Abstract
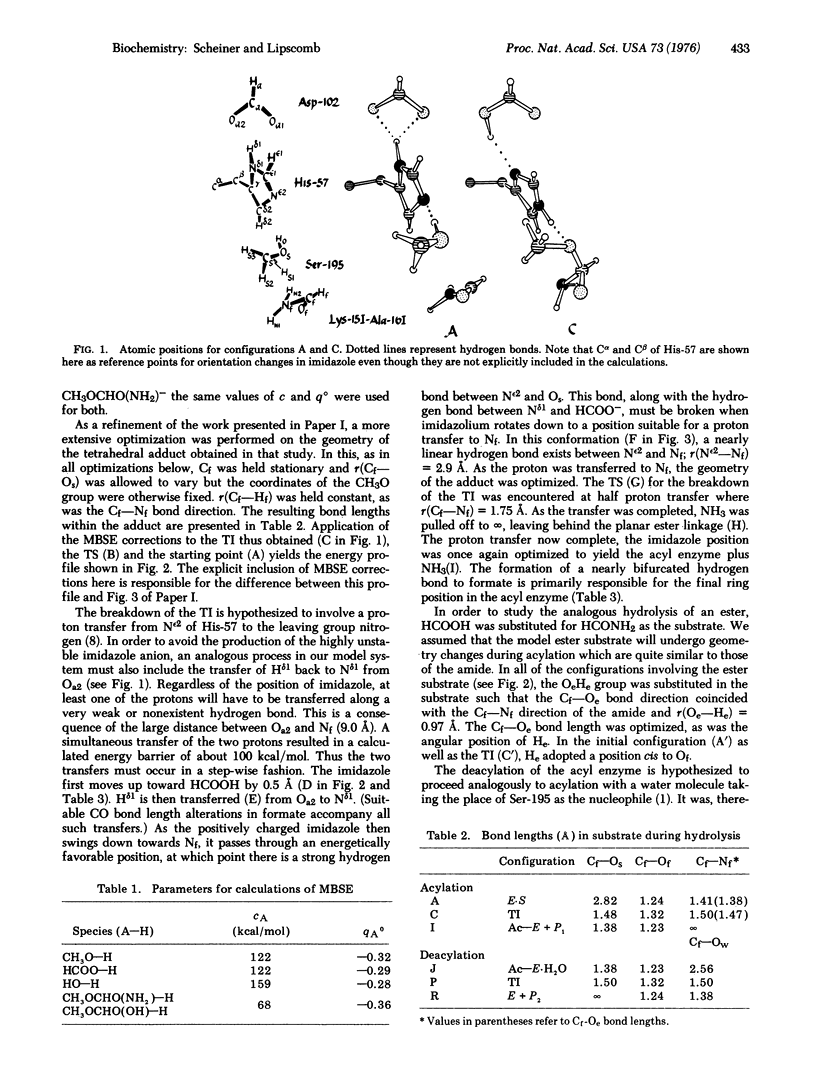
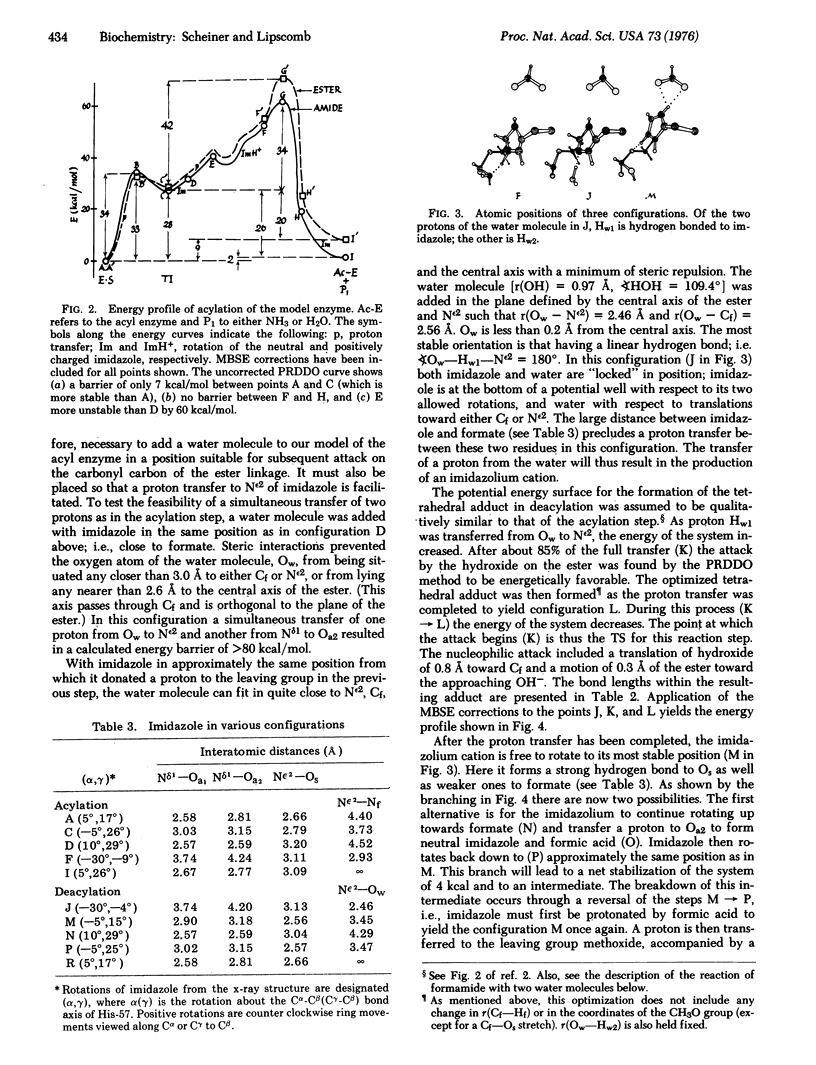
The catalytic activity of the serine proteinases is studied using molecular orbital methods on a model of the enzyme-substrate complex. A mechanism is employed in which Ser-195, upon donating a proton to the His-57-Asp-102 dyad, attacks the substrate to form the tetrahedral intermediate. As His-57 then donates a proton to the leaving group, the intermediate decomposes to the acyl enzyme. An analogous process takes place during deacylation, as a water molecule takes the place of Ser-195 as the nucleophile. The motility of the histidine is found to be an important factor in both steps. An attempt is made to include the effects of those atoms not explicitly included in the calculations and to compare the reaction rate of the proposed mechanism with that of the uncatalyzed hydrolysis. This mechanism is found to be in good agreement with structural and kinetic data.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. L., Killheffer J. V. Chymotrypsins. CRC Crit Rev Biochem. 1973 Apr;1(2):149–199. doi: 10.3109/10409237309102546. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Caplow M. Chymotrypsin catalysis. Evidence for a new intermediate. J Am Chem Soc. 1969 Jun 18;91(13):3639–3645. doi: 10.1021/ja01041a037. [DOI] [PubMed] [Google Scholar]
- Henderson R. Catalytic activity of -chymotrypsin in which histidine-57 has been methylated. Biochem J. 1971 Aug;124(1):13–18. doi: 10.1042/bj1240013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M., Kay L. M., Stroud R. M. Structure and specific binding of trypsin: comparison of inhibited derivatives and a model for substrate binding. J Mol Biol. 1974 Feb 25;83(2):209–230. doi: 10.1016/0022-2836(74)90388-x. [DOI] [PubMed] [Google Scholar]
- Rühlmann A., Kukla D., Schwager P., Bartels K., Huber R. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. Crystal structure determination and stereochemistry of the contact region. J Mol Biol. 1973 Jul 5;77(3):417–436. doi: 10.1016/0022-2836(73)90448-8. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Kleier D. A., Lipscomb W. N. Molecular orbital studies of enzyme activity: I: Charge relay system and tetrahedral intermediate in acylation of serine proteinases. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2606–2610. doi: 10.1073/pnas.72.7.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]


