Abstract
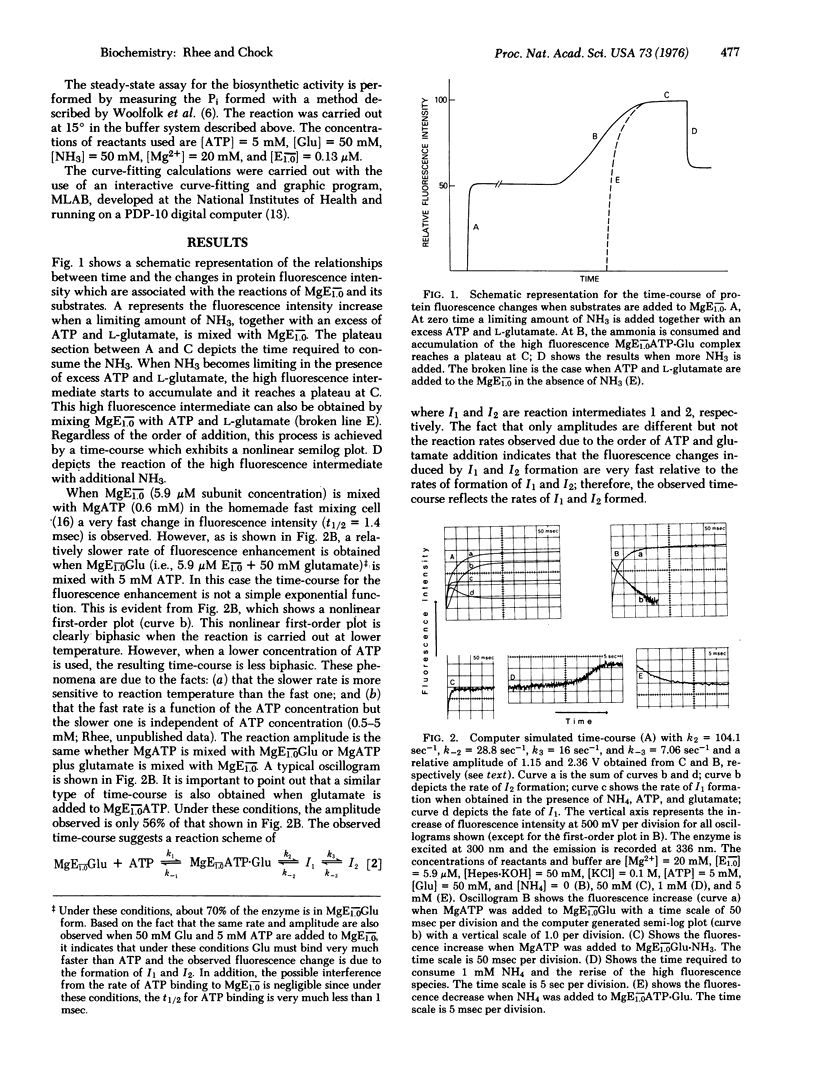
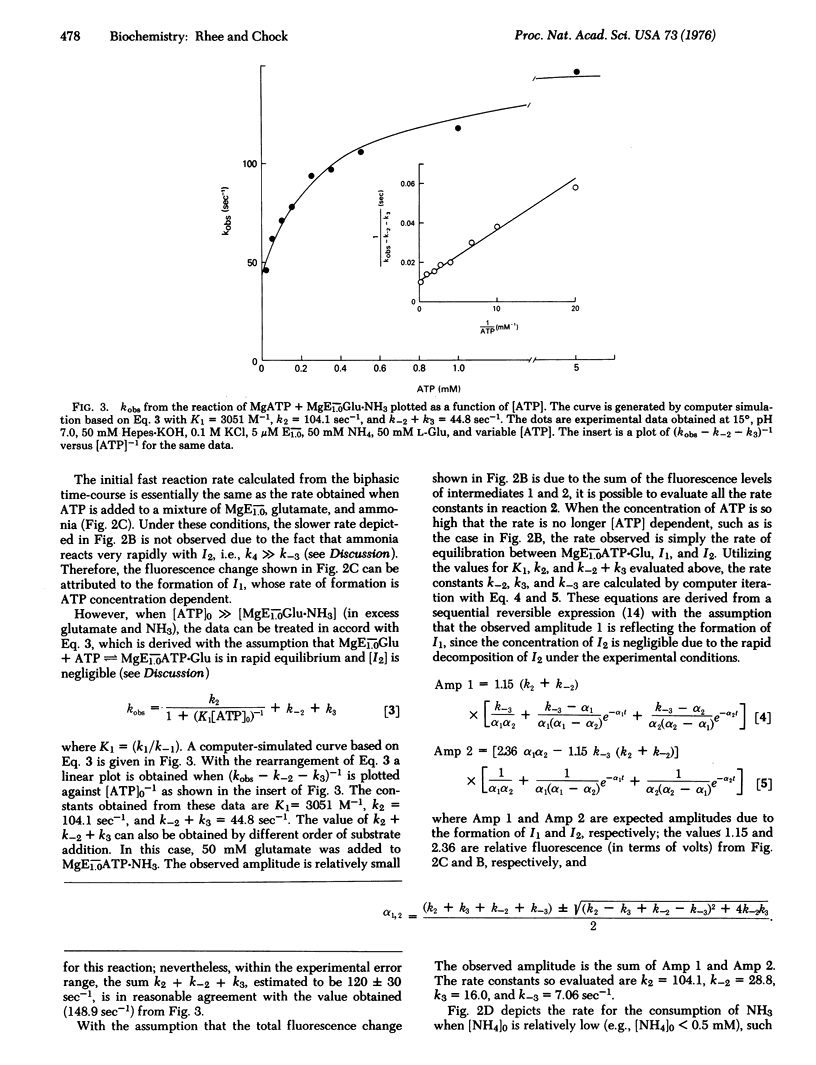
Fast reaction techniques were used to study the kinetics of protein fluorescence intensity changes that are associated with the reactions of unadenylylated Escherichia coli glutamine synthetase [L-glutamate: ammonia ligase (ADP-forming), EC 6.3.1.2] with its substrates. It was established that the synthesis of glutamine occurs by a stepwise mechanism. During the catalytic process two fluorometrically distinct intermediates were observed. Both forward and reverse rate constants which lead to the formation and consumption of these intermediates were evaluated. The catalytic rate constant, kc, which was calculated from these rate constants agrees well with the values of kc which were determined by direct measurement of the overall biosynthetic activities by means of stopped-flow technique or the steady-state assay method.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Ginsburg A., Yeh J., Hennig S. B., Denton M. D. Some effects of adenylylation on the biosynthetic properties of the glutamine synthetase from Escherichia coli. Biochemistry. 1970 Feb 3;9(3):633–649. doi: 10.1021/bi00805a025. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Shelton E., Stadtman E. R. Zinc-induced paracrystalline aggregation of glutamine synthetase. Arch Biochem Biophys. 1974 Jul;163(1):155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- Wedler F. C., Boyer P. D. Substrate binding and reaction intermediates of glutamine synthetase (Escherichia coli W) as studied by isotope exchanges. J Biol Chem. 1972 Feb 25;247(4):984–992. [PubMed] [Google Scholar]
- Wedler F. C. Mechanisms of substrate binding with glutamine synthetase. Equilibrium isotope exchanges with the ovine brain, pea seed, and Escherichia coli enzymes. J Biol Chem. 1974 Aug 25;249(16):5080–5087. [PubMed] [Google Scholar]
- Weisbrod R. E., Meister A. Studies on glutamine synthetase from Escherichia coli. Formation of pyrrolidone carboxylate and inhibition by methionine sulfoximine. J Biol Chem. 1973 Jun 10;248(11):3997–4002. [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]


