Abstract
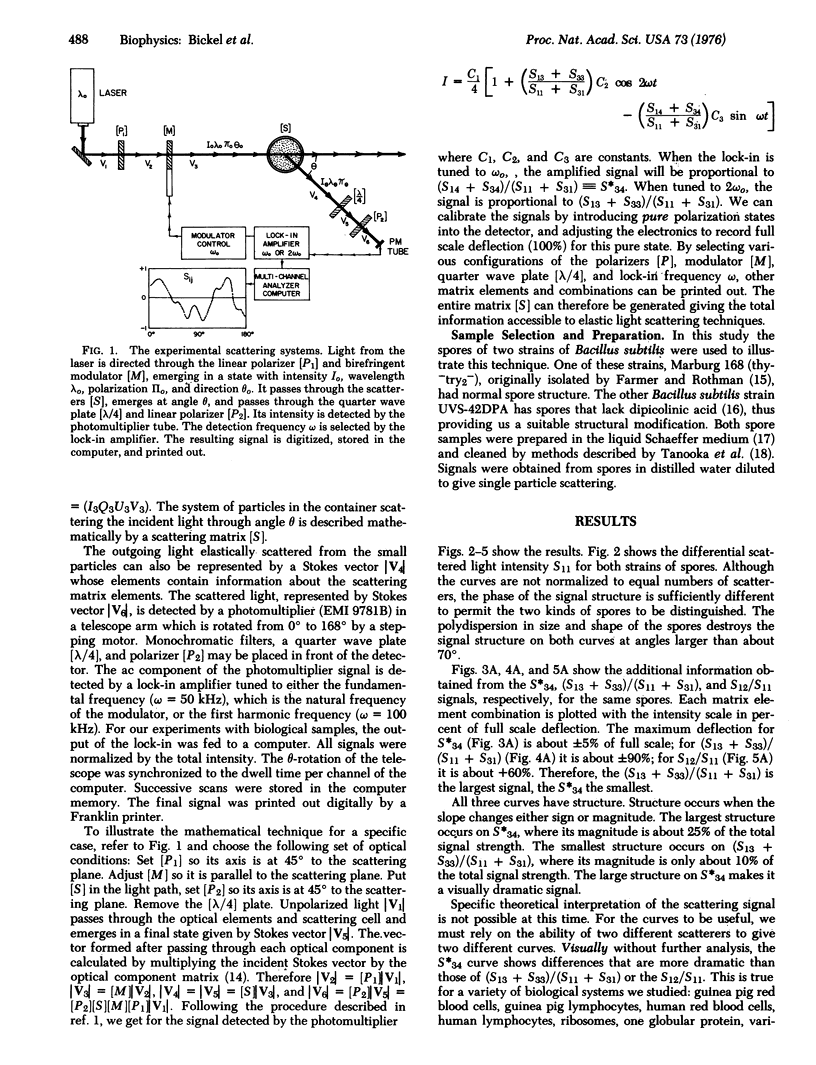
We demonstrate that a newly developed instrument which measures all polarization and intensity information contained in differentially and elastically scattered light has valuable applications in biology. The polarization states of light scattered differentially from suspensions of biological scatters are shown to contain structural information about those systems. The scatterers are discussed in the context of a 16 component matrix which completely characterizes the scattering process. The instrument and method are described in terms of the corresponding matrix algebra. We also discuss the use of the instrument as a device for distinguishing between closely related structural systems and as a tool for following time-dependent structural changes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FARMER J. L., ROTHMAN F. TRANSFORMABLE THYMINE-REQUIRING MUTANT OF BACILLUS SUBTILS. J Bacteriol. 1965 Jan;89:262–263. doi: 10.1128/jb.89.1.262-263.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J., Holzwarth G. Optical activity of membrane suspensions: calculation of artifacts by Mie scattering theory. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2365–2369. doi: 10.1073/pnas.68.10.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth G., Gordon D. G., McGinness J. E., Dorman B. P., Maestre M. F. Mie scattering contributions to the optical density and circular dichroism of T2 bacteriophage. Biochemistry. 1974 Jan 1;13(1):126–132. doi: 10.1021/bi00698a020. [DOI] [PubMed] [Google Scholar]
- MAESTRE M. F., TINOCO I., Jr OPTICAL ROTATORY DISPERSION OF BACTERIOPHAGES. J Mol Biol. 1965 May;12:287–289. doi: 10.1016/s0022-2836(65)80301-1. [DOI] [PubMed] [Google Scholar]
- Maestre M. F., Tinoco I., Jr Optical rotatory dispersion of viruses. J Mol Biol. 1967 Feb 14;23(3):323–335. doi: 10.1016/s0022-2836(67)80108-6. [DOI] [PubMed] [Google Scholar]
- Salzman G. C., Crowell J. M., Goad C. A., Hansen K. M., Hiebert R. D., LaBauve P. M., Martin J. C., Ingram M. L., Mullaney P. F. A flow-system multiangle light-scattering instrument for cell characterization. Clin Chem. 1975 Aug;21(9):1297–1304. [PubMed] [Google Scholar]
- TAKAHASHI I. TRANSDUCTION OF SPOROGENESIS IN BACILLUS SUBTILIS. J Bacteriol. 1965 Feb;89:294–298. doi: 10.1128/jb.89.2.294-298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanooka H., Terano H., Otsuka H. Increase of thymidine, thymidylate and deoxycytidine kinase activites during germination of bacterial spores. Biochim Biophys Acta. 1971 Jan 1;228(1):26–37. doi: 10.1016/0005-2787(71)90543-0. [DOI] [PubMed] [Google Scholar]
- Wyatt P. J., Phillips D. T. Structure of single bacteria from light scattering. J Theor Biol. 1972 Dec;37(3):493–501. doi: 10.1016/0022-5193(72)90087-2. [DOI] [PubMed] [Google Scholar]


