Abstract
The endoplasmic reticulum (ER) plays an essential role in the production of lipids and secretory proteins. Because the ER cannot be generated de novo, it must be faithfully transmitted or divided at each cell division. Little is known of how cells monitor the functionality of the ER during the cell cycle or how this regulates inheritance. We report here that ER stress in S. cerevisiae activates the MAP kinase Slt2 in a new ER Stress Surveillance (ERSU) pathway, independent of the Unfolded Protein Response. Upon ER stress, ERSU alters the septin complex to delay ER inheritance and cytokinesis. In the absence of Slt2 kinase, the stressed ER is transmitted to the daughter cell, causing the death of both mother and daughter cells. Furthermore, Slt2 is activated via the cell surface receptor Wsc1 by a previously undescribed mechanism. We conclude that the novel ERSU pathway ensures inheritance of a functional ER.
Keywords: ER stress, Cytokinesis, ER inheritance, Septin, MAP kinase, Slt2
INTRODUCTION
The ER functions as a gateway for newly synthesized secretory or membrane proteins. Upon synthesis, polypeptides coding for such proteins are targeted and translocated into the lumen of the ER as linear unmodified polypeptides. In the ER, the polypeptides undergo folding and modification processes to become their native functional structures. Only properly folded proteins can exit from the ER to their target sites (Bicknell and Niwa, 2009; Mori, 2000; Ron and Walter, 2007; Rutkowski and Kaufman, 2004). Misfolded proteins are toxic to the cell, and become marked within the ER and targeted for degradation by a process termed ERAD (ER-associated protein degradation) (Hampton, 2002; Bukau et al., 2006; Vembar and Brodsky, 2008). Additionally, the ER is the primary site of lipid biosynthesis, and thus influences the relative composition and overall abundance of lipids throughout the cell (McMaster, 2001). Essentially, the ER serves as a master regulator for the complex and error-prone process of protein maturation, quality control, and trafficking.
Morphologically, the ER is a continuous tubular-reticular network that is contiguous with the outer membrane of the nuclear envelope (Koning et al., 2002; Preuss et al., 1991). In yeast, the ER is comprised of two subdomains: the perinuclear ER/nuclear envelope and the reticulum of cortical ER tubules (cER), which is found near the plasma membrane or the cortex of the cell. ER tubules, approximately 50–100 nm in diameter, connect the cER to the perinuclear ER (Voeltz et al., 2002). Most ER proteins analyzed to date can migrate freely between perinuclear ER and cER. The functional implication for the distinction between these ER domains is unclear.
Paradoxically, the mechanism of inheritance of perinuclear ER and cER appears to be different. The perinuclear ER remains closely associated with the nucleus and becomes segregated and partitioned between the two cells in a microtuble-dependent manner. In contrast, the inheritance of cER is actin-based and powered by a myosin motor (Du et al., 2001; Estrada et al., 2003; Prinz et al., 2000). Recent genetic studies have begun to elucidate the molecular events of cER inheritance. Very early in the cell cycle, ER cytoplasmic tubules align along the mother-bud axis and extend to the newly developed bud. These tubules become anchored at the bud tip (Du et al., 2001; Huffaker et al., 1988; Jacobs et al., 1988), followed by the tubules spreading along the periphery of the bud to form the cER of the daughter cell (Estrada et al., 2003 & 2005; Prinz et al., 2000, Wiederkehr et al., 2003). While interesting molecular components and mechanisms involved in ER transmission have been identified, the extent of regulation imposed on this process remains largely unexplored.
Proper segregation of cellular components is the essence of cell division and is critical to sustain life. In addition to genomic materials in the nucleus, cytoplasmic components must also be separated properly so that the newly generated daughter cells can autonomously carry out cellular events immediately after cell division (Peng and Weisman, 2008). Given the critical nature of ER function in the cell and as ER is not synthesized de novo but arises only from existing ER, we reasoned that a surveillance mechanism may exist to ensure that a minimal threshold of ER functional capacity is inherited by each daughter cell during the cell cycle.
In S. cerevisiae, we have previously shown that ER stress causes cell cycle delay with high DNA content, large buds and divided nuclei. Further analyses have revealed that ER stress does not alter mitotic events including a major cell cycle regulator Clb2 production/degradation, mitotic phosphatase Cdc14 release into the cytoplasm, mitotic spindle formation/depolymerization. ER stressed cells are unable to divide even after lyticase treatment, revealing that ER stress causes a cytokinesis defect rather than a problem in cell separation (Bicknell et al., 2007).
Here, we set out to investigate molecular events leading to the ER stress induced cytokinesis delay. We find that the cytokinesis delay is part of a multi-faceted cell cycle response to ER stress including septin alteration and ER inheritance delay. Furthermore, this ER surveillance response ensuring cER inheritance is independent of the canonical Unfolded Protein Response pathway, but rather coordinated by MAP kinase Slt2.
RESULTS
ER stress alters the localization and morphology of the septin ring
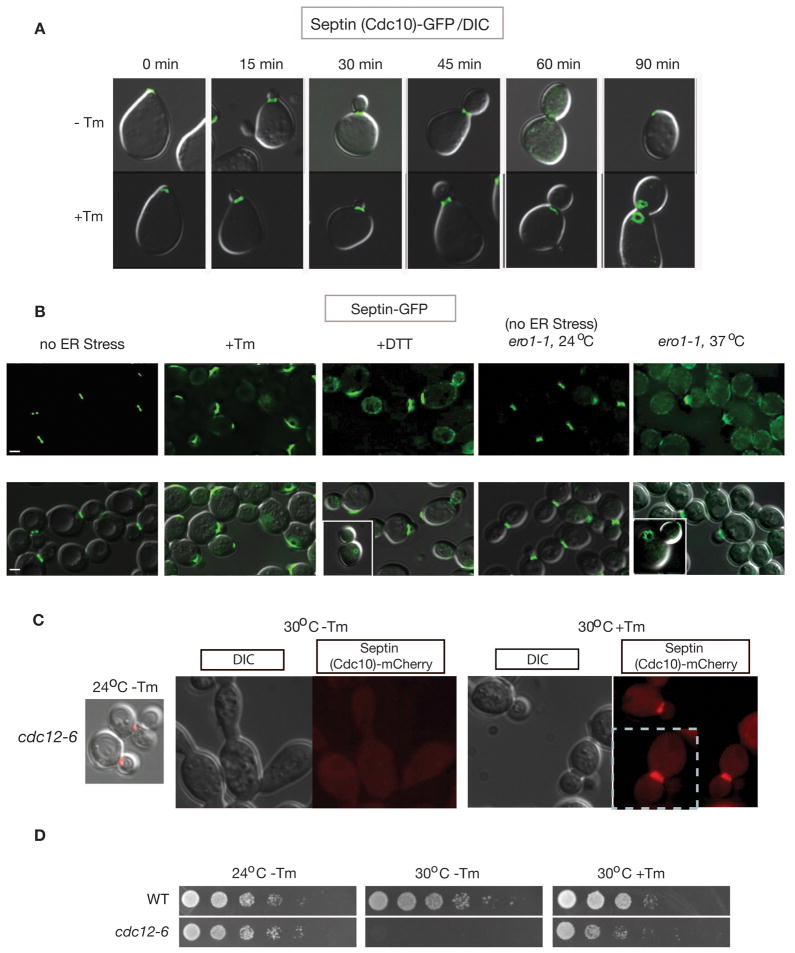
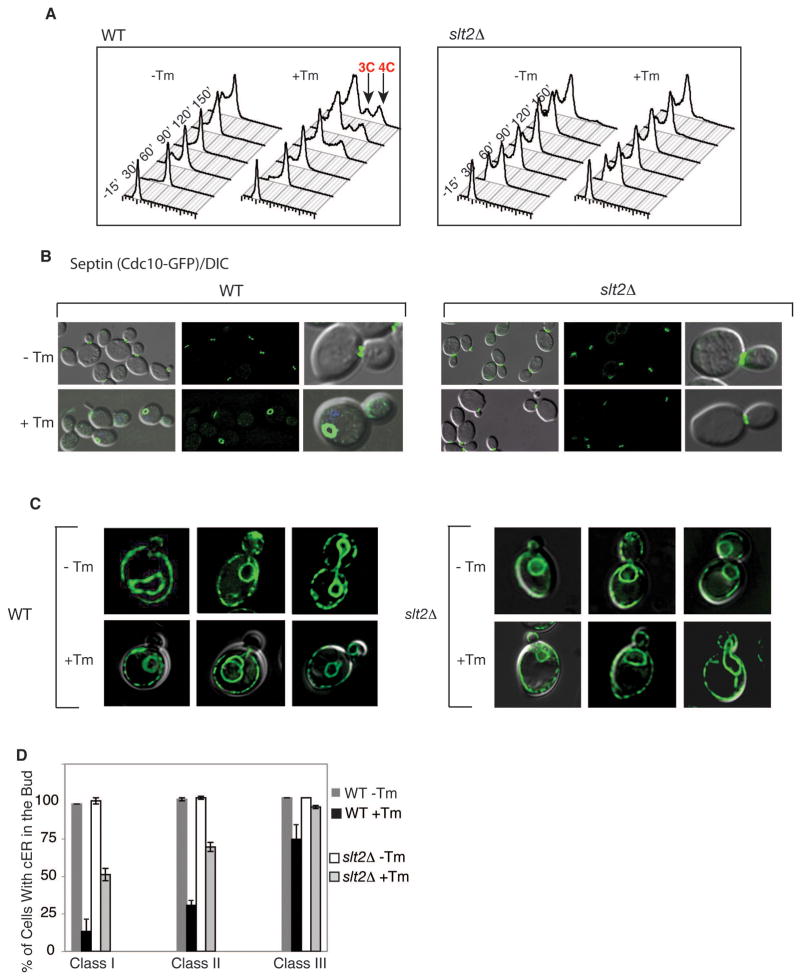
Previously we showed that cytokinesis block in cells experiencing ER stress is not due to delayed or altered actin patch distribution. To probe the molecular basis of blocked cytokinesis in ER-stressed cells, we examined the localization dynamics of the septin complex during the cell cycle after treating cells with tunicamycin (Tm). Tm is a well-characterized inducer of ER stress that inhibits N–linked glycosylation, leading to an accumulation of unfolded protein in the ER. As currently understood, assembly of the five septin complex subunits Cdc3, Cdc10, Cdc11, Cdc12, and Shs1 at the bud neck establishes a septation site for cytokinesis and is thought to act at one of the most upstream levels of the yeast cytokinesis pathway (Bertin et al., 2008; Gasper et al., 2009; Gladfelter et al., 2001; Iwase et al., 2006; Kim et al., 1991; McMurray and Thorner, 2009). We monitored the localization of the septin subunit Cdc10-GFP (Cid et al., 1998) in synchronized cells following treatment with tunicamycin (Tm). In unstressed cells, we observed septin ring formation at the bud neck of each mother cell during bud formation with conversion to hourglass structures over time. (Caviston et al., 2003; Dobbelaere et al., 2003; McMurray and Thorner, 2009) (Figure 1A). ER stress did not affect septin subunit targeting or ring formation, which were similar in normal and stressed cells. In unstressed cells, the septin ring went on to disperse toward the end of the cell cycle as cytokinesis progressed (Figure 1A; 60 min after release from mating pheromone). In contrast, in stressed cells, the septin ring did not disperse and cytokinesis was not observed even after 90 minutes (Figure 1A; compare −Tm and +Tm at 60 & 90 min). Ultimately, the septin fluorescence was observed distal from the bud neck in stressed cells, a localization that was never seen in unstressed cells. The aberrant behavior of the septin complex in Tm-treated cells was a general consequence of ER stress. This behavior was also observed when ER stress was induced by two other well-characterized means: DTT treatment, which disrupts disulfide bonds, or inactivation of the Ero1 protein (endoplasmic reticulum oxidoreductin I) through expression of the ero1-1 temperature sensitive allele by shifting from permissive temperature (at 24 °C) to non-permissive temperature (at 37 °C) (Frand and Kaiser, 1998; Pollard et al., 1998) (Figure 1B). This effect of ER stress that we observed on septin was not specific to the Cdc10-GFP reporter, as we observed similar changes in strains expressing Cdc11-GFP and Shs1-GFP fusion proteins (described in Figure 6D for Shs1-GFP).
Figure 1. ER stress alters the localization and morphology of the septin ring.
(A) Septin ring morphology during normal growth (−Tm) and under ER stress (+Tm). Upon release, α-factor synchronized cells expressing genomically GFP-tagged CDC10 (Jimenez et al., 1998)) were treated with or without tunicamycin (Tm) for the indicated amount of time (Bicknell et al., 2007).
(B) Genomic loci encoding septin subunits, either SHS1 or CDC11, were C-terminally tagged with GFP. ER stress was induced in asynchronous populations of WT cells with 1 μg/ml Tm (3 hrs) or 2 mM DTT (3 hrs), or in ero1-1 cells by shifting from the permissive temperature (24°C) to the non-permissive temperature (37°C) for 3 hrs. Note that similar conditions were used for ER stress induction throughout this study unless otherwise stated. GFP alone (upper panels) and GFP merged with a DIC image (lower panel) are shown. Bars, 2 μm.
(C) (D) Septin morphology in cdc12-6 cells carrying CDC10-mCherry in YPD or YPD+Tm at the indicated temperature. Upon shift to the non-permissive temperature without addition of Tm in the growth media (30°C, 3 hrs, −Tm), cells showed loss of normal septin structure (dispersed) and abnormal elongated morphology. However, addition of Tm (1 μg/ml, 3 hrs at 30°C) caused recovery of the septin ring and normal cell morphology to majorities of cdc12-6 cells, consistent with the partial recovery of cell growth with Tm at 30°C as shown in (D). cdc12-6 cells were not able to sustain their growth at 30°C without Tm. Cells were spotted on YPD or YPD+Tm (0.2 μg/ml) plates after five-fold serial dilutions and incubated at the indicated temperature for 3 days. See also Figure S1.
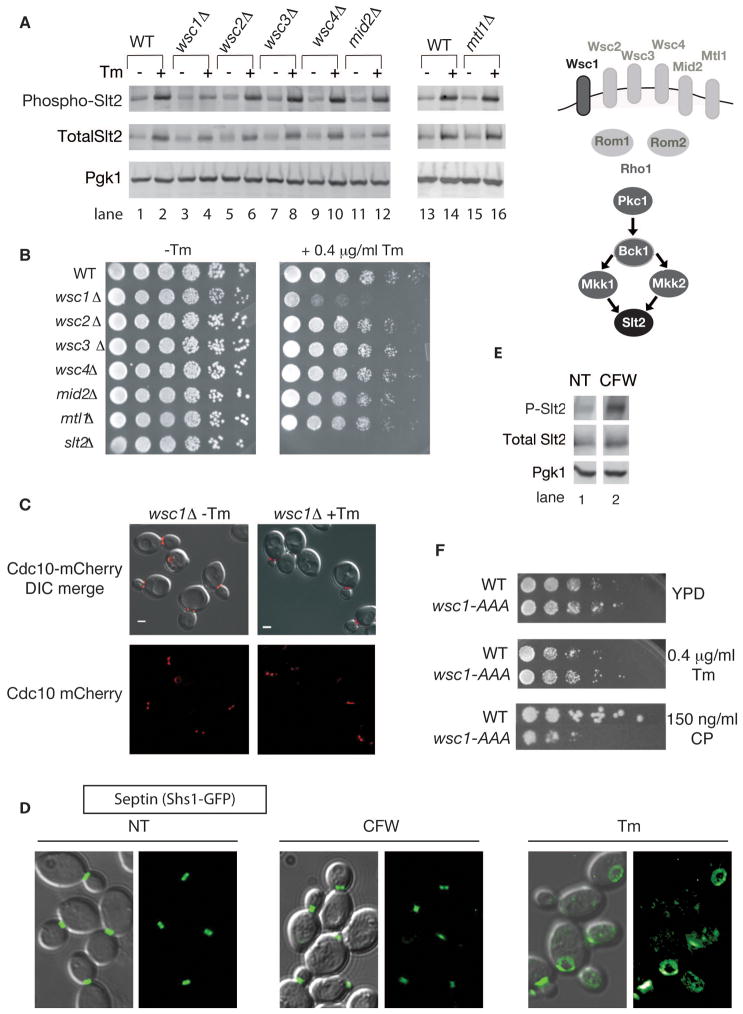
Figure 6. The ERSU pathway is activated by Wsc1 and is independent of the cell wall integrity and arrest of secretion response pathways.
(A) WSC1 is required for phosphorylation of Slt2 upon ER stress. Tm treated (+Tm) or untreated (−Tm) cells of indicated genotypes were collected and analyzed by western blot for Slt2 phosphorylation, total Slt2, and Pgk1.
(B) wsc1Δ cells did not support growth on a YPD plate containing 0.4 μg/ml Tm. Fivefold serial dilutions of the indicated mutant cells were grown on medium with and without Tm.
(C) In the absence of WSC1, septin rings (Cdc10-mCherry reporter) did not show altered morphology upon ER stress induced by Tm (1 μg/ml for 3 hrs), but were only observed at the bud neck (a similar septin phenotype as what was seen in slt2Δ cells).
(D) Not all stresses that induce Slt2 activation affect septin ring dynamics. Wild-type cells expressing Shs1-GFP were visualized after 2 hours of treatment with 10μg/ml calcofluor white (CFW) or 1 μg/ml Tm for 3 hrs. NT, no treatment. GFP alone, and GFP merged with a DIC image are shown.
(E) Samples from the experiment described in (D) were collected and analyzed by western blot for Slt2 phosphorylation.
(F) Endocytosis of Wsc1 does not play a role in the Wsc1 function in ERSU signaling, although it is known to be involved in the cell wall integrity (CWI) pathway. Five-fold serial dilutions of WT cells, and cells with the genomic copy of WSC1 replaced by the endocytosis mutant wsc1-AAA (Piao et al., 2007) were spotted onto plates with and without Tm (0.4 μg/ml) and the CWI pathway activator, caspofungin (CP) (150 ng/ml). Growth was monitored after 2 days. See also Figure S5.
The morphology of and choreographed changes in the septin ring that are observed in normal cells as the cell cycle progresses are known to be regulated by post-translational modifications that affect the stability of interactions between septin subunits (Dobbelaere et al., 2003). To test the possibility that ER stress stabilizes the septin complex, giving rise to the persistent septin ring appearance observed (Figure 1A), cells bearing the cdc12-6 mutation were examined. This temperature-sensitive mutation of the septin subunit CDC12 is known to cause septin ring disassembly at the restrictive temperature (at 30°C), presumably by destabilizing interactions between septin subunits (Dobbelaere et al., 2003). Thus, we reasoned that ER stress might stabilize the septin ring sufficiently to rescue cdc12-6 cell growth at the restrictive temperature. Growth of the cdc12-6 mutant at the restrictive temperature is known to result in the formation of elongated cells that fail to undergo cytokinesis (Figure 1C, 30°C −Tm) (Dobbelaere et al., 2003; Kim et al., 1991). Remarkably, addition of the ER stress inducer Tm to cdc12-6 cells at 30°C resulted in cells with a normal septin ring morphology and, ultimately, normal cell shape and cytokinesis (Figure 1C, 30°C +Tm), resulting in the rescue of overall cell growth (Figure 1D, 30°C +Tm).
Thus, ER stress suppressed the cytokinesis defect due to the cdc12-6 mutation. Similarly, we found that Tm treatment also rescued aberrant septin localization & morphology, and elongated shape and growth of cells deleted for SHS1 (Figures S1A & S1B), which encodes a subunit of the septin ring. These observations suggest that ER stress stabilizes the abnormal septin rings of cdc12-6 and shs1Δ cells sufficiently to allow normal septin behavior and cytokinesis to occur. Furthermore, this observation suggests that in WT cells ER stress delays cytokinesis by stabilizing the septin ring.
ER stress induces an inhibition of cortical ER (cER) inheritance
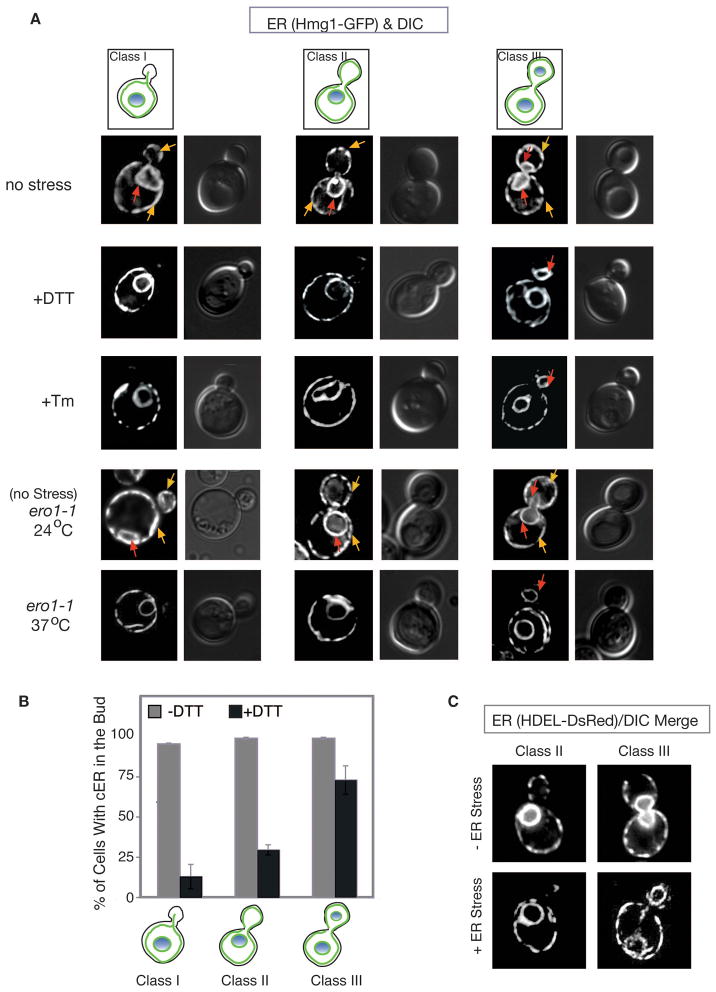
Since ER stress delays cell cycle progression, we asked whether ER stress also affects ER inheritance. Using the ER marker GFP-HMG CoA reductase (Hmg1-GFP) (Du et al., 2001; Hampton et al., 1996), we examined the distribution of both cortical and perinuclear ER in mother and daughter cells in the presence and absence of ER stress. In the absence of stress, cortical ER (cER) was delivered to the daughter cell very early in the cell cycle, consistent with previous reports (Estrada de Martin et al., 2005). As soon as a bud was visible, 96% of buds contained some cER (Figure 2A, yellow arrows: no stress or ero1-1 at 24 °C, Class I). As the bud grew, the cER began to spread along the cortex of the bud (Figure 2A, no stress or ero1-1 at 24 °C, class II&III). Perinuclear ER was inherited later in the cell cycle, during mitosis, along with the nucleus (Figure 2A; red arrows: no stress or ero1-1 at 24 °C, class III).
Figure 2. ER stress induces a delay in cortical ER (cER) inheritance.
(A) ER stress was induced (by Tm, DTT, or ero1-1) in cells expressing the ER membrane marker, Hmg1-GFP, for examination by fluorescence microscopy. Three categories of cells are depicted for each condition and as described in Results section. Yellow arrow shows cER, while red arrow represents perinuclear ER.
(B) For each of the three classes of cells, the number of cells containing elements of cER in the daughter cell was counted (n=300) during growth under normal conditions (grey bars) or in the presence of 2 mM DTT (3 hrs) (black bars). The average of 3 independent experiments is depicted; error bars represent standard deviation (SD).
(C) ER visualized by a HDEL-DsRed ER reporter confirmed that cER inheritance was delayed when ER stress was induced by Tm. Images shown are HDEL-DsRed alone. See also Figure S2.
When ER stress was induced, whether with Tm, DTT, or the ero1-1 allele grown at 37 °C, cER entry into the daughter cell was significantly inhibited (Figure 2A; quantified for DTT- and Tm-induced ER stress in Figure 2B and S2A, respectively). Early in the cell cycle, prior to nuclear division, only 13% of ER-stressed cells with small buds (Figure 2A left panels, +DTT, +Tm, ero1-1 at 37 °C, defined as Class I) and 30% of cells with medium buds (Figure 2A middle panels, +DTT, +Tm, ero1-1 at 37 °C, Class II) contained any cER. Even after mitosis (i.e. completed nuclear division) (Class III), 27% of ER-stressed cells still contained no visible cER in the bud (Figure 2A, right panels). In contrast, the perinuclear ER was inherited normally during ER stress. The inhibition of cER inheritance was also seen after Tm treatment using another ER reporter, HDEL-DsRed, which marks the lumen of the ER (Figure 2C and Figure S2B), demonstrating that the effect is independent of the ER reporter used. We conclude that ER stress inhibits the inheritance of cER. Finally, we found that ER stress did not affect the inheritance of the vacuole (Figure S2C) and the mitochondria (Figure S2D) although their morphologies differed between ER-stressed and unstressed cells.
The UPR is not involved in the septin stabilization, cytokinesis block, or ER inheritance delay observed during ER stress
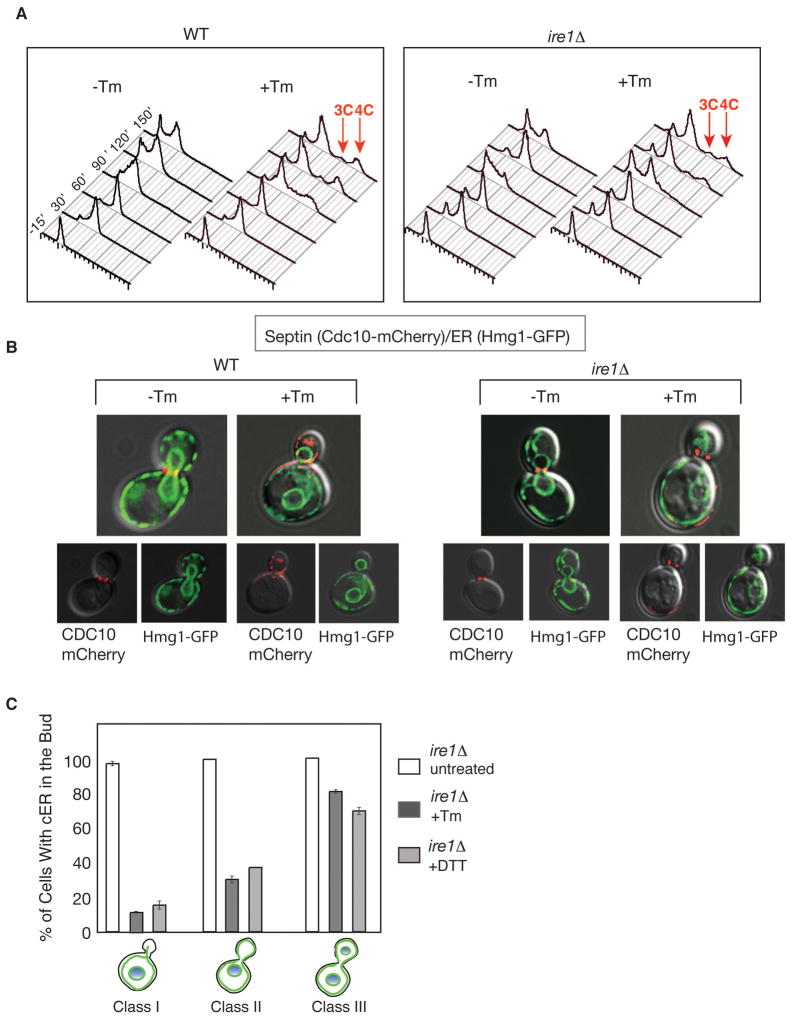
ER stress impacts the cell cycle in three ways: it alters septin structures, it inhibits cER inheritance, and it delays cytokinesis. We reasoned that these events might be a part of a surveillance mechanism to monitor the ER’s functional capacity and to prevent propagation of a compromised ER. Because of the physical location and functional roles of the septin complex, we reasoned that it might provide a pivotal point for integrating the functional state of the ER, its inheritance, and cell cycle progression. To date, the only signaling pathway known to be initiated by ER stress is the Unfolded Protein Response (UPR) pathway. In S. cerevisiae, the UPR is set in motion by Ire1p, an ER transmembrane receptor kinase/riboendonuclease (RNase) that senses ER stress, and signals to downstream components in order to help cells cope with the stress (Cox et al., 1993; Mori et al., 1993). Surprisingly, we found that cells lacking IRE1 (ire1Δ) continued to exhibit a delay in cytokinesis, observed as an increase in the 3C/4C DNA content (Bicknell et al., 2007) (Figure 3A), and aberrant septin morphology (Figure 3B, Cdc10-mCherry). Furthermore, a delay in cER inheritance still occurred in the bud of ER stressed ire1Δ cells (Figure 3B & quantified in Figure 3C) and did so at levels similar to those of ER stressed WT cells. Thus, the ER surveillance mechanism responsible for these events must be signaled via a mechanism independent of the IRE1-mediated UPR pathway.
Figure 3. The UPR does not signal septin stabilization, cytokinesis block or ER inheritance delay during ER stress.
(A) DNA content of α-factor synchronized wild-type and ire1Δ cells were monitored by FACS upon Tm treatment as described in experimental procedures. After 90 minutes, both cell types showed populations of cells with 3C or 4C DNA content (red arrows), indicating a cytokinesis delay (Bicknell et al., 2007).
(B) Cortical cER inheritance (visualized by Hmg1-GFP) and septin morphology (by Cdc10-mCherry) were perturbed in both WT and ire1Δ cells when grown in Tm. DIC pictures merged with either Cdc10-mCherry or Hmg1-GFP are shown for both WT and ire1Δ cells.
(C) Quantitation of ire1Δ cells with cER in the bud under ER stress inducing conditions (n=300 for each class, under normal conditions (white bars) or in the presence of 1 μg/ml Tm (dark gray bars) and 2 mM DTT (light gray bars). Error bars represent standard deviation (SD) of three independent experiments.
Slt2 MAP kinase is required for ER Stress Surveillance
In search of a regulatory molecule controlling the observed cell cycle defects above, we tested a number of candidate mutants. These included mutations in genes encoding proteins residing on the ER membrane, proteins involved in cER movement, and canonical signaling molecules such as kinases and phosphatases (Supplemental Table 1). We screened for mutants that had lost the characteristic responses to ER stress described above. Our search revealed a linkage to the Slt2 MAP kinase. Slt2 was originally included in our screen as it was known to be phosphorylated during ER stress (Bonilla and Cunningham, 2003; Chen et al., 2005), and had been genetically, albeit separately, linked to septins (Longtine et al., 1998a) and to ER inheritance (Du et al., 2006), although the functional significance was unknown.
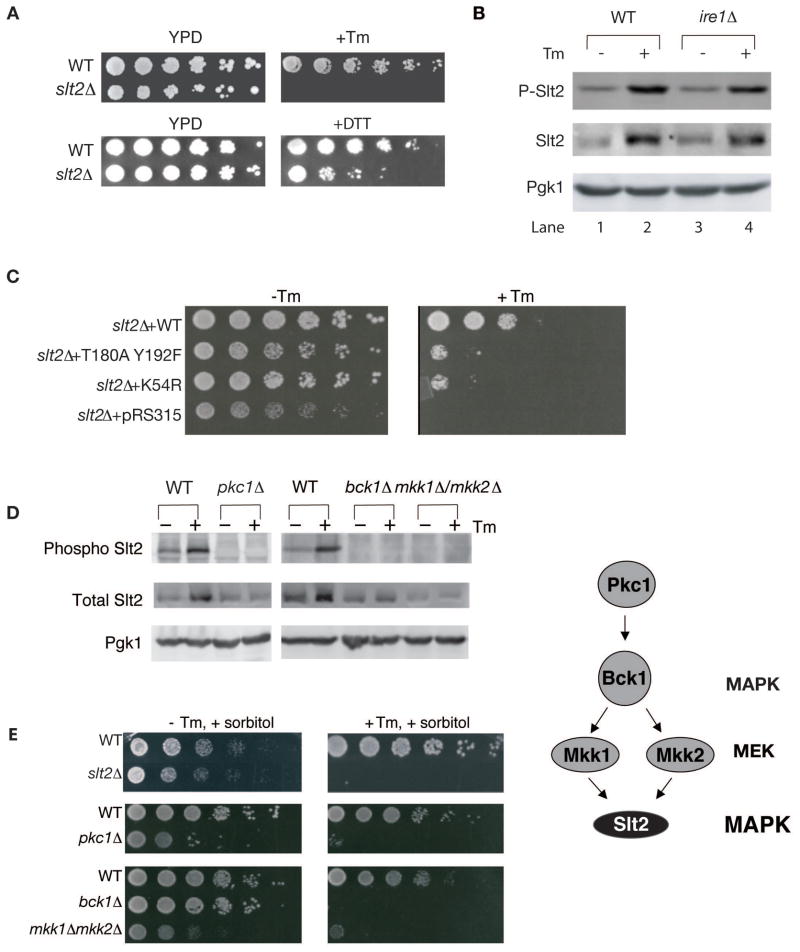
If Slt2 were mediating the ER surveillance mechanism, we expected that slt2Δ cells exposed to ER stress would not induce a cytokinesis defect, septin alterations, or delay the inheritance of cER. Indeed, synchronized slt2Δ cells did not exhibit 3C/4C DNA content after Tm treatment (Figure 4A; slt2Δ +Tm). The septin ring of slt2Δ cells also appeared normal after Tm treatment (Figure 4B). Moreover, cER inheritance was delayed significantly less in slt2Δ cells than in WT cells exposed to ER stress (Figure 4C; quantified in Figure 4D). The failure to induce these events was not caused by an inability of tunicamycin (Tm) to induce ER stress in slt2Δ cells because slt2Δ cells efficiently activated the separate UPR pathway under these conditions (Figure S3A, lanes 3 and 4). Taken together the data indicate that SLT2 mediates the previously undefined ER surveillance (ERSU) pathway that links ER stress with the cell cycle and ER inheritance.
Figure 4. Slt2 MAP kinase mediates septin alterations, cytokinesis delay, and ER inheritance delay during ER stress.
(A) ER stress did not induce a cytokinesis delay in slt2Δ cells, shown by no slt2Δ cells with 3C or 4C DNA content FACS peaks, in contrast to ER stressed wild-type cells (red arrows). Experimental conditions were as described in Figure 3A.
(B) Unlike in ER-stressed WT cells, the septin ring remained at a normal position in ER-stressed slt2Δ cells. Septin was visualized by Cdc10-GFP in WT (left) and slt2Δ cells (right) grown with or without 1 μg/ml Tm, as indicated. Additional genes tested are shown in Supplemental Table 1.
(C) cER entered the bud of slt2Δ cells treated with Tm (1 μg/ml) as visualized by Hmg1-GFP.
(D) Quantitation of wild-type and slt2Δ cells with elements of cER in the daughter cell under normal (−Tm) or ER stress (+Tm) (1 μg/ml) conditions in each class (n=300). The average of three independent experiments is depicted; error bars represent SD. See also Figure S3.
ER surveillance (ERSU) signaling is necessary for survival of ER stress
Induction of ER stress arrests the cell cycle. However, this arrest is not permanent; over a longer period of time, wild-type cells can recover and are observed to grow on Tm or DTT plates (Figure 5A). We hypothesized that the ERSU response, like the UPR response, allows cells to first adapt to ER stress, allowing them to grow under constant stress. Consistent with this hypothesis, slt2Δ cells failed to grow on plates containing Tm or DTT (Figure 5A, +Tm or +DTT). This suggests that the output of the ERSU pathway allows cells to cope with long-term stress.
Figure 5. Slt2p phosphorylation and kinase activity are essential for ER stress surveillance.
(A) slt2Δ cells were not able to sustain growth on a plate containing Tm or DTT. Fivefold serial dilutions of WT and slt2Δ cells were spotted on plates containing no drug (YPD) or +Tm (0.1 μg/ml), or +DTT (4 mM).
(B) Slt2 MAP kinase was phosphorylated upon ER stress in WT (lanes 1&2) and ire1Δ cells (lanes 3&4). Total cell lysate of either WT or ire1Δ cells with (+) or without (−) Tm treatment were blotted with anti-phospho specific Slt2, total Slt2, or Pgk1 (loading control) antibodies. In addition to increase in phosphorylation of Slt2, total protein level was also increased, although ER stress-induced phosphorylation does not require increase in Slt2 protein levels (see Figure S3B).
(C) slt2Δ cells were transformed with empty plasmid, or plasmid containing WT SLT2, T180AY192F slt2, or K54R slt2. Cells were grown to log phase in synthetic complete (SC)-leu medium, diluted serially 5-fold, and spotted onto plates with or without 0.1 μg/ml Tm.
(D) pkc1Δ and corresponding WT cells were grown in the presence of 1M sorbitol, while bck1Δ, mkk1Δ /mkk2Δ, and corresponding WT cells were grown in the absence of sorbitol.
(E) Cells of the indicated genotype were grown to log phase in YPD + 1M sorbitol, diluted serially 5 fold, and spotted onto 0.2 μg/ml Tm or no Tm plates containing 1M sorbitol. Sorbitol had no effect on sensitivity of slt2Δ on Tm plate (compare Figures 5E vs 5A & 6B), revealing lack of the CWI pathway involvement. See also Figure S4.
Slt2 phosphorylation and kinase activity are essential for ER stress surveillance
Slt2 was phosphorylated upon ER stress (Figure 5B, lanes 1& 2). Phosphorylation of Slt2 still occurred in ire1Δ cells (Figure 5B, lanes 3 & 4), revealing that Ire1 is not required for Slt2 phosphorylation and providing further support that ERSU is independent of UPR. It should be noted that, in addition to Slt2 phosphorylation, the total Slt2 protein level also increased upon ER stress. This was the consequence of a transcriptional increase of SLT2 during ER stress that was, as previously reported (Chen et al., 2005 independent of Hac1, a UPR transcription factor (Figure S3B, lanes 1 & 2 vs, lanes 3 & 4). Instead, Rlm1, one of the transcription factors reported to be downstream of Slt2 (Levin, 2005) was found to be responsible for the transcription increase in Slt2 (Figure S3B). Furthermore, in rlm1Δ cells, while ER stress did not induce SLT2 mRNA transcript levels, the increase in Slt2 phosphorylation still took place, revealing that Slt2 phosphorylation is independent of SLT2 mRNA or protein increase (see Figure S3C lanes 3 & 4). We therefore tested whether Slt2 phosphorylation and/or Slt2 kinase activity are important for surviving ER stress. slt2Δ cells transformed with wild type SLT2 regained their ability to grow on Tm plates. However, slt2Δ cells transformed with either the kinase-dead slt2-K54R mutant or the phosphorylation site slt2-T190A/Y192F mutant (Kim et al., 2008) failed to grow on Tm plates (Figure 5C). Therefore, we conclude that both Slt2 kinase activity and Slt2 phosphorylation are required to survive ER stress.
We next investigated the mechanism of Slt2 phosphorylation. Slt2 is a MAP kinase, and multiple upstream activators of Slt2 have been identified (Levin, 2005). We found that both ER stress-induced phosphorylation of Slt2 (Figure 5D) and growth in the presence of Tm (Figure 5E) require Pkc1 (MEKK activator), Bck1 (MEKK), and either Mkk1 or Mkk2 (redundant MEKs). Furthermore, septin ring and daughter cell cER inheritance of pkc1Δ and bck1Δ cells were not disturbed, even upon ER stress induction; similar to what we observed in slt2Δ cells (Figure S4). This suggests that the ERSU signaling pathway is activated upstream of Pkc1.
The ERSU pathway is activated by Wsc1 and is distinct from the cell wall integrity and arrest of secretion response pathways
The Pkc1-Slt2 pathway can be activated by any one of six upstream components, Wsc1, Wsc2, Wsc3, Wsc4, Mid2, and Mtl1, which reside in the plasma membrane (de Nobel et al., 2000; Gray et al., 1997; Ketela et al., 1999; Philip and Levin, 2001; Verna et al., 1997; Zu et al., 2001). We found that, of these six sensors, only wsc1Δ cells displayed reduced Slt2 phosphorylation during Tm treatment (Figure 6A) and Tm sensitivity on plates (Figure 6B). Furthermore, wsc1Δ cells did not display any aberrant septin morphology (Figure 6C) and no delay in cER inheritance (Figure S5A) during Tm treatment, indicating that ERSU relies on Wsc1 activation.
Wsc1 is known to mediate the cell wall integrity (CWI) pathway that allows a cell to respond to excess turgor pressure against the cell wall. Its activation during cell wall stress results in phosphorylation of Slt2 via Pkc1 (Levin, 2005). We therefore asked whether the ERSU signal originates as a defect in CWI. We found that stimulation of cell wall stress induced by the chitin antagonist calcoflour white (CFW) did not affect septin dynamics, even though it induced Slt2 phosphorylation as previously reported (Figure 6D & 6E). Thus, Slt2 phosphorylation is not sufficient to activate the ERSU response and the ERSU pathway is not induced by cell wall stress. Moreover, a recent report has found that CFW-induced cell wall stress activates the UPR response (Bonilla and Cunningham, 2003; Scrimale et al., 2009). In this case, however, UPR was mediated by Mid2, and not by Wsc1, differentiating the ERSU pathway from cell wall stress.
Additional support for the distinction between the ERSU pathway and the CWI pathway came from our observation that sorbitol, an osmotic stabilizer known to suppress signaling through the CWI pathway (Verna et al., 1997), did not alter Tm sensitivity of slt2Δ cells (compare Figure 5A; no sorbitol & 5E; with sorbitol). Furthermore, it has been observed that in the CWI pathway, the GDP exchange factors Rom1 and Rom2 mediate signaling from the cell surface components (Philip and Levin, 2001). We found, however, that they were not involved in the ERSU pathway, as neither rom1Δ nor rom2Δ cells were sensitive to Tm, and Slt2 underwent phosphorylation at similar efficiency in these mutant cells as in WT cells (Figure S5B & S5C). Lack of Rom1 and Rom2 involvement also provides further support for difference in the CWI pathway and the ERSU signaling.
A previous report has shown that the arrest of secretion response (ASR) is caused by secretory block and is mediated by Pkc1 and Wsc proteins trapped along the secretory pathway (Nanduri and Tartakoff, 2001). Because the ER plays roles in maturation of secretory proteins, ER stress may indirectly cause secretory block and therefore, ER surveillance may be induced by activation of the arrest of secretion response (ASR) pathway. To distinguish between ERSU and ASR pathways, we induced a secretory block that was independent of ER stress, using the sec1-1 temperature sensitive allele (Figure S5D) and asked whether this caused visible defects in the septin ring. sec1-1 is one of the best-characterized secretory block mutants (Novick and Schekman, 1983). Shifting of sec1-1 cells to the restrictive temperature (37°C) has been shown to result in a reduction of mRNA transcripts coding for ribosomal proteins including RPL32 (Nanduri and Tartakoff, 2001). Although we confirmed reduction in RPL32 mRNA transcript when sec1-1 cells were grown at 37°C (Figure S5E), septin morphology and localization remained normal (Figure S5F), distinguishing ERSU signaling from ASR pathway. Additionally, ASR signal is mediated via Pkc1 but it does not involve Slt2, while ERSU is mediated by Wsc1, Pkc1 and Slt2 upon ER stress, providing further distinction from the ASR pathway.
Finally, during cell wall stress, Wsc1’s function in sensing the stress relies on its localization to sites of polarized growth on the plasma membrane (Piao et al., 2007). This localization requires constitutive endocytosis of Wsc1 from the cell surface. Indeed, wsc1 mutants defective in endocytosis (wsc1AAA) cannot establish a polarized localization and cannot sense cell wall stress (Piao et al., 2007). They are therefore hypersensitive to caspofungin (CP) treatment, which induces cell wall stress (Figure 6F, compare WT and wsc1-AAA with CP) (Piao et al., 2007). In contrast, we found that cells expressing an endocytosis-defective mutant form of WSC1 (wsc1AAA) were able to grow in the presence of Tm at the rate similar to wild-type cells (Figure 6F). Therefore, taken together, the data indicate that Wsc1 senses ER stress by mechanisms distinct from cell wall stress and the ERSU represents a novel utilization of this MAP kinase cascade.
ERSU promotes mother cell viability during ER stress
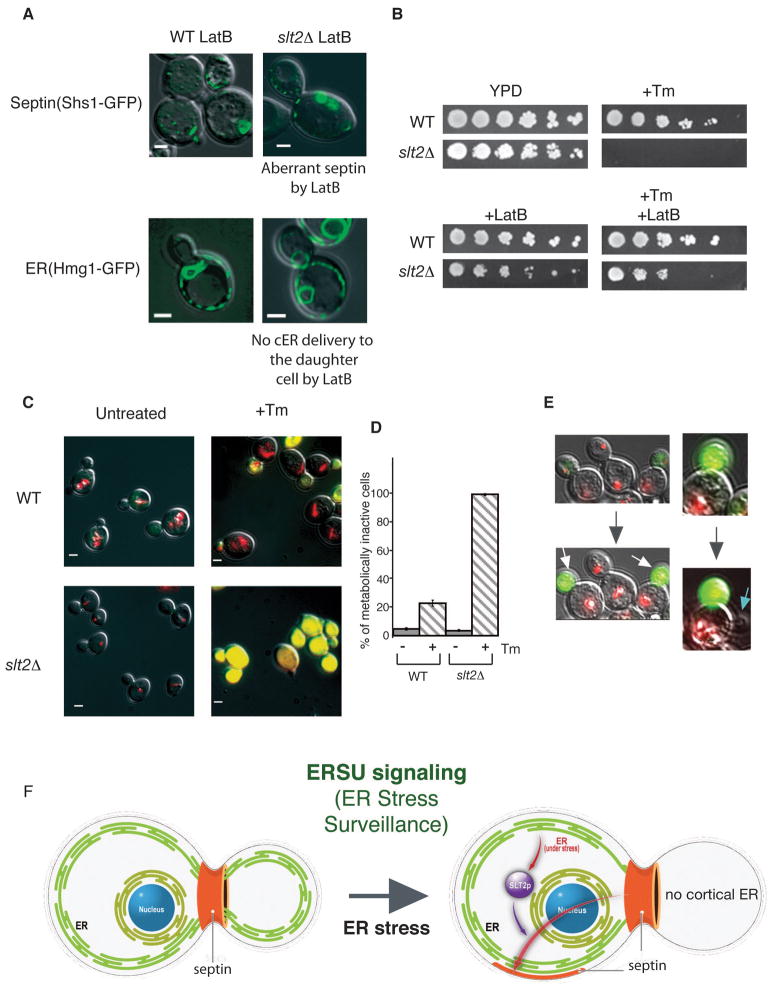
We have shown that Slt2’s function in linking the cell cycle with ER stress is important for long-term survival during ER stress (Figure 5A). To further determine if the output of the ERSU pathway that prevents stressed ER from entering into the daughter cell is protective, we asked if we could mimic the ERSU response in the slt2Δ mutant by inhibiting cER entry into the daughter cell. Since both cER movement and septin morphology are actin-dependent (Estrada et al., 2003; Kozubowski et al., 2005), we asked if treatment of slt2Δ cells with the actin depolymerizing agent Latrunculin B (LatB) (Spector et al., 1983) would prevent ER inheritance, alter septin structure, and allow growth in Tm in slt2Δ cells. Remarkably, LatB treatment suppressed the cER inheritance (Figure 7A; Hmg1-GFP) and altered the septin morphology (Figure 7A; Shs1-GFP). Quantification indicated that the treatment of slt2Δ cells with LatB + Tm reduced the number of daughter cells containing cER (Figure S6B, class I and II) to numbers similar to wild-type cells treated only with Tm. Furthermore, LatB also rescued the tunicamyin-sensitivity of slt2Δ cells (Figure 7B), i.e., slt2Δ cells were able to grow on Tm plates when LatB prevented cER entering into the daughter cell. Similarly, prevention of cER inheritance by MYO4 gene deletion (Estrada et al., 2003) mimicked the ability of LatB to rescue slt2Δ cell growth on Tm (Figure S6C). In addition, mild actin defect helps cER inheritance delay upon induction of ER stress, allowing act1-1 to grow better than WT cells (Figure S6E). Taken together, these results are consistent with the idea that ERSU signaling ultimately protects cells from deleterious effects of inheriting stressed ER.
Figure 7. ERSU signaling protects mother cells during ER stress.
(A) The effect of Latrunculin B (LatB) on ER and septin distribution was visualized with the septin reporter Shs1-GFP and the ER reporter Hmg1-GFP in wild-type (left) or slt2Δ cells (right) treated with 1 μg/ml Tm & 400 μM LatB for 2 hrs. In both cell types, septin morphology was altered and cER inheritance was inhibited by the presence of LatB.
(B) Lat B rescues growth of slt2Δ cells during ER stress. Five-fold serial dilutions of WT and slt2Δ cells were spotted on plates containing no drug (YPD), +Tm (0.1 μg/ml) alone, +LatB alone (6 μM) or +Tm (0.1 μg/ml)/+LatB (6 μM). See also Figure S6.
(C) & (D) Untreated and Tm treated WT and slt2Δ cells were stained with FUN-1. Upon ER stress induction by Tm treatment (+Tm) (1 μg/ml, 3 hrs), the daughter cells showed yellow staining, while the larger mother cells showed intravacuolar red rod-like structures (CIVSs), indicating that they were metabolically active. In contrast, slt2Δ cells following Tm treatment were stained yellow for both mother and daughter cells. Untreated WT and slt2Δ cells showed red CIVSs. Quantitation of yellow metabolically inactive cells is shown in (C). For WT and slt2Δ cells, the numbers of cells with yellow staining were counted.
(E) Continuous observation of mother and daughter cell fate during ER stress. Following staining with Fun-1, Tm treated cells (1 μg/ml for 30 min) were spread onto a gelatine-YPD pad containing 0,1 μg/ml Tm for microscopic observation. Initially both mother and daughter cells were alive (stained red), although the daughter cell (white arrow) became metabolically inactive. Furthermore, a blue arrow indicates a new daughter cell started to emerge from the original mother cell, showing asymmetric protection of the mother cell. See also Figure S7.
(F) During the cell cycle, the functional capacity of the ER is under surveillance and when ER stress is detected, ERSU activates Slt2, leading to septin re-organization and alteration so that the aberrant septin ring (shown in orange) is found in unusual places and is no longer at the bud neck. Concomitantly, ER inheritance, and cytokinesis are delayed. Thus, ERSU plays a critical role in correct transmission of a functional ER into the progeny cell.
We next asked whether this survival is achieved through specific preservation of either the mother or daughter cell. To distinguish the viability of mother and daughter cells, we stained both WT and slt2Δ cells with the vital dye FUN-1 following Tm treatment (Figure 7C & 7D). This dye generates differential staining patterns in metabolically active and inactive cells (Millard et al., 1997): metabolically active cells exhibit red-fluorescent cylindrical intravacuolar structures (see Figure S7A, upper panel (b)), whereas metabolically inactive cells display diffuse bright green and red cytoplasmic fluorescence, which appears yellow when merged (see Figure S7A, lower panel (d)). Upon induction of ER stress, 22% of cells became metabolically inactive, while less than 5% were metabolically inactive in an asynchronous population of normally grown WT cells (Figure 7C & quantified in Figure 7D (n=300)). Strikingly, in budded cells, the mother cell remained metabolically active, whereas the daughter cell appeared metabolically inactive. In contrast, in the slt2Δ mutant, ER stress caused a much higher fraction of the cells to become metabolically inactive (Figure 7C & 7D) and there was no preference for the daughter over the mother. Similar results were obtained with WT and slt2Δ cells when non-viable cells were enumerated by propidium iodide (PI) dye, a well-established nucleic acid binding agent that is only permeable to dead cells (Figure S7B & S7C). Furthermore, when ER stress was induced by ero1-1 cells grown at non-permissive temperature (37°C), we observed a similar increase of PI stained cells (Figure S7D). We also examined the viability of WT and slt2Δ cells treated with Tm in the presence of LatB and compared this to treatment with Tm alone (Figure S7B & S7C). Whereas ~95% of slt2Δ cells treated with Tm alone were stained by PI (both mother cells and daughter cells), only ~25% of cells were stained (mostly daughter cells with some both mother and daughter cells) in the presence of Tm and LatB. These results are in agreement with the growth rescue of slt2Δ cells observed on YPD medium containing Tm and LatB (Figure 7B). Finally, the selective inactivation of the daughter cell was further confirmed by continuous observation of live cells using FUN-1 staining after ER stress induction (Figure 7E). We observed FUN-1 stained daughter cells attached to a viable mother cell from which ultimately, a new bud started to emerge. This suggests that ERSU signaling specifically promotes mother cell viability, at the expense of the daughter cell.
DISCUSSION
A minimum level of ER functionality is required for cell viability. Thus, the functional capacity and timing of cell division and cER inheritance may be coordinated by a checkpoint that ensures a minimum ER functionality before cell division. We report here the identification of an ER surveillance (ERSU) pathway that may function as the gatekeeper for this checkpoint and monitors the functional capacity of the ER during cell division. When ER stress is induced, ERSU causes cytokinesis delay and cER to be retained in the mother cell until it replenishes ER function (Figure 7F). The delay in cytokinesis correlates with, and is likely caused by, altered dynamics of the septin complex. ERSU is independent of UPR signaling, and instead relies upon the MAP kinase Slt2, ensuring that only functional ER is transmitted to daughter cells. Thus, ER stress activates both the ERSU pathway, which controls cell cycle progression, and UPR pathways which re-establishes ER functions. Once the ER capacity is re-established, we expect that the ERSU pathway will be turned off. Thus, although the ER may not be delivered to the daughter cell during ER stress, subsequent daughter cells will receive functional ER. In ERSU deficient cells (for example, slt2Δ cells), this regulation is lost, and mother cells distribute cER into the daughter cells irrespective to functional state of the ER. As a consequence, the level of ER functionality in the mother cell may drop below the minimum requirement causing both cells to undergo cell death. Thus, during ER stress, in slt2Δ cells both the mother and daughter cell are inviable, whereas in WT cells, when the ER is retained in the mother cell, only the daughter cell is inviable. In support of this model, inhibiting ER inheritance in slt2Δ cells through treatment with LatB allowed restoration of mother cell viability (Figure 7A & 7B). While we described in this report conditions in which ER stress is highly induced, we believe that the ERSU pathway may also function during the normal cell cycle, serving as a cell cycle “checkpoint”, that assures generation of progeny cells with functional ER.
One intriguing observation is that the ERSU pathway causes stressed cER to be preferentially retained in the mother cell. This type of mother cell retention has also been seen for factors that contribute to cell aging, such as extra-chromosomal rDNA circles (ERCs), maternal nuclear pores and carbonylated proteins (Tessarz et al., 2009) (Shcheprova et al., 2008). Such toxic factors accumulate with age and ultimately lead to cell death; they are observed to be retained in the mother cell presumably to increase the lifespan of the newly born daughters (Erjavec et al., 2007; Murray and Szostak, 1983; Sinclair and Guarente, 1997). It has been shown that the retention in the mother cells of nuclear pores and ERCs requires a septin-dependent diffusion barrier within the nuclear envelope (Shcheprova et al., 2008). Taken together, these data point to a general evolutionary rationale that uses multiple critical mechanisms to assure the asymmetric distribution of elements to the mother cell. At first glance, ERSU seems to act differently from these ageing pathways, because it allows preferential protection of mother cells rather than daughter cells. However, unlike the accumulation of ageing factors, ER stress is reversible. Thus, the ERSU pathway ultimately promotes conservation of offspring by protecting the mother cell and allowing it to generate subsequent generations of daughter cells.
Another intriguing feature of the ERSU pathway is the different behavior of cortical and nuclear ER. We observed that perinuclear ER was inherited normally with the nucleus during ER stress, while cER delivery to the daughter cell was inhibited, suggesting a potential distinction between cortical and perinuclear ER. A recent report using an ER stress reporter indicates that ER stress is not transmitted to daughter cells (Merksamer et al., 2008). As we have found that perinuclear ER along with the nucleus is transmitted into daughter cell even during ER stress, such observation suggests that perinuclear ER is free of ER stress. It prompts one to ask whether ER stress could somehow be partitioned to the cER, which is retained in the mother cell. Future studies will test such ideas and explore the mechanism and functional implications of this distinction between the two subdomains of the ER.
ERSU controls cell cycle in response to ER stress via Wsc1 and Slt2, distinct from the UPR, CWI and ASR pathways. Currently, we do not know how ER stress is signaled through Wsc1. The mechanism is unlikely to involve gross changes in Wsc1 localization, as we have found that the steady state localization of Wsc1 during ER stress does not change (data not shown). One possibility is that ER stress activates Wsc1 at the cell surface through an unknown mechanism. Alternatively, as Wsc1 transits the ER during its folding process, it might directly detect ER stress and initiate the ERSU pathway. For example, during ER stress, Wsc1 protein might be modified within the ER lumen before exiting from the ER to initiate ERSU. In addition, we do not know how Slt2 kinase activation leads to the cER inheritance delay and septin alteration. Slt2 kinase may directly phosphorylate cER inheritance components or septin subunits. Future studies will be required to uncover the molecular mechanism of Wsc1 and Slt2 activation during ER stress.
In summary, our study has described the discovery of an ER surveillance (ERSU) pathway in yeast. We have mapped a number of the components of the ERSU pathway but anticipate that future studies will provide additional components involved in the pathway. For example, a recent report describes a novel molecular mechanism involving the polarisome, a multi-protein complex that regulates actin cytoskeleton restricting apical growth of S. cerevisiae (Sheu et al., 2002), that prevents protein aggregates from staying in the daughter cell (Liu et al., 2010). It may also be possible that the polarisome functions to establish the cER inheritance delay in response to ER stress. In addition, Ptc1, a phosphatase that is thought to negatively regulate Pkc1 and thus ultimately Slt2 kinase (Nanduri and Tartakoff, 2001), may also function in the ERSU pathway. A recent genetic screen identified Ptc1 is a component with an as yet unknown role in ER inheritance during normal cell growth (Du et al., 2006b). Ptc1 also regulates inheritance of the mitochondria (Roeder et al., 1998) and the vacuole (Jin et al., 2009). Therefore, Ptc1 may be a part of a master regulator choreographing different mechanisms that modulate the transmission of organelles and the cytoplasmic components to the daughter cell.
A mechanism of ER surveillance similar to ERSU may exist in mammalian cells. Since the fundamental mechanisms of cytokinesis differ between yeast and mammalian cells, the details of ERSU may differ between the two cell types. However, the failure to properly regulate ER functional capacity in vertebrate cells is increasingly recognized as contributing to the pathophysiology of a number of human diseases, including diabetes and certain cancers. Thus, further understanding of the cellular mechanisms of the ERSU Response that we have reported here, and investigation of the mammalian counterpart may allow for the development of previously unrecognized strategies for therapeutic intervention.
EXPERIMENTAL PROCEDURES
Cell Manipulations
The S. cerevisiae strains used in this study are described in Supplemental Table 2. All cells were grown in YPD at 30°C and were examined during log phage unless otherwise noted. ER stress was induced upon addition of Tm, or DTT, or shifting the temperature of ero1-1 cells to 37°C, as described detail in Figure legends and in Supplemental Experimental Procedures.
Northern, and Western blots
Both Northern and western blotting were performed as described previously (Bicknell et al., 2007).
Microscopy
All cells were visualized using a microscope (Axiovert 200M; Carl Zeiss MicroImaging, Inc.) with a 100x 1.3 NA objective. Images were captured using a monochrome digital camera (Axiocam; Carl Zeiss MicroImaging, Inc.) and analyzed using Axiovision software (Carl Zeiss MicroImaging, Inc.).
DNA Staining and Flow Cytometry
106 cells were fixed in 70% ethanol overnight, treated with 1 mg/ml RNase A at 37°C for 2 hours, 5 mg/ml pepsin for 20 min at 37°C, and then stained with 10 μM Sytox Green (Invitrogen), as described previously (Bicknell et al., 2007). Data were collected using a flow cytometer (FACSCalibur; BD Biosciences) and analyzed using FlowJo software (TreeStar).
Supplementary Material
Acknowledgments
We are grateful to Dr. Douglass Forbes for critical reading, many insightful suggestions and support throughout the study. We also thank Dr. Lorraine Pillus and Rei Otsuka for their help for generating a strain critical for the experiments and Dr. Pillus for support throughout the study. We thank Drs. Randy Hampton, and Jim Umen for their suggesting and careful reading of the manuscript, Dr. David E. Levin, for providing the bck1Δ, mkk1Δ mkk2Δ yeast strains, pkc1Δ yeast strain, and plasmids p2188, p2190 and p2193 and Dr. Enrique Herrero for providing the pkc1Δ strain. We also thank Dr. Peter Novick for providing the sec1-1 strain and Dr Yves Barral for providing the cdc12-6 strain. We thank Drs. Randy Hampton, Jodi Nunnari and Svetlana Dokudovskaya for providing plasmids pRH475 and pRH1827, pVT100-ds-RedT1, and Vph1-mCherry, respectively. This work was supported from NIH (RO1GM087415), the American Cancer Society (ACS RSG-05-01GMC), Searle 03-G107, and CRCC 6-447140-34384 to M.N.
Abbreviations
- ERSU
ER Surveillance
- UPR
Unfolded Protein Response
- ER
Endoplasmic Reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bertin A, McMurray MA, Grob P, Park SS, Garcia G, III, Patanwala I, Ng H-l, Alber T, Thorner J, Nogales E. Saccharomyces cerevisiae septins: Supramolecular organization of heterooligomers and the mechanism of filament assembly. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8274–8279. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell AA, Babour A, Federovitch CM, Niwa M. A novel role in cytokinesis reveals a housekeeping function for the unfolded protein response. J Cell Biol. 2007;177:1017–1027. doi: 10.1083/jcb.200702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell AA, Niwa M. Regulating Endoplasmic Reticulum Function through the Unfolded Protein Response. Handbook of Cell Signaling. 2009:2511–2525. [Google Scholar]
- Bonilla M, Cunningham KW. Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell. 2003;14:4296–4305. doi: 10.1091/mbc.E03-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular Chaperones and Protein Quality Control. Cell. 2006;123:443–551. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, Shi G, Le QT, Brown JM, Koong AC. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Molecular Cancer Research. 2005;3:669–677. doi: 10.1158/1541-7786.MCR-05-0181. [DOI] [PubMed] [Google Scholar]
- Cid VJ, Adamikova L, Cenamor R, Molina M, Sanchez M, Nombela C. Cell integrity and morphogenesis in a budding yeast septin mutant. Microbiology. 1998;144(Pt 12):3463–3474. doi: 10.1099/00221287-144-12-3463. [DOI] [PubMed] [Google Scholar]
- Dobbelaere J, Gentry MS, Hallberg RL, Barral Y. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev Cell. 2003;4:345–357. doi: 10.1016/s1534-5807(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Du Y, Pypaert M, Novick P, Ferro-Novick S. Aux1p/Swa2p is required for cortical endoplasmic reticulum inheritance in Saccharomyces cerevisiae. Mol Biol Cell. 2001;12:2614–2628. doi: 10.1091/mbc.12.9.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Walker L, Novick P, Ferro-Novick S. Ptc1p regulates cortical ER inheritance via Slt2p. Embo J. 2006a;25:4413–4422. doi: 10.1038/sj.emboj.7601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essary BD, Marshall PA. Assessment of FUN-1 vital dye staining: Yeast with a block in the vacuolar sorting pathway have impaired ability to form CIVS when stained with FUN-1 fluorescent dye. Journal of microbiological methods. 2009;78:208–212. doi: 10.1016/j.mimet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Estrada P, Kim J, Coleman J, Walker L, Dunn B, Takizawa P, Novick P, Ferro-Novick S. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J Cell Biol. 2003;163:1255–1266. doi: 10.1083/jcb.200304030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand AR, Kaiser CA. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell. 1998;1:161–170. doi: 10.1016/s1097-2765(00)80017-9. [DOI] [PubMed] [Google Scholar]
- Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. It takes two to tango: regulation of G proteins by dimerization. Nature Reviews Molecular Cell Biology. 2009;10:423–429. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Current Opinion in Microbiology. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Hampton RY. ER-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 2002;14:476–482. doi: 10.1016/s0955-0674(02)00358-7. [DOI] [PubMed] [Google Scholar]
- Huang KN, Symington LS. Suppressors of a Saccharomyces cerevisiae pkc1 mutation identify alleles of phosphatase gene PTC1 and of a novel gene encoding a putative basic leucine zipper protein. Genetics. 1995;141:1275–1285. doi: 10.1093/genetics/141.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker TC, Thomas JH, Botstein D. Diverse Effects of Beta Tubulin Mutations on Microtubule Formation and Function. J Cell Biol. 1988;106:1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M, Luo J, Nagaraj S, Longtine M, Kim HB, Haarer BK, Caruso C, Tong Z, Pringle JR, Bi E. Role of a Cdc42p effector pathway in recruitment of the yeast septins to the presumptive bud site. Mol Biol Cell. 2006;17:1110–1125. doi: 10.1091/mbc.E05-08-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs CW, Adams AEM, Szaniszlo PJ, Pringle JR. Functions of Microtubules in the Saccharomyces-Cerevisiae Cell Cycle. J Cell Biol. 1988;107:1409–1426. doi: 10.1083/jcb.107.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J, Cid VJ, Cenamor R, Yuste M, Molero G, Nombela C, Sanchez M. Morphogenesis beyond cytokinetic arrest in Saccharomyces cerevisiae. J Cell Biol. 1998;143:1617–1634. doi: 10.1083/jcb.143.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Taylor Eves P, Tang F, Weisman LS. PTC1 is required for vacuole inheritance and promotes the association of the myosin-V vacuole-specific receptor complex. Mol Biol Cell. 2009;20:1312–1323. doi: 10.1091/mbc.E08-09-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Truman AW, Levin DE. Yeast mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal. Mol Cell Biol. 2008;28:2579–89. doi: 10.1128/MCB.01795-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HB, Haarer BK, Pringle JR. Cellular Morphogenesis in the Saccharomyces-Cerevisiae Cell Cycle Localization of the Cdc3 Gene Product and the Timing of Events at the Budding Site. J Cell Biol. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning AJ, Larson LL, Cadera EJ, Parrish ML, Wright RL. Mutations that affect vacuole biogenesis inhibit proliferation of the endoplasmic reticulum in Saccharomyces cerevisiae. Genetics. 2002;160:1335–1352. doi: 10.1093/genetics/160.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, Nystrom T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Fares H, Pringle JR. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J Cell Biol. 1998a;143:719–736. doi: 10.1083/jcb.143.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998b;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Martin H, Flandez M, Nombela C, Molina M. Protein phosphatases in MAPK signalling: we keep learning from yeast. Mol Microbiol. 2005;58:6–16. doi: 10.1111/j.1365-2958.2005.04822.x. [DOI] [PubMed] [Google Scholar]
- McMaster CR. Lipid metabolism and vesicle trafficking: More than just greasing the transport machinery. Biochemistry and Cell Biology. 2001;79:681–692. doi: 10.1139/o01-139. [DOI] [PubMed] [Google Scholar]
- McMurray MA, Thorner J. Reuse, replace, recycle Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast. Cell Cycle. 2009;8:195–203. doi: 10.4161/cc.8.2.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Nanduri J, Tartakoff AM. The arrest of secretion response in yeast: signaling from the secretory path to the nucleus via Wsc proteins and Pkc1p. Mol Cell. 2001;8:281–289. doi: 10.1016/s1097-2765(01)00312-4. [DOI] [PubMed] [Google Scholar]
- Novick P, Schekman R. Export of Major Cell Surface Proteins Is Blocked in Yeast Saccharomyces-Cerevisiae Secretory Mutants. J Cell Biol. 1983;96:541–547. doi: 10.1083/jcb.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuni K, Okuda A, Kikuchi A. Yeast Nap1-binding protein Nbp2p is required for mitotic growth at high temperatures and for cell wall integrity. Genetics. 2003;165:517–529. doi: 10.1093/genetics/165.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Weisman LS. The cyclin-dependent kinase Cdk1 directly regulates vacuole inheritance. Dev Cell. 2008;15:478–485. doi: 10.1016/j.devcel.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip B, Levin DE. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Molecular and Cellular Biology. 2001;21:271–280. doi: 10.1128/MCB.21.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao HL, Machado IM, Payne GS. NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol Biol Cell. 2007;18:57–65. doi: 10.1091/mbc.E06-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard MG, Travers KJ, Weissman JS. Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol Cell. 1998;1:171–182. doi: 10.1016/s1097-2765(00)80018-0. [DOI] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Kaiser CA, Orlean P, Albright C, Rose MD, Robbins PW, Botstein D. Structure of the Yeast Endoplasmic Reticulum Localization of Er Proteins Using Immunofluorescence and Immunoelectron Microscopy. Yeast. 1991;7:891–911. doi: 10.1002/yea.320070902. [DOI] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder AD, Hermann GJ, Keegan BR, Thatcher SA, Shaw JM. Mitochondrial inheritance is delayed in Saccharomyces cerevisiae cells lacking the serine/threonine phosphatase PTC1. Mol Biol Cell. 1998;9:917–930. doi: 10.1091/mbc.9.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007:8. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends in Cell Biology. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Scrimale T, Didone L, Bentley KLdM, Krysan DJ. The Unfolded Protein Response Is Induced by the Cell Wall Integrity Mitogen-activated Protein Kinase Signaling Cascade and Is Required for Cell Wall Integrity in Saccharomyces cerevisiae. Mol Biol Cell. 2009a;20:164–175. doi: 10.1091/mbc.E08-08-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimale T, Didone L, de Mesy Bentley KL, Krysan DJ. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae. Mol Biol Cell. 2009b;20:164–175. doi: 10.1091/mbc.E08-08-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y. A mechanism for asymmetric segregation of age during yeast budding. Nature. 2008;454:728–734. doi: 10.1038/nature07212. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Tessarz P, Schwarz M, Mogk A, Bukau B. The yeast AAA+ chaperone Hsp104 is part of a network that links the actin cytoskeleton with the inheritance of damaged proteins. Mol Cell Biol. 2009;29:3738–3745. doi: 10.1128/MCB.00201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drogen F, Peter M. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr Biol. 2002;12:1698–1703. doi: 10.1016/s0960-9822(02)01186-7. [DOI] [PubMed] [Google Scholar]
- Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nature Reviews Molecular Cell Biology. 2008:9. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna J, Lodder A, Lee K, Vagts A, Ballester R. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13804–13809. doi: 10.1073/pnas.94.25.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Chen CY, Levin DE. Saccharomyces cerevisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J Biol Chem. 1994;269:16829–16836. [PubMed] [Google Scholar]
- Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell. 2003;14:4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.