Abstract
Background
The exact mechanism of the effects of hypoxia on the proliferation and apoptosis in carcinoma cells is still conflicting. This study investigated the variation of hypoxia-inducible factor-1α(HIF-1α) expression and the apoptosis effect of hypoxia stimulated by cobalt chloride (CoCl2) in pancreatic cancer PC-2 cells.
Methods
PC-2 cells were cultured with different concentration (50-200 μmol/L) of CoCl2 after 24-120 hours to simulate hypoxia in vitro. The proliferation of PC-2 cells was examined by MTT assay. The cellular morphology of PC-2 cells were observed by light inverted microscope and transmission electron microscope(EM). The expression of HIF-1α on mRNA and protein level was measured by semi-quantitive RT-PCR and Western blot analysis. Apoptosis of PC-2 cells were demonstrated by flow cytometry with Annexin V-FITC/PI double staining.
Results
MTT assay showed that the proliferation of PC-2 cells were stimulated in the first 72 h, while after treated over 72 h, a dose- dependent inhibition of cell growth could be observed. By using transmission electron microscope, swollen chondrosomes, accumulated chromatin under the nuclear membrane and apoptosis bodies were observed. Flow cytometer(FCM) analysis showed the apoptosis rate was correlated with the dosage of CoCl2. RT-PCR and Western blot analysis indicated that hypoxia could up-regulate the expression of HIF-1α on both mRNA and protein levels.
Conclusion
Hypoxic microenvironment stimulated by CoCl2 could effectively induce apoptosis and influence cell proliferation in PC-2 cells, the mechanism could be related to up-expression of HIF-1α.
Keywords: Pancreatic carcinoma, Hypoxia, Cobalt chloride, HIF-1α, Apoptosis, Proliferation
Background
Hypoxia is one of the most important pathological characteristics of solid tumor which is the result of imbalance between tumor cell proliferation and blood supply [1]. As solid tumor growing, its center becomes a hypoxic area because of lacking blood and oxygen. The hypoxic status of various solid tumor has been recognized as an important determinant for the outcome of anti-cancer therapies in a number of tumors [2].
Hypoxia-inducible factor-1 (HIF-1) was found in the 1992 when Semenza [3] researched the expression of erythropoietin gene induced by hypoxia. Human HIF-1 has been depurated and isolated, it is a heterodimeric transcription factor composed of oxygen-dependent HIF-1α and constitutively expressed HIF-1β subunits, HIF-1 transcriptional activity is largely determined by regulated expression of the HIF-1α subunit [4]. HIF-1α over-expression has been detected in various tumors including breast, oropharyngeal, nasopharyngeal, prostate, brain, lung, stomach cancer and so on, and has been associated with tumor aggressiveness, vascularity, treatment failure and mortality [5-7]. Interestingly, HIF-1α can also over-expressed under normoxic conditions in some human tumors [8].
In this research, we treated a human pancreatic cancer cell line (PC-2) with cobalt chloride (CoCl2) to stimulate hypoxia in vitro. Under the hypoxic condition, we observed the proliferation of PC-2 cells by MTT assay. Meanwhile, RT-PCR and Western blot analysis were conducted to measure the expression of HIF-1α on mRNA and protein level. Furthermore, we discussed the effect of hypoxic microenvironment on apoptosis and its mechanism.
Materials and methods
Reagents
Fetal bovine serum (Gibco, USA); RPMI1640 medium (Gibco, USA); 3-(4,5) -dimethylthiahiazo(-z-y1)-3,5- diphenyte- trazoliumromide (MTT) (Gibco, USA); annexin V-FITC/PI apoptosis detection kit (Becon Dickinson Facsalibur, USA); RT-PCR kit (ampliqon, Denmark); Trizol (Invitrogen, USA); HIF-1α monoclonal antibody (Santa Cruz Biotechnology, USA); 3-(5'-hydroxymethyl-2'-furyl)-1 -benzylindazole (YC-1) (Shanghai Shenggong Biological Engineering Technology&Service, China); CoCl2 (Shanghai Shenggong Biological Engineering Technology&Service, China).
Cell line and cell culture
Human pancreatic cancer cell line, PC-2, was purchased from the medical experimental animal center of the fourth military medical university. Cells were cultured in RPMI 1640 maximal medium containing 10% inactived fetal bovine serum (56°C, 30 min), 1 × 105 U/L penicillin and 100 mg/L streptomycin in a humidified atmosphere with 5% CO2 incubator at 37°C.
MTT assay for the proliferation of PC-2 cells
The proliferation of PC-2 cells was assessed using MTT dye reduction assay (Sigma, USA), which was conducted as described previously [9]. PC-2 cells were seeded in a 96-well plate at a density of 1 × 104 cells/well, cultured for 12 h under 37°C in 5% CO2, then treated with different concentration (50, 100, 150, 200 μmol/L) CoCl2 for 24-120 h. At the end of the treatment, MTT, 50 μg/10 μL, was added and the cells were incubated for another 4 hours. Dimethylsufloxide (DMSO; 200 μl) was added to each well after removal of the supernatant. After shaking the plate for 10 min, cell viability was assessed by measuring the absorbance at 490 nm using an Enzyme-labeling instrument (EX-800 type); all measurements were performed three times. Cell growth curve was completed using time as the abscissa and A value (mean ± SD) as the ordinate.
Detection of morphological change by transmission electron microscope
Uranyl acetate and lead citrate staining of cells were performed to detect morphological changes. Briefly, adherent PC-2 cells were treated with 200 μmol/L CoCl2 for 48 hours. After treatment, the treated cells were digested with pancreatin and fixed with 3% glutaraldehyde precooled in 4°C for 2 hours. To make ultra-thin sections of copper, cells were washed with phoisphate-buffered salein (PBS) once, fixed with 1% osmic acid for 1 hour, dehydrated by acetone and embedded in epoxide resin. After staining with uranyl acetate and lead citrate, the sections were examined by a Hitachi-800 transmission electron microscope [10].
Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) assay
PC-2 cells were seeded in 6 cm culture capsules and treated with concentration gradient CoCl2 (0, 50, 100, 150, 200 μmol/L) separately for 8 h. In the group of 200 μmol/L, we selected cells at 0 h, 4 h, 8 h and 12 h point for further experiment. And then treated with 2.0 μmol/L YC-1 (0, 50, 100, 150,) for 2 h. As previously described [11], cells collected at specified time were used to extract total RNA using the Trizol reagent following the manufacturer's instructions. 1 μgRNA synthetized cDNA through reverse transcriptase undergo listed below condition: 70°C 5 min, 42°C extended for 60 min, 95°C enzyme inactivated for 3 min and 4°C terminated reaction. Synthetical cDNA as template to carry out polymerase chain reaction. HIF-1α primer sequence (Invitrogen CO): 5'-ACTTCTGGATGCTGGTGATT-3' (sense) and 5'-TCCTCGGCTAGTTAG GGTAC -3' (anti-sense), amplification fragment was 325 bp, renaturation temperature was 55°C (cycling 35 times). β-actin, its primer sequence was 5'-GTTGCGTTACACCCTTTCTTG-3' (sense), 5'-TGCTGTCACCTTCACCGT TC-3' (anti-sense), amplification fragment was 133 bp, and renaturation temperature was 55°C (cycling 40 times). Amplification condition was below: pre-denaturized for 3 min at 95°C, denaturized for 30s at 95°C, renaturated for 30s at 55°C and extended for 30s at 72°C. PCR product was detected on agarose gel electrophoresis and ethidium bromide imaging system was used to make density index analysis. The expression intensity of HIF-1α mRNA was denoted with the ratio of the photodensity of the RT-PCR products of HIF-1α and β-actin.
Western blot analysis
As previously described [12], cells were washed with ice-cold PBS twice and lysed with lysis buffer containing 1% NP40, 137 mM NaCL, 20 mM Tris base(pH7.4), 1 mM DTT, 10% glycerol, 10 mg/mL Aprotinin, 2 mM sodium vanadate and 100 μM PMSF. Protein concentrations were determined using the PIERCE BCA protein assay kit. Protein was separated by 10% SDS-PAGE under denaturing conditions and transferred to nitrocellulose membranes. Membranes were incubated with an mouse HIF-1α monoclonal antibody (1:1000; Santa Cruz Biotechnology), followed by incubation in goat antimouse secondary antibody conjugated with horseradish peroxidase (1:1000; Santa Cruz Biotechnology). Immunoreactive proteins were visualized using enhanced chemiluminescence detection system (Amersham Biosciences)
Apoptosis detection by FCM
Apoptotic cells were differentiated from viable or necrotic ones by combined application of annexin V-FITC and propidium iodide (PI) (BD Biosciences Clontech, USA) [13]. The samples were washed twice and adjusted to a concentration of 1 × 106 cells/mL with 4°C PBS. The Falcon tubes (12 mm × 75 mm, polystyrene round-bottom) were used in this experiment, 100 μL of suspensions was added to each labeled tube, 10 μL of annexin V-FITC and 10 μL PI(20 μg/mL) were added into the labeled tube, incubated for at least 20 min at room temperature in the dark, then 400 μL of PBS binding buffer was added to each tube without washing and analyzed using FCM analysis (BD Biosciences Clontech, USA) as soon as possible (within 30 min). This assay was done quintuplicate.
Statistical analysis
All data were expressed by mean ± S.E.M. Statistical analyses were performed using SPSS 11.0 for Windows software. ANOVA (one-way analysis of variance) and Student's t-test were used to analyze statistical differences between groups under different conditions. P-value < 0.05 was considered statistically significant.
Results
The influence of hypoxia on PC-2 cells proliferation
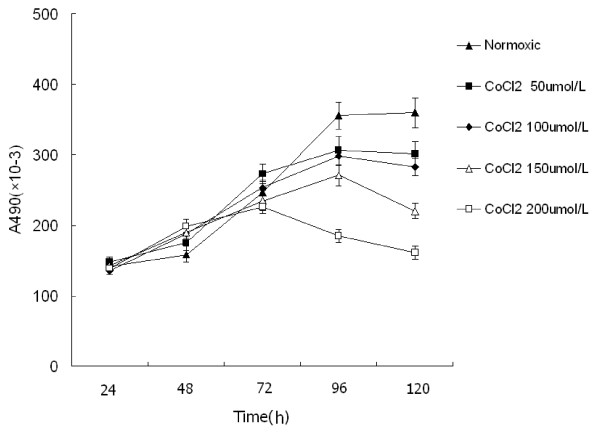
We studied the proliferation of PC-2 cells under hypoxia simulated by CoCl2 using MTT assay. As shown in Figure 1, the growth curve of cells under normoxia showed "S" shape: 24-48 h was detention period (slowly grow), 72-96 h were exponential phase of growth (rapidly proliferate), the following 24 h was platform period. Compared with the normoxic group, the cells of hypoxic group didn't show "S" shape. Following a 72 h hypoxic exposure, the proliferation speed of cells under hypoxia was faster, 72 h later, the speed was slower, achieved saturation density in advanced, went into platform period but gradually degraded at 96-120 h. Meanwhile, as the hypoxia became serious, this phenomenon was more conspicuous. After treated over 72 h, a dose- dependent inhibition of cell growth could be observed.
Figure 1.
The growth curve of PC-2 cells treated with different dose of CoCl2. Cell viability was determined by MTT method. This assay was performed in triplicate. Dose- dependent inhibition of cell growth could be observed after 72 h (P < 0.05, ANOVA analysis).
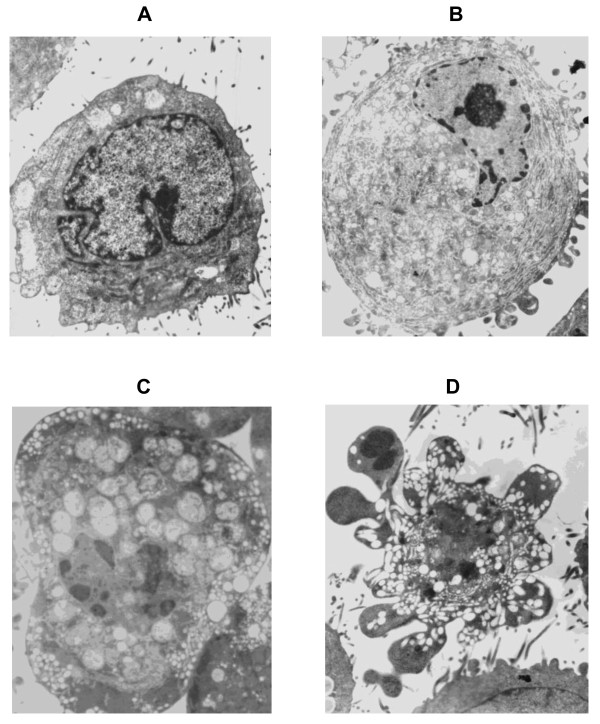
Morphological changes of PC-2 cells induced by hypoxia
By using transmission electron microscope, normal PC-2 cells were round and regular, with abundant organelles, the chromatin margination showed in few cells (Figure 2A). After treated with CoCl2 for 48 hours, part of nuclear membrane domed outward with a sharp angle. The following different apoptotic periods could be observed. (1) Early stage of apoptosis: the nuclei showed chromatin pyknosis, and were clustered on the inner border of karyotheca; cytoplasm condensation and swelling of mitochondria were observed in the inner segment; the nucleus was at one end of the cell with complete karyotheca and many mitochondria in the cytoplasm showed the early ultrastructure changes of apoptosis (Figure 2B). (2) Middle stage of apoptosis: in addition to the swelling of mitochondria and many vacuoles, the surface of cellular membrane process to crassitude, and the endoplasmic reticulum was abundant; the typical changes were karyopyknosis or karyorrhexis (Figure 2C). (3) Late stage of apoptosis: characterized by changes such as shrinkage, condensation of nuclear chromatin, fragmentation of nuclei and formation of apoptotic bodies (showed in Figure 2D)
Figure 2.
Morphological changes of PC-2 cells induced by hypoxia by transmission electron microscope. A: Normal pancreatic cancer PC-2 cells(×6000); B: PC-2 cells in early stage of apoptosis (×6000); C: PC-2 cells in middle stage of apoptosis cell(×6000); D: Apoptotic body(×6000).
Expression of HIF-1α mRNA detected by semi-quantitive RT-PCR
RT-PCR revealed HIF-1α mRNA expressed rarely in normoxic PC-2 cells, as CoCl2 density increased its expression gradually increased (Figure 3A). When cells treated with 200 μmol/L CoCl2, accompanied with the action time extended the expression of HIF-1αmRNA increased (Figure 3B). The correlation of CoCl2 and HIF-1α mRNA was a dose- and time-dependent manner. After treated with YC-1 for 2 h, overexpression of HIF-1αmRNA induced by CoCl2 was significantly down-regulated (Figure 3B).
Figure 3.
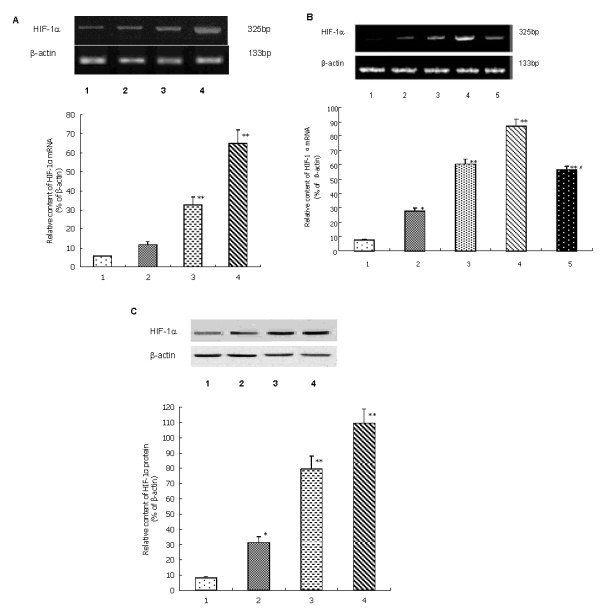
A: The expression of HIF-1α mRNA in PC-2 cells treated with different concentration of CoCl2. 1. Normoxia group; 2. CoCl2 100 μmol/L group; 3. CoCl2 150 μmol/L group; 4. CoCl2 200 μmol/L group. This assay was done quintuplicate. Values represent means ± standard deviations (n = 5) and were determined using the Student's t-test. *P < 0.05 and **P < 0.01 versus Normoxia group. B: The expression of HIF-1α mRNA in PC-2 cells treated with 200 μmol/L CoCl2 for different time. 1. 0 h; 2. 4 h; 3. 8 h; 4. 12 h; 5. YC-1 2 h. This assay was done quintuplicate. Values represent means ± standard deviations (n = 5) and were determined using the Student's t-test. *P < 0.05, **P < 0.01 versus 0h, #P < 0.05 versus 12h. C: The expression of HIF-1α protein in PC-2 cells treated with different concentration of CoCl2. 1. Normoxia group; 2. CoCl2 100 μmol/L group; 3. CoCl2 150 μmol/L group; 4. CoCl2 200 μmol/L group. This assay was done quintuplicate. Values represent means ± standard deviations (n = 5) and were determined using the Student's t-test. *P < 0.05 and **P < 0.01 versus Normoxia group.
Expression of HIF-1α protein detected by western blot analysis
The protein level of HIF-1α was measured in PC-2 cells treated with different doses of CoCl2 by Western blot analysis employing mouse monoclonal HIF-1α antibodies. As shown in Figure 3C, the amount of HIF-1α protein after CoCl2 treatment was significantly increased in a dose-dependent manner (P < 0.05). These data demonstrated that hypoxic microenvironment simulanted by CoCl2 could up-regulate HIF-1α expression.
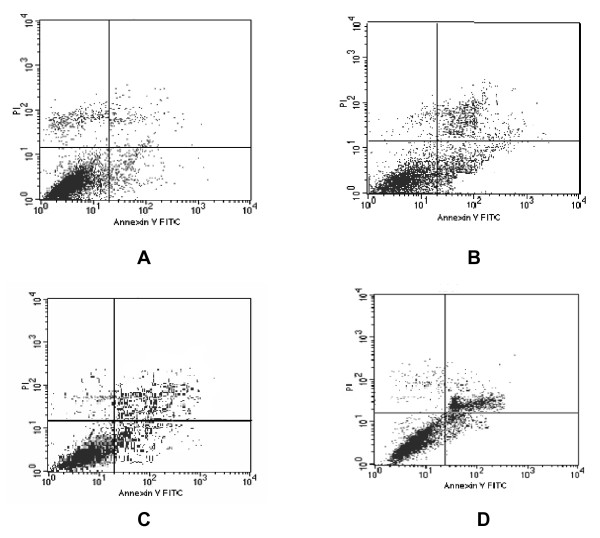
FCM analysis of cell apoptosis induced by hypoxia
After treatment with different doses of CoCl2 for 72 h, apoptosis induction was demonstrated using FCM analysis. Apoptotic cells were differentiated from viable or necrotic ones by combined application of annexin V-FITC and PI. Apoptotic and necrotic cells were distinguished according to annexin V-FITC reactivity and PI exclusion. As shown in Figure 4, in normoxic group, there were almost normal cells, rarely viable apoptotic cells; while in hypoxic group, the rate of apoptotic cells was gradually increased along with increasing concentrations of CoCl2. The rate of apoptosis in normoxic, 100-200 μmol/L CoCl2 group were 10.77%, 34.32%, 40.17%, 52.30%, respectively. Furthermore, apoptotic cells gradually increased in a dose-dependent manner.
Figure 4.
Flow cytometry was used to observe the apoptosis of PC-2 cells by staining with annexinV-FITC/PI. A. Normoxia group; B. CoCl2 100 μmol/L group; C. CoCl2 150 μmol/L group; D. CoCl2 200 μmol/L group.
Discussion
More recently, experimental and clinical studies demonstrated that intra-tumor hypoxia might be a key factor in tumor microenvironment promoting invasive growth and metastasis [14]. The increased malignancy of hypoxic tumors has been attributed to the ability of hypoxia to select for cells with diminished apoptotic potential and to induce their clonally expansion [15]. Since the hypoxic phenomenon in tumors was revealed, more and more evidence indicated hypoxia existed in solid tumor generally [16].
Pancreatic cancer is common malignant tumor of digestive system which has high malignancy, difficulty in treatment and poor prognosis. And less than 10% of pancreatic cancer is resectable when being diagnosised and 5-year overall survival rate is less than 5% [17]. During the development of pancreatic cancer, the blood can't supply the tumor nourishment, thus the tumor are hypoxic partly, while hypoxia makes the tumor cell more malignant. In this way, the rapid growth and the hypoxia are unity of opposites in tumors [18].
CoCl2 is a chelator which instead of Fe2+ in hemoglobin, and then damage cell's reception of oxygen [19]. The mechanism of CoCl2 simulating hypoxia is similar with hypoxic microenvironment in vivo, because they have identical signal transduction and transcription regulation. Moreover previous research demonstrated CoCl2 correlated with proliferation and apoptosis in human carcinoma cells [20,21]. In our study, we treated PC-2 cells with CoCl2 to simulate hypoxic microenvironment, MTT assay revealed along with the increased CoCl2 concentration, the exponential phase of PC-2 cells was earlier in advanced and persisted shorter, cells grew slower and went into platform period early(Figure 1). It is reasonable to assume that the step down in PC-2 cell proliferation correlated with the increased hypoxia, hypoxic microenvironment could slow down the speed of tumor growth.
HIF-1α, a transcription factor regulating genes' expression induced by hypoxia, is a key molecular player in the hypoxic response [22]. HIF-1α is generally resided in mammal and human tissue in hypoxic condition, it has been found over-expressed in about 70% tumor [5-7]. Experiment showed that under hypoxic the transcriptive activity of HIF-1α was increasing, which indicated that hypoxic microenvironment might increase the genetic transcriptional level of HIF-1α to regulate the expression of downstream gene [22,23]. However, some scholars presumed hypoxic microenvironment could enhance the stability of HIF-1α [24]. Our present research indicated HIF-1α obviously increased at both protein level and mRNA level in PC-2 cells under hypoxic microenvironment, and it was positive correlated with the hypoxic time and the density of CoCl2. This suggested the level of hypoxia was coinciding with the expression of HIF-1α.
Whether HIF-1α can promote tumor cell apoptosis or anti- apoptosis, the opinion didn't reach unify, different research suggest converse results. Some date indicated overexpressed HIF-1α could promote apoptosis by activating Bcl-2 and Bcl-Xl or enhancing the stability of p53 [25]. On the other hand, experiment displayed HIF-1α could up-regulate the VEGF and GLUT1 to make tumor cell resist to apoptosis, inhibition of HIF-1α could promote apoptosis [26]. In our research, under electron microscope, PC-2 cells in hypoxic microenvironment were found in different apoptotic stage (Figure 2A-D), most were in early stage. The FCM analysis showed that the apoptotic rate of normal control group, 100 μmol/L group, 150 μmol/L group and 200 μmol/L group, was 10.77%, 34.32%, 40.17%, 52.30%, respectively. These results were consistent with Luo's research [27].
In conclusion, our study suggested that hypoxic microenvironment can effectively induce apoptosis and influence cell proliferation in PC-2 cells, and the mechanism may be concerned with the up-regulation of HIF-1α.
Conflicts of interest
The authors declare that they have no competing interests.
Authors' contributions
DZJ and WXJ designed the research. DZJ, GJ, MXB, YK and KHF performed the experiments throughout this research. LXX, JZZ and GHT contributed to the reagents, and participated in its design and coordination. DZJ and GJ analyzed the data; DZJ and MXB wrote the paper. Co-first authors: DZJ and GJ. All authors have read and approved the final manuscript.
Contributor Information
Zhi-Jun Dai, Email: dzj0911@126.com.
Jie Gao, Email: Gxej_cn@sina.com.
Xiao-Bin Ma, Email: binbinmxb@sohu.com.
Kun Yan, Email: topestyk@163.com.
Xiao-Xu Liu, Email: xiaoxuliu@yahoo.com.
Hua-Feng Kang, Email: kanghf73@yahoo.com.cn.
Zong-Zheng Ji, Email: zongzj@126.com.
Hai-Tao Guan, Email: guanhaitao@csco.org.cn.
Xi-Jing Wang, Email: Wangxijing@21cn.com.
Acknowledgements
This work was supported by The Science and Technology Foundation of Shaanxi Province, China, No. 2010 K01-138 and Sci-tech Program of Xi'an City, China, No. HM1117.
References
- Piret JP, Mottet D, Raes M, Michiels C. CoCl2, a chemical inducer of hypoxia inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann N Y Acad Sci. 2002;973(5):443–447. doi: 10.1111/j.1749-6632.2002.tb04680.x. [DOI] [PubMed] [Google Scholar]
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56(19):4509–4515. [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via denovoprotein synthesis binds to the human erythropoietin gene enhance ratasite required for transcriptional activation. Mol Cell Bio. 1992;12(12):5447–5456. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology. 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia inducible factors HIF-1 alpha and HIF-2 alpha in normal human tissues, cancers and tumor-associated macrophages. Am J Pathol. 2000;157(2):411–421. doi: 10.1016/S0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami K, Kakeji Y, Oda S, Irie K, Yonemura T, Konishi F, Maehara Y. Clinicopathologic significance of hypoxia inducible factor 1alpha overexpression in gastric carcinomas. J Surg Oncol. 2006;94(2):149–154. doi: 10.1002/jso.20568. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Hiroi S, Tominaga S, Aida S, Kasamatsu H, Matsuyama S, Matsuyama T, Kawai T. Expression of hypoxia-inducible factor-1alpha protein predicts survival in patients with transitional cell carcinoma of the upper urinary tract. Clin Cancer Res. 2005;11(7):2583–2590. doi: 10.1158/1078-0432.CCR-04-1685. [DOI] [PubMed] [Google Scholar]
- Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1 a expression by the epidermal growth factor phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60(6):1541–1545. [PubMed] [Google Scholar]
- Ma L, Xie YL, Yu Y, Zhang QN. Apoptosis of human gastric cancer SGC-7901 cells induced by mitomycin combined with sulindac. World J Gastroenterol. 2005;11(12):1829–1832. doi: 10.3748/wjg.v11.i12.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Yang CL, Qu Y, Wei HY, Zhang TT, Zhang NJ. The flavonoid component isorhamnetin in vitro inhibits proliferation and induces apoptosis in Eca-109 cells. Chem Biol Interact. 2007;167(2):153–160. doi: 10.1016/j.cbi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Guan HT, Xue XH, Dai ZJ, Wang XJ, Li A, Qin ZY. Downregulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J Gastroenterol. 2006;12(18):2901–2907. doi: 10.3748/wjg.v12.i18.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Mallick SK, Maiti S, Maiti TK. Antitumor and proapoptotic effect of Abrus agglutinin derived peptide in Dalton's lymphoma tumor model. Chem Biol Interact. 2008;174(1):11–18. doi: 10.1016/j.cbi.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Dai ZJ, Gao J, Ji ZZ, Wang XJ, Ren HT, Liu XX, Wu WY, Kang HF, Guan HT. Matrine Induces Apoptosis in Gastric Carcinoma Cells via Alteration of Fas/FasL and Activation of Caspase-3. Journal of Ethnopharmacology. 2009;123:91–96. doi: 10.1016/j.jep.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Furlan D, Sahnane N, Carnevali I, Cerutti R, Uccella S, Bertolini V, Chiaravalli AM, Capella C. Up-regulation and stabilization of HIF-1a in colorectal carcinomas. Surg Oncol. 2007;16:S25–27. doi: 10.1016/j.suronc.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Thomlinson RH. An experimental method for comparing treatments of intact malignant tumours in animals and its application to the use of oxygen in radiotherapy. Br J Cancer. 1960;14(6):555–576. doi: 10.1038/bjc.1960.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azria D, Ychou M, Jacot W, Thezenas S, Lemanski C, Senesse P, Prost P, Delard R, Masson B, Dubois JB. Treatment of unresectable, locally advanced pancreatic adenocarcinoma with combined radiochemotherapy with 5-fluorouracil and cisplatin. Pancreas. 2002;25(4):360–365. doi: 10.1097/00006676-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Ardyanto TD, Osaki M, Tokuyasu N, Nagahama Y, Ito H. CoCl2-induced HIF-1alpha expression correlates with proliferation and apoptosis in MKN-1 cells: a possible role for the PI3K/Akt pathway. Int J Oncol. 2006;29(3):549–555. [PubMed] [Google Scholar]
- Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994;269(6):4355–4359. [PubMed] [Google Scholar]
- Liu XH, Kirschenbaum A, Yao S, Stearns ME, Holland JF, Claffey K, Levine AC. Upregulation of vascular endothelial factor by cobalt chloride-simulated hypoxia is mediated by persistent induction of cyclooxygenase-2 in metastatic human prostate cancer cell line. Clin Exp Metastasis. 1999;17(8):687–694. doi: 10.1023/A:1006728119549. [DOI] [PubMed] [Google Scholar]
- Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42(3):358–364. doi: 10.1016/j.jhep.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Covello KL, Simon MC, Keith B. Targeted replacement of hypoxia-inducible factor-1 alpha by a hypoxia-inducible factor-2alpha knock-in allele promotes tumor growth. Cancer Res. 2005;65(6):2277–2286. doi: 10.1158/0008-5472.CAN-04-3246. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88(4):1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Nat Acad Sci USA. 1997;94(11):5667–5672. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DL, Li MY, Luo JY, Gu W. Direct interactions between HIF-1alpha and Mdm2 modulate p53 function. J Biol Chem. 2003;278(16):13595–13598. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- Dai S, Huang ML, Hsu CY, Chao KS. Inhibition of hypoxia inducible factor lalpha causes oxygen-independent cytotoxicity and induces p53 independent apoptosis in glioblastoma cells. Int J Radiat Oncol Bio Phys. 2003;55(4):1027–1036. doi: 10.1016/S0360-3016(02)04507-8. [DOI] [PubMed] [Google Scholar]
- Luo FM, Liu XJ, Yan NH, Li SQ, Cao GQ, Cheng QY, Xia QJ, Wang HJ. Hypoxia-inducible transcription factor-1alpha promotes hypoxia- induced A549 apoptosis via a mechanism that involves the glycolysis pathway. BMC Cancer. 2006;6:26–32. doi: 10.1186/1471-2407-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]