and 8 M urea]. The mixture was loaded on to a SAX (strong-anion exchange) spin column (Vivapure Maxi Q; Sartorius Stedim) pre-equilibrated with 3 ml of 8 M urea containing 2% (w/v) CHAPS, and centrifuged at 500 g for 5 min. The bound pG was washed once with 3 ml of 8 M urea containing 2% (w/v) CHAPS and three times with 3 ml of 100 mM NaCl. Decorin pG was released with three 1 ml volumes of 2 M NaCl, desalted using a 10 kDa MWCO (molecular-mass cut-off) centrifugal filter (YM-10; Millipore), lyophilized and stored at − 20 °C.
Preparation of GAG
The GAG component of decorin pG was released by base-catalysed β-elimination under reducing conditions. Decorin pG was dissolved in a 0.5 M NaOH solution containing 0.5 M NaBH4. The reaction was allowed to proceed overnight at 4 °C and neutralized with 1 M HCl. The resulting GAG mixture was purified using a 10 kDa MWCO centrifugal filter (YM-10; Millipore).
PAGE analysis
Products of the decorin GAG enzymatic depolymerization were analysed by native PAGE using 0.75 mm × 6.8 cm × 8.6 cm minigels cast from 15% T resolving gel monomer solution and 5% T stacking gel monomer solution [16]. Heparin oligosaccharide ladder and bikunin GAG CS-A-type chains of known MMs were used as molecular markers [17]. The mini-gels were subjected to electrophoresis at a constant 200 V for 30 min and visualized with 0.5% (w/v) Alcian Blue in 2% (v/v) aqueous acetic acid solution. Intact decorin GAG was analysed using precast 4–15% gradient Tris/HCl mini-gels (Ready-gels; Bio-Rad Laboaratories). The gradient gels were subjected to electrophoresis at 200 V for 20 min. A 25% (v/v) ethanol and 10% (v/v) acetic acid solution was used for washing the gels prior to the Alcian Blue staining for 1 h at room temperature (23 °C) and for destaining. Silverammine staining of the Alcian Blue-stained gels was used to improve visualization [18]. MM analysis was performed with the aid of UNSCANIT software (Silk Scientific) using the logarithmic relationship between the GAG MM and its migration distance.
1H-NMR spectroscopy
Decorin pG was dissolved in 0.4 ml of [2H]H2O (99.96%;Sigma), freeze-dried three times from [2H]H2O, and re-dissolved in 0.4 ml of [2H]H2O for one-dimensional and two-dimensional NMR experiments. One-dimensional 1H-NMR and two-dimensional H-H COSY spectra were recorded on a Bruker Avance II 600 MHz spectrometer equipped with a cryogenically cooled HCN probe with a z-axis gradient. All NMR experiments were recorded at 40 °C (313 K), because at 25 °C the chemical shift of the IdoA H1 overlaps HO2H signal. One-dimensional 1H spectra were recorded with 128 scans with a spectral width of 12 kHz and an acquisition time set to 2.7 s. Two-dimensional H-H COSY spectra were recorded with 16 scans with a spectral width of 7.4 kHz and an acquisition time set to 1.1 s.
Enzymatic depolymerization of decorin GAG for PAGE analysis
Two 10 μg portions of decorin GAG were exhaustively treated with 5 m-units of chondroitin ACI endolyase in 50 mM ammonium bicarbonate buffer, pH 8, at 37 °C overnight to obtain DS-type domains, or 5 m-units of chondroitin B endolyase in 50 mM ammonium bicarbonate buffer, pH 8, at 37 °C overnight to obtain CS-type domains. Products of the depolymerization reactions were analysed by isocratic 15% T PAGE using approx. 5 μg/lane.
Enzymatic depolymerization and analysis of the non-reducing end
A 200 μg portion of decorin GAG was exhaustively digested with 50 milli-units of chondroitin ACII exolyase in 50 mM Tris/HCl, pH 8, and 60 mM sodium acetate buffer. After an overnight incubation, the enzyme was denatured by heating in a boiling water bath for 5 min and precipitated by centrifugation at 12 000 g. The digestion mixture was passed through a 30 kDa MWCO centrifugal filter (YM-30; Millipore) to separate the disaccharides (flow-through) from the high-MM components (retentate) of the digestion mixture. The disaccharide composition of the NRE (non-reducing end) was determined by LC-MS. The retentate containing ACII exolyase-resistant reducing ends of decorin GAG was analysed by isocratic (15% T) and gradient (4–15% T) PAGE.
Decorin GAG domain mapping
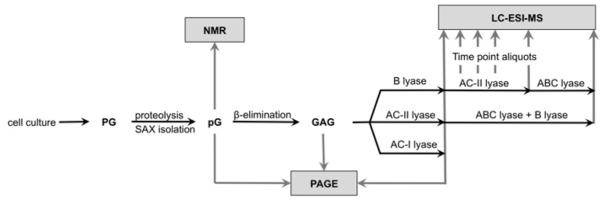
The overall workflow of the mapping experiment consisted of: (i) exhaustive degradation of the decorin DS-type domains with chondroitin B endolyase and the LC-MS analysis of digestion products; (ii) treatment of the resulting continuous CS-type domains with chondroitin ACII exolyase and the LC-MS analysis of digestion products removed at timed intervals; and (iii) exhaustive depolymerization of the remaining mixture with chondroitin ABC lyase and the LC-MS analysis of digestion products (Figure 2). The decorin GAG (1 ml of 0.4 mg/ml solution in deionized water) was treated with 0.1 unit of chondroitin B endolyase at 37 °C overnight. The enzyme was denatured by heating at 100°C and removed by centrifugation at 12 000 g. The supernatant containing CS-type domains was treated with 5 milli-units of chondroitin ACII lyase at 37 °C. During the digestion, 100 μl aliquots were removed at 0, 10, 30, 90 and 120 min, corresponding to 0%, 20%, 40%, 60% and 100 % digestion completion; at each time-point, the enzyme was thermally denatured and removed by centrifugation. The aliquot removed at zero time contained disaccharides obtained by the exhaustive chondroitinase B treatment. The supernatants were lyophilized, reconstituted in water and analysed by LC-MS. After 2 h of chondroitinase ACII digestion, 50 milliunits of chondroitin ABC lyase was added to the remaining 500 μl of the digestion mixture, and the mixture was incubated at 37 °C overnight. The enzyme was thermally inactivated and removed by centrifugation, and the supernatant was lyophilized.
Figure 2.
Overall experimental workflow
Action pattern and activity of the chondroitin ACII exolyase were verified using CS-A and CS-C standard polysaccharides. A 1 ml aliquot of a 10 mg/ml solution of CS-A or CS-C was treated with 0.4 unit of chondroitin ACII lyase at 37 °C, and 100 μl aliquots were removed at 0, 30, 60, 120 and 240 min and after an overnight incubation. Each time, the enzyme was thermally inactivated and removed by centrifugation. The A232 of each supernatant was measured after 100-fold dilution.
LC-MS analysis
The LC-MS disaccharide analysis was performed as described previously [19] on an Agilent 1100 LC-MSD mass spectrometer equipped with an ion-trap mass analyser. An Agilent 1100 LC system was used for delivering a two-step mobile-phase gradient consisting of an isocratic 10 min segment of solution A followed by a linear gradient from 10 to 40 min of 0–50% solution B. Solutions A and B were 0% and 75% acetonitrile respectively, containing 15 mM n-hexylamine as an ion-pairing reagent and 100 mM 1,1,1,3,3,3-hexafluoro-2-propanol as an organic modifier. Disaccharides were separated on an Acquity UPLC BEH C18 column (2.1 mm×150 mm, 1.7 μm; Waters). The column temperature was maintained at 45 °C and the mobile-phase flow rate was 100 μl/min. The disaccharide elution was monitored by the A232 (4,5-unsaturated uronic acid) and by MS. Mass spectra were acquired in the positive ionization mode over a 350–2000 m/z range at a rate of 10 scans/s. Nitrogen was used as the drying and nebulizing gas.
RESULTS AND DISCUSSION
The recombinant decorin used in the present study was produced by culturing stably transfected, transformed, HEK cells (HEK-293-EBNA) and represents a single lot of PG sample. The N-terminal sequence of the recombinant decorin core protein contained six histidine residues (His6-tag), facilitating its purification. The decorin pG, a decorin core protein peptide with a GAG chain attached to Ser21, was prepared by treating the PG with a non-specific protease, actinase E. The GAG chain was then released from the purified pG by reductive β-elimination. The MM and dispersivity of the pG and GAG, examined using PAGE, were very similar, indicating that peptide represents a small percentage of the pG MM, which is consistent with the actinase E action pattern [20–22].
Intact decorin GAG
The MM of decorin GAG was analysed by PAGE (Figure 3) using a ladder of heparin oligosaccharides and bikunin GAG chains with known MMs as standards [16,17,23]. It should be noted that neither the heparin oligosaccharide ladder nor bikunin GAG polysaccharides used in the present study as the standards cover the entire gel range. MM values of decorin GAG and its degradation products with MM > 9 kDa were derived by extrapolating a linear function of log(MM) against migration distance plotted using available standards, which entails a fairly wide degree of uncertainty in the extrapolated MM distributions. The MW (weight-average MM) of the decorin GAG chain was determined to be 30 kDa and the MN (number-average MM) was 22 kDa, corresponding to the chain length of ~50 disaccharides based on the monosulfated disaccharide residue mass (459 Da).
Figure 3. PAGE analysis of decorin pG and enzyme-treated decorin GAG and pG.
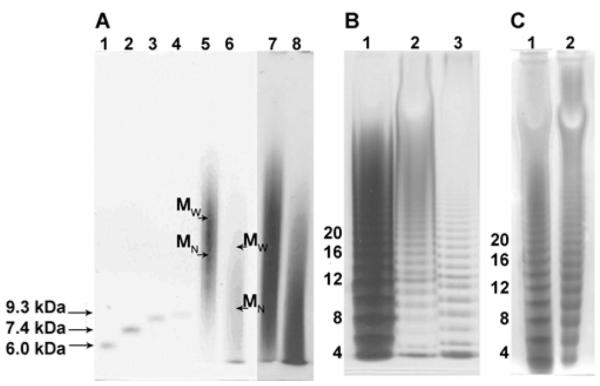
(A) PAGE using a 4–15% gradient gel: lanes 1–4, bikunin GAG MM markers 6 kDa–9.3 kDa; lanes 5 (Alcian Blue-stained) and 7 (silver-stained), intact decorin pG; lanes 6 (Alcian Blue-stained) and 8 (silver-stained), chondroitinase ACII-treated decorin pG. (B) PAGE using a 15% isocratic gel: lane 1, heparin oligosaccharide standard ladder (MM in kDa); lane 2, chondroitinase B-treated decorin pG; lane 3, chondrotinase ACI-treated decorin GAG. (C)PAGE using a 15% isocratic gel: lane 1, heparin oligosaccharide standard ladder (MM in kDa); lane 2, chondroitinase ACII-treated decorin GAG oligosaccharides. MM of intact decorin and high-MM products of its digestion with chondroitinase ACII were calculated using the gradient gel in (C).
The uronic acid epimers D-GlcA and L-IdoA are distinguishable by 1H-NMR. One-dimensional 1H-NMR and two-dimensional COSY spectra of the decorin pG were used to determine the ratio of GlcA to IdoA (Supplementary Figure S1 at http://www.BiochemJ.org/bj/431/bj4310199add.htm). In the one-dimensional 1H experiments, the H1 signals of GlcA and IdoA were observed at 4.55 and 4.95 p.p.m. respectively. Because of the partial overlap of these signals with the HO2H peak (4.74 p.p.m.) in one-dimensional experiments, two-dimensional COSY was used to estimate their intensity [24,25,26]. Based on the integration of the GlcA and IdoA H1 signals in the two-dimensional COSY NMR spectra, the ratio of GlcA to IdoA was determined to be 5:1. Because this ratio was obtained for a collection of GAG chains as opposed to a single GAG chain, it represents a statistical approximation rather than an exact number. Additional uncertainties in the NMR-based relative quantification experiments inevitably arise from varying the instrumental conditions and integration parameters. These deviations fall within ±7% of the mean value (results not shown).
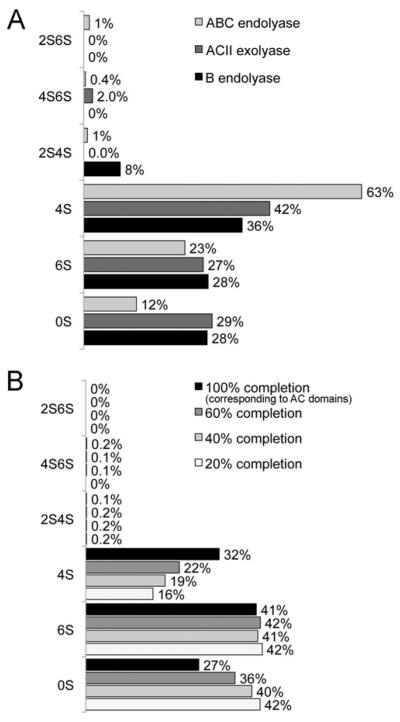
LC-MS analysis of products of the exhaustive depolymerization with chondroitinase ABC afforded overall disaccharide composition of decorin GAG: 12% 0S, 23% 6S, 63% 4S, 1% 2S6S, 1% 2S4S and 0.4% 4S6S (Figure 4A).
Figure 4. Disaccharide composition of the decorin GAG and GAG domains determined by LC-MS.
(A) Disaccharide composition of continuous DS-type domains (black), CS-type domains resistant to chondroitinase B (dark grey) and overall disaccharide composition (light grey). (B) Digestion of the chondroitinase B-resistant domains with exolytic chondroitinase ACII; the disaccharide compositions at 20%, 40%, 60% and 100% reaction completion are shown.
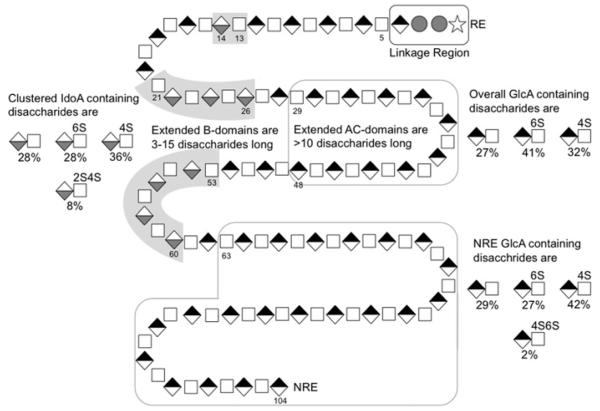
Based on the results of PAGE, NMR and LC-MS disaccharide-composition analyses, an average hypothetical decorin GAG chain consists of 50 disaccharides, 42 of which contain GlcA and eight contain IdoA, six disaccharides are unsulfated, 43 disaccharides are monosulfated and one disaccharide is disulfated (Figure 5).
Figure 5. A composite average GAG chain of decorin.
Symbols correspond to xylose (stars), galactose (grey circles), N-acetylgalactosamine (white squares), GlcA (black-and-white diamonds) and iduronic acid (white-and-grey diamonds).
DS-type domains
Exhaustive treatment of the decorin GAG mixture with endolytic chondroitinase ACI afforded IdoA-rich DS-type domains, resistant to depolymerization by this enzyme. The products of the depolymerization reaction were analysed by PAGE (Figure 3B, lane 3) using heparin oligosaccharides of known MM as standards. The results of PAGE analysis indicate that continuous DS-type domains in the decorin GAG chain contain 3–15 disaccharides. Based on the proportion of DS-type disaccharides determined by NMR, the majority of IdoA-containing disaccharides appear to be present in the continuous DS-type domains.
Exhaustive treatment of decorin GAG with endolytic chondroitinase B affords disaccharides originating from continuous DS-type domains. The disaccharide composition of these DS-type domains was determined using LC-MS (Figure 4A). Continuous DS-type domains of decorin GAG contained 28% 0S, 28% 6S, 36% 4S and 8% 2S4S disaccharides. Neither 2S6S nor 4S6S disaccharides were detected in DS-type domains. Since exhaustive treatment of decorin GAG with chondroitinase ABC shows that 2S4S disaccharide constitutes 1% of the overall decorin GAG disaccharide composition, its presence in the continuous DS-type domains at a relatively high proportion of 8% suggests that essentially all 2S4S disaccharides originate from continuous DS-type domains.
CS-type domains
GlcA-containing disaccharides constitute 83% of decorin GAG, according to the NMR analysis, suggesting that there is a high probability of finding continuous CS-type domains in a decorin GAG chain. Exolytic chondroitinase ACII acts on CS by removing one GlcA-containing disaccharide at a time from the NRE of GAG, and does not act on IdoA-containing disaccharides, i.e. DS-type domains [27,28]. Exhaustive treatment of decorin GAG with exolytic chondroitinase ACII followed by the analysis of products demonstrated that most decorin GAG chains are terminated with a CS-type NRE and allowed us to estimate the average length of the CS-type domain at the NRE and to determine its disaccharide composition.
The low-MM products of chondroitinase ACII digestion were resolved using isocratic 15% PAGE, whereas 4–15% gradient PAGE was used for determining the size of the high-MM products (Figures 3C, lane 2, and 3A, lane 6). The downward shift in chain size from MN 22 kDa to MN 12 kDa (MW 28 kDa) after the exolyase treatment suggests that the NRE consists of up to 20 CS-type disaccharides. The low-MM products of the ACII exolyase treatment contain oligosaccharides with dp6 (degree of polymerization 6, i.e. hexasaccharide) and higher, as evident from the isocratic PAGE analysis (Figure 3C, lane 2). These oligosaccharides could originate from the reducing end and either contain a penultimate DS-type disaccharide or, in case of dp6, represent the LR (linkage-region) hexasaccharide which is usually obtained by exhaustive enzymatic depolymerization of a GAG [11]. This suggests that some decorin GAG chains may be entirely composed of CS-type building blocks.
The disaccharide compositional profile of the NRE, as determined by LC-MS analysis of products obtained on chondroitinase ACII treatment, differs from the inner-chain CS-type disaccharide compositional profile in that the GlcA2S-containing disaccharides were not detected in the decorin GAG NRE domain, whereas 4S6S disaccharides were present in the highest proportion, 2% (Figure 4A) compared with 0.2% in the overall disaccharide profile (Figure 4B). These results suggest that virtually all 4S6S disaccharides originate from the NRE CS-type domain of decorin GAG. Other disaccharides present in the NRE domain included 42% 4S, 27% 6S and 29% 0S (Figure 4A).
Exhaustive depolymerization of the DS-type domains of decorin GAG with endolytic chondroitinase B generated DS-type disaccharides as described above, as well as CS-type domains, resistant to the degradation by chondroitinase B. These CS-type domains were then treated with exolytic chondroitinase ACII, and during the first 2 h of the digestion, aliquots were removed at timed intervals for LC-MS disaccharide composition analysis. LC-MS disaccharide profiles corresponding to various time-points throughout the digestion provided information about changes in disaccharide composition in going from the NRE towards the RE (reducing end) in these CS-type domains. The results obtained during this experiment are summarized in Figure 4(B), which shows, for example, that whereas the proportion of 6S disaccharide remain essentially unchanged, the proportion of 4S disaccharide doubles. Thus a more highly sulfated portion of the CS-type domain lies toward its RE.
Domain map of a decorin GAG chain
Based on the information gathered through sequential enzymatic treatment of decorin GAG with specific CS lyases and the analysis of the digestion products, a feature map of an ‘average’ decorin GAG chain was constructed (Figure 5). This chain is dp104 with the first four saccharide residues at the RE corresponding to the linkage region that attaches to Ser34 (Ser21 in the recombinant decorin) of the PG core protein. The dp100 chain that follows (residues 5–104) consists of three types of domain: isolated DS-type domains (residues 13–14), clustered DS-type domains (residues 21–26 and 53–60), and extended CS-type domains (residues 29–48 and 63–104). The clustered DS-type domains contain essentially all of the 2S4S disaccharides. The extended internal CS-type domain (residues 29–48) is enriched in 6S disaccharides, whereas the extended CS-type domain at the NRE (residues 63–104) is enriched in 4S and contains most of the 4S6S disaccharides. It should be noted that the chain drawn in Figure 5 is a composite average and may not correspond to any chain found in the decorin PG. Moreover, we see evidence of chains that are entirely CS in composition, containing no IdoA residues and devoid of a DS domain.
In conclusion, the present study provides an initial rough map of the GAG chain from decorin PG isolated from a single lot of cultured HEK cells. Biosynthesis of GAGs is a non-template process, and the structural features of CS/DS GAGs vary between different species: across a population of the same species, depending on age, environmental stimuli, physiological or pathophysiological state; within an organism, depending on tissue type; and, at a molecular level, depending on the type of PG. Thus the composite average chain of decorin GAG will vary depending on the source. The strategy described in the present work can be applied to constructing and comparing the domain maps of CS/DS chains isolated from different sources as well as to examining CS/DS components of other PGs. Future studies are underway to isolate individual GAG chains from decorin PG for structure and sequence determination.
Supplementary Material
Figure S1 NMR analysis of decorin peptidoglycan (one-dimensional 1H-NMR and two-dimensional H-H COSY)
Acknowledgments
FUNDING This work was supported by the National Institutes of Health [grant number GM38060].
Abbreviations used
- CS
chondroitin sulfate
- dp
degree of polymerization
- DS
dermatan sulfate
- EC
Enzyme Commission
- EGF
epidermal grouth factor
- GAG
glycosaminoglycan
- GalNAc
2-deoxy-2-acetamido galactopyranose
- GlcA
glucuronic acid
- HEK
human embryonic kidney
- IdoA
iduronic acid
- MM
molecular mass
- MN
number-average MM
- MW
weight-average MM
- MWCO
molecular-mass cut-off
- NRE
non-reducing end
- pG
peptidoglycan
- PG
proteoglycan
- SAX
strong-anion exchange
- RE
reducing end
- ΔUA
4-deoxy-α-L-threo-hex-4-enopyranosyl uronic acid
Footnotes
AUTHOR CONTRIBUTION Tatiana Laremore performed isocratic PAGE analysis of the chondroitinase ACI and chondroitinase B digestions of decorin GAG and interpreted and organized LC-MS and PAGE data. Mellisa Ly determined the decorin MM and polydispersity by PAGE, analysed products of decorin GAG enzymatic digestions by isocratic and gradient PAGE, and interpreted and organized PAGE results. Zhenqing Zhang performed enzymology studies and disaccharide analysis of the decorin GAG by LC-MS and analysed decorin GAG by one-dimensional and two-dimensional NMR to determine GlcA/IdoA ratios. Kemal Solakyildirim performed one-dimensional and two-dimensional NMR experiments on decorin peptidoglycan under optimized conditions to determine GlcA/IdoA ratios. Scott McCallum shared his expertise in the area of one-dimensional and two-dimensional NMR. Richard Owens expressed and purified the human recombinant decorin proteoglycan used in the present study. Robert Linhardt, the principal investigator with expertise in glycosaminoglycan characterization and structure–function relationships, designed and led the study.
REFERENCES
- 1.Heinegard D. Proteoglycans and more – from molecules to biology. Int. J. Exp. Pathol. 2009;90:575–586. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malavaki C, Mizumoto S, Karamanos N, Sugahara K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect. Tissue Res. 2008;49:133–139. doi: 10.1080/03008200802148546. [DOI] [PubMed] [Google Scholar]
- 3.Iozzo RV, Zoeller JJ, Nystrom A. Basement membrane proteoglycans: modulators par excellence of cancer growth and angiogenesis. Mol. Cells. 2009;27:503–513. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hocking AM, Shinomura T, Mcquillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 5.Fransson LA, Belting M, Jonsson M, Mani K, Moses J, Oldberg A. Biosynthesis of decorin and glypican. Matrix Biol. 2000;19:367–376. doi: 10.1016/s0945-053x(00)00083-4. [DOI] [PubMed] [Google Scholar]
- 6.Seo NS, Hocking AM, Hook M, Mcquillan DJ. Decorin core protein secretion is regulated by N-linked oligosaccharide and glycosaminoglycan additions. J. Biol. Chem. 2005;280:42774–42784. doi: 10.1074/jbc.M511531200. [DOI] [PubMed] [Google Scholar]
- 7.Ruoslahti E. Structure and biology of proteoglycans. Annu. Rev. Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- 8.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 9.Seidler DG, Dreier R. Decorin and its galactosaminoglycan chain: extracellular regulator of cellular function? IUBMB Life. 2008;60:729–733. doi: 10.1002/iub.115. [DOI] [PubMed] [Google Scholar]
- 10.Mcewan PA, Scott PG, Bishop PN, Bella J. Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J. Struct. Biol. 2006;155:294–305. doi: 10.1016/j.jsb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa H, Oyama M, Masayama K, Yamaguchi Y, Sugahara K. Structural variations in the glycosaminoglycan-protein linkage region of recombinant decorin expressed in Chinese hamster ovary cells. Glycobiology. 1997;7:1175–1180. doi: 10.1093/glycob/7.8.1175. [DOI] [PubMed] [Google Scholar]
- 12.Seidler DG, Peter-Katalinic J, Zamfir AD. Galactosaminoglycan function and oligosaccharide structure determination. ScientificWorldJournal. 2007;7:233–241. doi: 10.1100/tsw.2007.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamfir AD, Flangea C, Sisu E, Serb AF, Sinca N, Bruckner P, Seidler DG. Analysis of novel over- and under-sulfated glycosaminoglycan sequences by enzyme cleavage and multiple stage MS. Proteomics. 2009;9:3435–3444. doi: 10.1002/pmic.200800440. [DOI] [PubMed] [Google Scholar]
- 14.Chi L, Wolff JJ, Laremore TN, Restaino OF, Xie J, Schiraldi C, Toida T, Amster IJ, Linhardt RJ. Structural analysis of bikunin glycosaminoglycan. J. Am. Chem. Soc. 2008;130:2617–2625. doi: 10.1021/ja0778500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, Campbell S, Iozzo RV. Biologically active decorin is a monomer in solution. J. Biol. Chem. 2004;279:6606–6612. doi: 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- 16.Laremore TN, Ly M, Solakyildirim K, Zagorevski DV, Linhardt RJ. High-resolution preparative separation of glycosaminoglycan oligosaccharides by polyacrylamide gel electrophoresis. Anal. Biochem. 2010;401:236–241. doi: 10.1016/j.ab.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laremore TN, Leach FE, Solakyildirim K, Amster IJ, Linhardt RJ. Glycosaminoglycan characterization by electrospray ionization mass spectrometry including Fourier transform mass spectrometry. Methods Enzymol. 2010;478:79–108. doi: 10.1016/S0076-6879(10)78003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ly M, Wang Z, Laremore TN, Zhang F, Zhong W, Pu D, Zagorevski DV, Dordick JS, Linhardt RJ. Analysis of E. coli K5 capsular polysaccharide heparosan. Anal. Bioanal. Chem. 2010 doi: 10.1007/s00216-010-3679-7. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solakyildirim K, Zhang Z, Linhardt RJ. Ultraperformance liquid chromatography with electrospray ionization ion trap mass spectrometry for chondroitin disaccharide analysis. Anal. Biochem. 2010;397:24–28. doi: 10.1016/j.ab.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yum DY, Chung HC, Bai DH, Oh DH, Yu JH. Purification and characterization of alkaline serine protease from an alkalophlic Streptomyces sp. Biosci. Biotechnol. Biochem. 1994;58:470–474. doi: 10.1271/bbb.58.470. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida N, Tsuruyama S, Nagata K, Noda K, Makisumi S. Purification and characterization of an acidic amino acid specific endopeptidase of Streptomyces griseus obtained from a commercial preparation (Pronase) J. Biochem. 1988;104:451–456. doi: 10.1093/oxfordjournals.jbchem.a122488. [DOI] [PubMed] [Google Scholar]
- 22.Edens RE, Al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ. Gradient polyacrylamide gel electrophoresis for determination of molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J. Pharm. Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 23.Jandik KA, Gu K, Linhardt RJ. Action pattern of polysaccharide lyases on glycosaminoglycans. Glycobiology. 1994;4:289–296. doi: 10.1093/glycob/4.3.289. [DOI] [PubMed] [Google Scholar]
- 24.Guerrini M, Naggi A, Guglieri S, Santarsiero R, Torri G. Complex glycosaminoglycans: profiling substitution patterns by two-dimensional nuclear magnetic resonance spectroscopy. Anal. Biochem. 2005;337:35–47. doi: 10.1016/j.ab.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Giraudeau P, Guignard N, Hillion E, Baguet E, Akoka S. Optimization of homonuclear 2D NMR for fast quantitative analysis: application to tropine-nortropine mixtures. J. Pharm. Biomed. Anal. 2007;43:1243–1248. doi: 10.1016/j.jpba.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Sudo M, Sato K, Chaidedgumjorn A, Toyoda H, Toida T, Imanari T. 1H Nuclear magnetic resonance spectroscopic analysis for determination of glucuronic and iduronic acids in dermatan sulfate, heparin, and heparan sulfate. Anal. Biochem. 2001;297:42–51. doi: 10.1006/abio.2001.5296. [DOI] [PubMed] [Google Scholar]
- 27.Gu K, Liu J, Pervin A, Linhardt RJ. Comparison of the activity of two chondroitin AC lyases on dermatan sulfate. Carbohydr. Res. 1993;244:369–77. doi: 10.1016/0008-6215(83)85014-9. [DOI] [PubMed] [Google Scholar]
- 28.Gu K, Linhardt RJ, Laliberte M, Zimmermann J. Purification, characterization and specificity of chondroitin lyases and glycuronidase from Flavobacterium heparinum. Biochem. J. 1995;312:569–577. doi: 10.1042/bj3120569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 NMR analysis of decorin peptidoglycan (one-dimensional 1H-NMR and two-dimensional H-H COSY)