Abstract
In an initial preliminary screen we identified factors associated with controlling Drosophila aging by examining longevity in adults where EP elements induced over-expression or antisense-RNA at genes adjacent to each insertion. Here, we study 45 EP lines that initially showed at least 10% longer mean lifespan than controls. These 45 lines and a daughterless (da)-Gal4 stock were isogenized into a CS10 wild-type background. Sixteen EP lines corresponding to 15 genes significantly extended lifespan when their target genes were driven by da-Gal4. In each case, the target genes were seen to be over-expressed. Independently derived UAS-gene transgenic stocks were available or made for two candidates: ImpL2 which is ecdysone-inducible gene L2, and CG33138, 1,4-alpha-glucan branching enzyme. With both, adult lifespan was increased upon over-expression via the GeneSwitch inducible Gal4 driver system. Several genes in this set of 15 correspond to previously discovered longevity assurance systems such as insulin/IGF-1 signaling, gene silencing, and autophagy; others suggest new potential mechanisms for the control of aging including mRNA synthesis and maturation, intracellular vesicle trafficking, and neuroendocrine regulation.
Keywords: Aging, Misexpression screen, Longevity genes, ImpL2
1. Introduction
Aging involves progressive functional deterioration accompanying reduced reproduction, increased mortality and sensitivity to diseases with the advance of age (Kirkwood and Austad, 2000). Multiple genetic and environmental factors are thought to influence the progress of these phenotypes (Finch and Tanzi, 1997), and in recent years work with model organisms has described numerous genes that increase lifespan when mutated or misexpressed. Genes affecting lifespan have been isolated from studies with yeast, C. elegans, Drosophila and mice (Guarente and Kenyon, 2000). Many of these longevity genes comprise cellular signaling pathways including: insulin/IGF-1 signaling (Kenyon et al., 1993; Tatar et al., 2001), target of rapamycin signaling (Harrison et al., 2009; Lee et al., 2010a; Luong et al., 2006), and the c-Jun N-terminal kinase pathway (Wang et al., 2003). Others involve genome regulation, stress responses and the integration of systems, including: gene silencing/deacetylation (Rosenberg and Parkhurst, 2002; Tanny et al., 1999), control of telomerase (Blasco, 2005), oxidation responses and chaperones (Orr and Sohal, 1994; Tatar et al., 1997), DNA or protein repair (Matheu et al., 2007), reproduction (Flatt et al., 2008; Hsin and Kenyon, 1999), and neuron function (Cvejic et al., 2004; De Luca et al., 2003; Lin et al., 1998).
A key approach of such analyses with Drosophila involves the P-element modular-misexpression system (Rørth et al., 1998). This allows a conditional over-expression or knock-down of genes tagged by transpositional insertion of an engineered P-element that carries the enhancer and the basal promoter, thereby designated as EP. The EP contains 14 copies of Upstream Activator Sequence (UAS), to which Gal4 binds and drives transcription of flanking genomic DNA downstream to the basal promoter. When a fly has an EP element inserted in the 5’ untranslated region (UTR) or promoter region of a gene in the orientation of normal transcription (+), the gene will be over-expressed in the progenies of EP flies mated with a fly expressing Gal4. When a fly has an EP element inserted within a coding region of a gene in the orientation opposite to normal transcription, antisense RNA is produced in the presence of Gal4 causing reduced expression of the corresponding gene (Rørth et al., 1998).
Over a number of years we have performed a preliminary large-scale screen to find new longevity genes by analyzing lifespans of EP lines under control of a heat shock 70 (hsp70)-Gal4 driver that moderately induces the EP UAS elements when flies are maintained at 29 °C. Other results of this initial study that dealt with more than 27,000 EP lines will be reported elsewhere. Here, 45 lines were non-systematically selected from a large set of potential candidate lines that show at least 10% longer mean lifespan (MLS) when driven by an hsp70-Gal4 driver (hsp70-Gal4>EP) relative to controls (hsp70-Gal4/+). These 45 EP lines and flies possessing a ubiquitously expressing daughterless (da)-Gal4 driver were backcrossed to CS10 wild-type flies. The survival of adults from these isogenic EP lines driven by da-Gal4 was analyzed at 25 °C. This analysis confirmed 15 genes from this set to extend Drosophila lifespan when misexpressed, including genes with functions in chromatin remodeling/silencing, cell matrix, metabolism, and insulin/IGF ligand binding. This longevity assurance was further confirmed for two genes, ImpL2 and CG33138, with over-expression from independently generated UAS-transgenes.
2. Materials and Methods
2.1. Fly Stocks
EP of the GX series were generated at GenExel Inc. by mobilization of an EP element after crossing with P[ry+,Dr;Δ2-3] (Rørth, 1996). Some GX series lines are currently available from KAIST Bio Medical Research Center (http://genexel.kaist.ac.kr/mapview3/) or the Bloomington Drosophila Stock Center. Lines labeled only with EP numbers were generated by Rørth and were provided by the Szeged Stock Center (Rørth et al., 1998). Lines with hsp70-Gal4 (Brand and Perrimon, 1993) and S106-GeneSwitch (GS)-Gal4 (Roman et al., 2001) were from the Bloomington Drosophila Stock Center. CS10 wild-type, da-GS-Gal4, and UAS-ImpL2 flies were obtained from Minoru Saitoe (Yamazaki et al., 2007), Véronique Monnier (Tricoire et al., 2009), and Hugo Stocker (Honegger et al., 2008), respectively. We generated UAS-CG33138 (chromosome 3) for this study.
Males of all 45 homozygous EP lines and da-Gal4 flies were first crossed with virgin females of CS10. Their female progeny were mated with CS10 males, and this backcross was repeated for 6 to 8 times. After the final cross, red-eyed males and virgin females were mated to make homozygous EP lines which were then approximately isogenic with CS10. These EP lines and da-Gal4 flies are designated as EPCS10/EPCS10 and da-Gal4CS10/da-Gal4CS10.
2.2. UAS-transgenic Flies
To produce UAS-CG33138, the open reading frame of clone RE12027 (Drosophila Genomics Resource Center, Bloomington, USA) was inserted into BamHI/XhoI sites of pUAST vector. Transgenic flies were generated by standard germ line transformation in the w1118 background. Positions of the inserted UAS sequence were mapped by inverse PCR.
2.3. Longevity
In the preliminary screen, males from 27,157 EP lines were crossed to hsp70-Gal4 females in a series of blocks. From each cross, between 20 and 255 F1 male progeny were maintained in vials of 20 flies, with deaths counted weekly when adults were transferred to new vials. In every block, lifespan was recorded for contemporary, similarly handled control adults (hsp70-Gal4/+ from the cross of hsp70-Gal4 females and w1118 males). Overall, 8,736 EP lines had a MLS that was at least 10% greater than the across-block average MLS of the control cohorts. From these 8,736 EP lines, 45 lines were selected non-systematically for follow-up study in this report.
After backcrossing to CS10, virgins of da-Gal4 (da-Gal4CS10/da-Gal4CS10) were mated with males of each selected EP line (EPCS10/EPCS10). Male progeny of da-Gal4CS10/EPCS10 from each cross were collected within 48 hours after eclosion and maintained in a transparent polystyrene chamber with mesh ventilation (ø 40 mm, 72×72×100 mm; SPL, Republic of Korea). Near the bottom of the chamber, an adaptor connects to a vial of regular fly food. Each chamber contained about 100 (a range of 80-136) flies. Two to five chambers were allocated for each genotype. These chambers were maintained at 25°C with 60% relative humidity and 12 h light: 12 h dark. Dead flies were counted every 2-3 days and removed from the chamber, when fresh food (3% cornmeal, 10% sucrose and 10% yeast) was supplied. Each EP line was also crossed to the coisogenic CS10 (+CS10/+CS10) and to coisogenic da-Gal4 (da-Gal4CS10/da-Gal4CS10) to produce control EPCS10/+CS10 and da-Gal4CS10/+CS10 progeny. Lifespan data of all da-Gal4CS10/EPCS10 lines, their two controls and a cohort of the coisogenic CS10 stock were collected simultaneously.
To measure lifespan of the independently derived UAS-CG33138 and UAS-ImpL2 lines, females from these stocks were crossed to males from the da-GS-Gal4 (ubiquitous) and S106-GS-Gal4 (abdominal fat body-specific) stocks. Male and female offspring were maintained separately in cages as above but with food that either contained 200 μM RU486 (mifepristone, Sigma, USA) to induce gene expression or vehicle only (ethanol) as control. RU486 at this concentration alone does not affect longevity (Tricoire et al., 2009).
2.4. Real Time RT-PCR
Adults, 3-5 day after eclosion, were frozen in liquid nitrogen and stored at − 80°C until analysis. After treating with DNase I (Invitrogen, USA) to remove trace genomic DNA, total RNA from homogenized whole body lysates was prepared with RNAiso reagent (Takara, Japan). Total RNA (5 μg) was reverse-transcribed using the PrimeScript RT reagent Kit (Takara, Japan). Real-time RT-PCR was performed using SYBR Premix Ex-Taq II (Takara, Japan) on an ABI Prism 7000 Sequence Detection System (Applied Biosystems, USA). Mean induction folds were calculated from values of 3-6 independent experiments and statistically evaluated by chi-square test.
2.5. Survival statistics
Data from the assays with the 45 selected lines and with the UAS-transgene lines were converted to life tables by the extinct cohort method, and the mean life span (MLS) was estimated from Kaplan-Meier life tables (Rosner, 1995). The proportion change in MLS (increased lifespan, ILS) was estimated from the ratio of the MLS of EPCS10/da-Gal4CS10 to EPCS10/+CS10. Differences in mortality rate between genotypes were evaluated by Log-Rank tests (Rosner, 1995) for EPCS10/+CS10 versus da-Gal4CS10/EPCS10, da-Gal4CS10/+CS10 versus da-Gal4CS10/EPCS10, and +CS10/+CS10 versus da-Gal4CS10/EPCS10.
3. Results and Discussion
Forty-five EP lines (Table 1) were selected from the preliminary subset that lived at least 10% longer than controls. In the preliminary screen, the MLS of the control (hsp-Gal4/+) was 27.8 days, on average across blocks. The MLS of the 45 selected lines ranged from 30.5 to 43.1 days. The purpose of the current study was to determine for this subset whether and which of these preliminary longevity differences can be verified through independent, robust genetic experiments.
Table 1. Longevity of 45 EP lines induced by da-Gal4.
Forty-five isogenic EP lines, in which an EP is inserted in the same orientation of normal transcription, were crossed with either isogenic CS10 or isogenic da-Gal4. Lifespans of their male progeny were measured at 25°C. The target genes of these EP lines are categorized into 8 groups, based on their functions. Fifteen EP lines that as da-Gal4CS10 >EPCS10 presented significantly longer mean lifespan than EPCS10/+CS10, da-Gal4CS10/+CS10 (33.6 d) and +CS10/+CS10 (33.4 d) were selected as longevity candidates (bold type; p < 0.001 by Log-Lank test). Five EP lines (GX193, GX7554, GX5503-1, GX8277, and GX6548) were lethal when induced by da-Gal4. Human homologs of these genes are indicated in the parentheses. Mean lifespan (MLS), maximum lifespan (MaxLS, day when the final survivor died), and increased lifespan (ILS, ratio of da-Gal4CS10 >EPCS10 to EPCS10/+CS10) are presented with the number (n) of flies tested.
| EP line | Gene | Description (Human Homolog) | /+CS10 | /da- Gal4CS10 | ILS |
Log-Rank Probability |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +CS10 /+CS10 | Gal4CS10 /+CS10 | EPCS10 /+CS10 | ||||||||||
| MLS | MaxLS | (n) | MLS | MaxLS | (n) | vs. da -Gal4CS10 >EPCS10 | ||||||
| Transcription & translation | ||||||||||||
| GX193 | CG10209 | DNA binding | 39.4 | 63.0 | (361) | lethal | – | – | – | – | ||
| GX2970 | kis | DNA helicase (CDH7) | 37.8 | 62.0 | (357) | 51.4 | 76.0 | (382) | 1.36 | < 0.001 | < 0.001 | < 0.001 |
| GX6561 | kis | DNA helicase (CDH7) | 36.6 | 59.0 | (348) | 45.3 | 76.0 | (345) | 1.24 | < 0.001 | < 0.001 | < 0.001 |
| GX7554 | dan | Transcription factor | 42.7 | 71.0 | (360) | lethal | – | – | – | – | ||
| GX8261 | gug | Histone deacetylase | 38.7 | 62.0 | (385) | 37.7 | 80.0 | (358) | 0.97 | < 0.001 | < 0.001 | 0.293 |
| GX8304 | Sin3A | Transcription repressor (SIN3A) | 50.5 | 78.0 | (251) | 57.2 | 80.0 | (237) | 1.13 | < 0.001 | < 0.001 | < 0.001 |
| GX8403 | RpL13A | Ribosomal protein L13 (RPL13A) | 44.0 | 66.0 | (172) | 44.2 | 66.0 | (220) | 1.00 | < 0.001 | < 0.001 | 0.687 |
| GX21702 | Rat1 | 5’-3’ exoribonuclease (XRN2) | 39.4 | 62.0 | (285) | 34.8 | 56.0 | (323) | 0.88 | 0.051 | 0.320 | < 0.001 |
| GX21702-1 | sm | Heterogeneous nuclear ribonucleoprotein L (HNRNPL) | 40.4 | 62.0 | (383) | 52.1 | 72.0 | (257) | 1.29 | < 0.001 | < 0.001 | < 0.001 |
| GX25754 | CG4901 | ATP-dependent RNA helicase (DHX33) | 37.5 | 70.0 | (613) | 34.6 | 63.0 | (594) | 0.92 | 0.008 | 0.034 | < 0.001 |
| GX31631 | Thor | Eukaryotic initiation factor 4E binding | 46.2 | 75.0 | (593) | 46.7 | 77.0 | (567) | 1.01 | < 0.001 | < 0.001 | 0.567 |
| GX46252 | mam | Transcription coactivator | 43.3 | 70.0 | (611) | 41.8 | 72.0 | (586) | 0.97 | < 0.001 | < 0.001 | 0.107 |
| EP(2)2559 | CG3927 | RNA binding | 31.4 | 60.0 | (559) | 34.9 | 65.0 | (602) | 1.11 | 0.013 | 0.080 | < 0.001 |
| Cell cycle | ||||||||||||
| GX822 | CycG | Cyclin-dependent protein kinase regulator (CCNG2) | 40.2 | 69.0 | (577) | 38.1 | 73.0 | (549) | 0.95 | < 0.001 | < 0.001 | 0.420 |
| GX11242 | CycB3 | Cyclin B3 | 43.6 | 68.0 | (362) | 39.4 | 64.0 | (327) | 0.90 | < 0.001 | < 0.001 | < 0.001 |
| Signal transduction | ||||||||||||
| GX4499 | ImpL2 | Ecdysone-inducible gene L2 | 39.8 | 60.0 | (391) | 45.7 | 74.0 | (385) | 1.15 | < 0.001 | < 0.001 | < 0.001 |
| GX47280-1 | SIFR | Neuropeptide receptor (NPFFR2) | 38.4 | 66.0 | (364) | 47.1 | 68.0 | (381) | 1.23 | < 0.001 | < 0.001 | < 0.001 |
| GX5503-1 | Rapgap1 | Ras GTPase activator (RAP1GAP) | 36.3 | 58.0 | (350) | lethal | – | – | – | – | ||
| GX6589 | cv-2 | Cysteine-rich domain (BMPER) | 34.6 | 60.0 | (388) | 34.8 | 58.0 | (377) | 1.01 | 0.028 | 0.082 | 0.548 |
| GX8277 | gom | Calcium ion binding | 37.1 | 56.0 | (317) | lethal | – | – | – | – | ||
| GX8630 | Lrch | Leucine-rich-repeats and calponin homology domain protein (LRCH2) | 39.4 | 69.0 | (631) | 40.0 | 65.0 | (539) | 1.02 | < 0.001 | < 0.001 | 0.673 |
| GX8689 | vimar | Ral GTPase binding (RAP1GDS1) | 33.6 | 66.0 | (330) | 33.6 | 60.0 | (371) | 1.00 | 0.597 | 0.977 | 0.474 |
| GX16168 | CalpA | Calcium-dependent cysteine-type endopeptidase (CAPN9) | 37.5 | 64.0 | (368) | 42.0 | 60.0 | (347) | 1.12 | < 0.001 | < 0.001 | < 0.001 |
| EP(2)2612 | CG8155 | Rab GTPase activator (TBC1D25) | 39.7 | 76.0 | (369) | 45.6 | 74.0 | (294) | 1.15 | < 0.001 | < 0.001 | < 0.001 |
| Cellular component movement | ||||||||||||
| GX48290 | Dlc90F | Dynein intermediate chain binding (DYNLT1) | 36.5 | 56.0 | (197) | 39.8 | 64.0 | (224) | 1.09 | < 0.001 | < 0.001 | 0.002 |
| Cellular metabolism | ||||||||||||
| GX1008 | nemy | Carbon-monoxide oxygenase | 33.0 | 56.0 | (375) | 35.3 | 59.0 | (296) | 1.07 | 0.017 | 0.072 | 0.035 |
| GX1008-1 | CG42708 | Glutaminase | 40.4 | 66.0 | (317) | 40.3 | 61.0 | (342) | 1.00 | < 0.001 | < 0.001 | 0.731 |
| GX4385 | CG13890 | Dodecenoyl-CoA delta-isomerase | 36.8 | 60.0 | (626) | 36.5 | 63.0 | (546) | 0.99 | < 0.001 | < 0.001 | 0.297 |
| GX8295 | arf51F | NAD(P)+-protein-arginine | 35.2 | 62.0 | (280) | 33.9 | 58.0 | (336) | 0.96 | 0.032 | 0.518 | 0.111 |
| GX8331 | CG33138 | 1,4-alpha-glucan branching enzyme (GBE1) | 41.9 | 72.0 | (340) | 46.4 | 72.0 | (354) | 1.11 | < 0.001 | < 0.001 | < 0.001 |
| GX47642-1 | CG10383 | Hydrolase (SERAC1) | 42.0 | 68.0 | (372) | 47.2 | 74.0 | (285) | 1.12 | < 0.001 | < 0.001 | < 0.001 |
| GX56643 | eco | Acetyltransferase | 47.3 | 72.0 | (223) | 36.8 | 66.0 | (204) | 0.78 | < 0.001 | < 0.001 | < 0.001 |
| GX62810 | fabp | Fatty acid binding | 41.9 | 71.0 | (593) | 42.1 | 72.0 | (585) | 1.00 | < 0.001 | < 0.001 | 0.219 |
| EP(3)1250 | men | NADP-dependent malate dehydrogenase (ME3) | 39.5 | 72.0 | (350) | 52.4 | 76.0 | (374) | 1.33 | < 0.001 | < 0.001 | < 0.001 |
| EP(2)2086 | CG30427 | Oxidoreductase | 38.1 | 66.0 | (380) | 44.9 | 66.0 | (464) | 1.18 | < 0.001 | < 0.001 | < 0.001 |
| Transport | ||||||||||||
| GX26268 | Atpα | Na pump α subunit (ATP1A3) | 52.2 | 78.0 | (619) | 49.9 | 77.0 | (597) | 0.96 | < 0.001 | < 0.001 | 0.012 |
| EP(3)3232 | drip | Water channel (AQP4) | 48.0 | 76.0 | (330) | 42.3 | 74.0 | (344) | 0.88 | < 0.001 | < 0.001 | < 0.001 |
| Immunity | ||||||||||||
| GX5503 | PGRP-LF | Peptidoglycan recognition protein LF (PGLYRP3) | 31.3 | 51.0 | (356) | 35.5 | 63.0 | (351) | 1.13 | < 0.001 | 0.002 | < 0.001 |
| GX6548 | PGRP-LC | Peptidoglycan recognition protein LC | 44.3 | 70.0 | (372) | lethal | – | – | – | – | ||
| Others | ||||||||||||
| GX1042 | CG42268 | Unknown | 36.3 | 60.0 | (345) | 35.6 | 63.0 | (362) | 0.98 | < 0.001 | < 0.001 | 0.606 |
| GX2970-1 | CG42663 | Unknown | 36.0 | 60.0 | (368) | 42.5 | 64.0 | (356) | 1.18 | < 0.001 | < 0.001 | < 0.001 |
| GX3571 | Mbs | Myosin phosphatase (PPP1R12A) | 55.9 | 83.0 | (387) | 47.2 | 78.0 | (350) | 0.84 | < 0.001 | < 0.001 | < 0.001 |
| GX8400 | CG5861 | Transmembrane protein (TMEM147) | 40.3 | 66.0 | (347) | 37.3 | 62.0 | (324) | 0.93 | < 0.001 | < 0.001 | < 0.001 |
| GX47642 | CG10916 | Zinc ion binding | 32.0 | 62.0 | (361) | 40.8 | 78.0 | (293) | 1.28 | < 0.001 | < 0.001 | < 0.001 |
| GX62808 | CG5807 | Limb region 1 homolog-like (LMBR1L) | 47.5 | 75.0 | (596) | 42.6 | 70.0 | (572) | 0.90 | < 0.001 | < 0.001 | < 0.001 |
After backcrossing, the 45 EP lines were crossed to driver and wild-type control stocks to produce the Gal4CS10>EPCS10 genotype and two control genotypes (EPCS10/+CS10 and da-Gal4CS10/+CS10). Five EP lines were lethal when driven by da-Gal4 and were excluded from further study. Forty EP lines produced adults for survival analysis (Table 1). Wild-type CS10 (+CS10/+CS10) and driver da-Gal4CS10/+CS10 cohorts had nearly identical MLS (33.4 and 33.6 days, respectively). Some EPCS10/+CS10 cohorts lived longer than the parental, coisogenic CS10 or da-Gal4CS10/+CS10 cohorts (Table 1, Fig. 1). Heterosis is an unlikely explanation because these lines had been backcrossed, and the parental da-Gal4CS10/+CS10 genotype was also a composite genotype with chromosomes from two lines. Rather, several EPCS10/+CS10 flies appear to induce some over-expression of the target gene in the absence of Gal4 (Appendix A. Supplementary table 1). Accordingly, here we conservatively quantify longevity assurance by comparing progeny that always carry the EP construct: da-Gal4CS10/EPCS10 versus EPCS10/+CS10, and we infer that an EP line increases MLS only when da-Gal4CS10/EPCS10 has greater MLS (Log-Rank test with p < 0.001) than EPCS10/+CS10, da-Gal4CS10/+CS10, and +CS10/+CS10.
Fig. 1. Survival and mortality of long-lived 14 EP lines induced by da-Gal4.
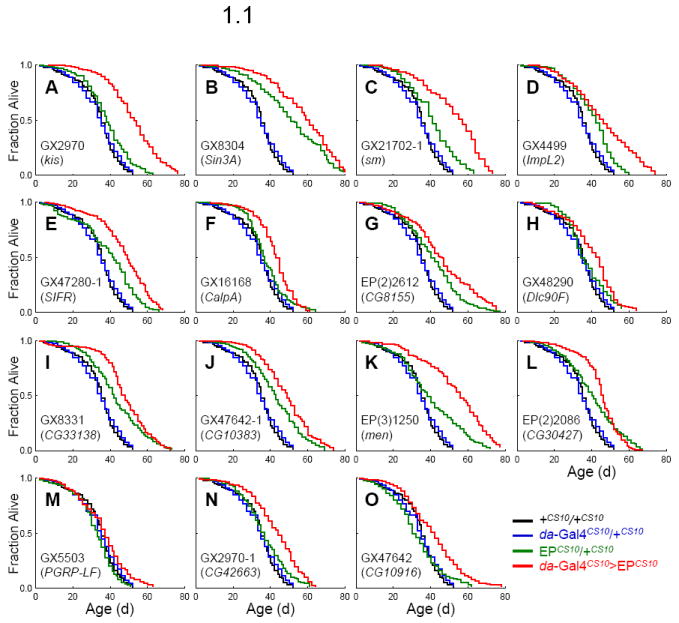
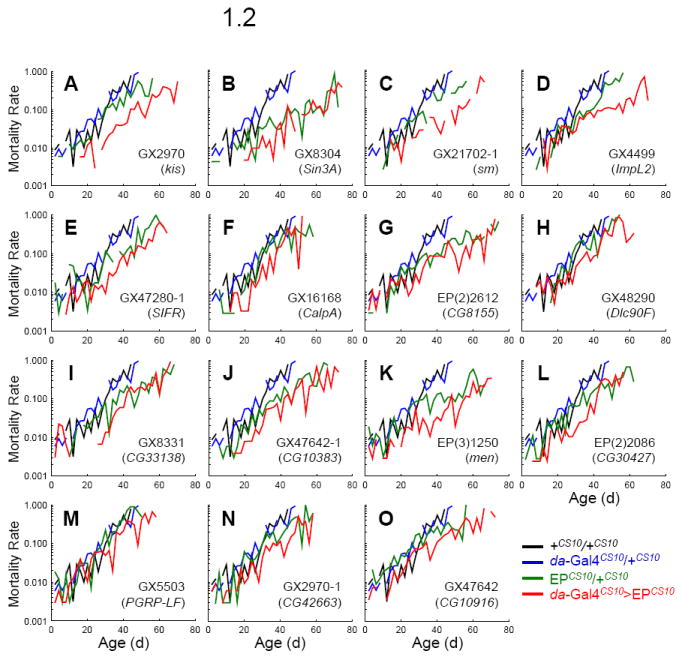
Survivorship (fraction alive, Fig 1.1) and mortality rate (Fig 1.2) of +CS10/+CS10 (black line), da-Gal4CS10/+CS10 (blue line), EPCS10/+CS10 (green line), and da-Gal4CS10 >EPCS10 (red line). Survival plots are estimated by the Kaplan-Meier method. Mortality rate (μx) is estimated as ln (− ln px), where px is the age-specific probability of survival.
We identified 16 EP lines that met these criteria (Table 1). These lines also extend maximum lifespan (MaxLS; Table 1 and Fig. 1.1) and consistently reduce mortality rate across adult ages (Fig. 1.2). These 16 EP lines represent 15 genes since GX2970 and GX6561 are inserted at kismet (kis). Only GX2970 was used for further validation. From Table 1 we do not include EP(2)2559 in the selected set because while these flies under da-Gal4 driver live significantly longer than +CS10/+CS10 and EPCS10/+CS10, they do not live longer than their da-Gal4CS10/+CS10 control.
To determine which gene was affected by the EP insertion of these 15 candidates, we compared mRNA levels of da-Gal4CS10/EPCS10 to EPCS10/+CS10 control (Fig. 3). mRNA from genes flanking the insertion on both strands were quantified by real time RT-PCR. When driven by da-Gal4 the EP insertions significantly increased the mRNA of the gene corresponding to the 5’ to 3’ direction of the insertion’s orientation upstream of transcription start site of the target gene in all cases (Fig. 3). Thus, lifespan extension in the 15 EP lines is associated with over-expression of downstream target genes of the EP elements.
Fig. 3. Over-expression of CG33138 and ImpL2 during adulthood extends Drosophila lifespan.
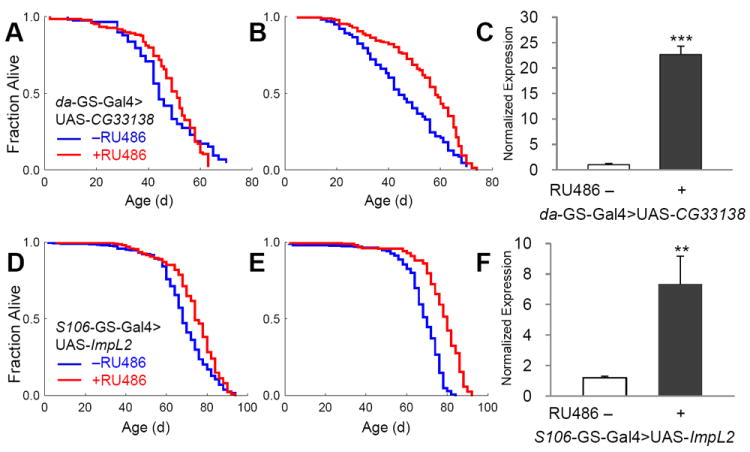
Using the inducible and ubiquitous da-GS-Gal4 with CG33138 and the adult fat cell specific S106-GS-Gal4 with ImpL2, over-expression was induced by feeding flies with 200 μM RU486 after eclosion in males (A and D) and females (B and E). Gene expression of CG33138 was enhanced more than 20 fold (C). Gene induction levels reached more than 7 fold by over-expression of ImpL2 (F). Survival curves show control cohorts treated with vehicle only (blue) and with RU486 (red). Asterisks indicate statistical significance by chi-square test (**, p < 0.01; ***, p < 0.001).
Stocks with UAS-transgene were available or generated for two of these longevity genes, UAS-ImpL2 and UAS-CG33138. To further confirm the extension of lifespan by these two candidates we analyzed adult survival when the transgenes were driven in adults with the conditional GS driver system. This method produces control and experimental cohorts with identical genetic backgrounds and permits analysis of the transgene specifically in the adult. CG33138 is a putative transcript for 1,4-alpha-glucan branching enzyme. Male offspring of the da-GS-Gal4>UAS-CG33138 genotype showed 4% longer MLS when the transgene was induced (Fig. 2A). Longevity was likewise increased in the females, by 20% (Fig. 2B). When these flies were fed with less RU486, thus inducing less expression of CG33138 gene, still MLS increased significantly in females (also refer to Supplementary Table 1). Mutations in 1,4-alpha-glucan branching enzyme 1 (GBE1), the human homologue of CG33138, cause glycogen storage disease type IV, which is characterized by tissue accumulation of abnormal glycogen accompanying liver disease, myopathy, or cardiomyopathy (Bruno et al., 2004; Tay et al., 2004). On the other hand, expression of GBE1 is dramatically enhanced by hypoxic stresses in mammalian cells and tissues (Zhao et al., 2004), which might be simply the consequence of increased anaerobic glycolysis and glycogen remodeling. Recently, it has been reported that hypoxia extends worm lifespan and this is mediated through increased expression of HIF-1α (Lee et al., 2010b). Thus, GBE1 might be a downstream target of HIF-1α through which it controls longevity.
Fig. 2. Insertion position of each EP line and gene expression induction by da-Gal4.
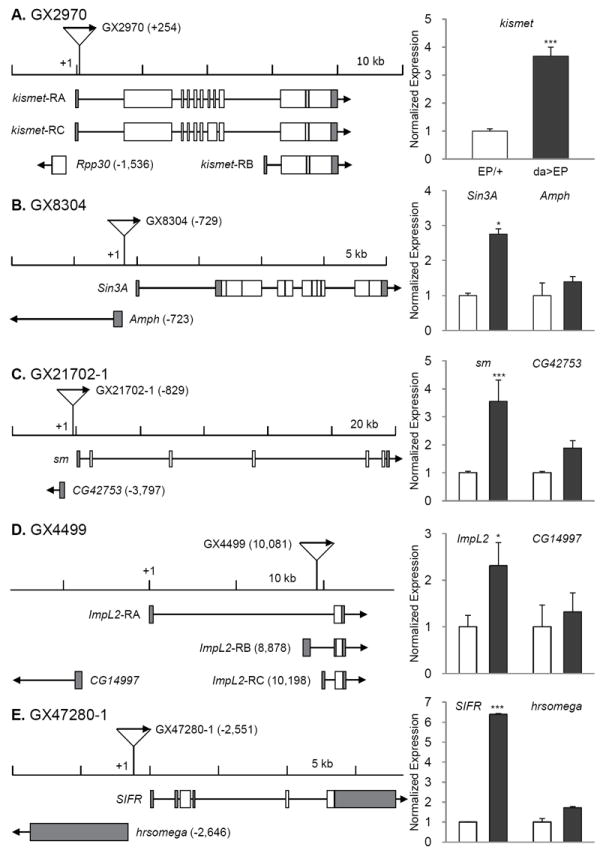
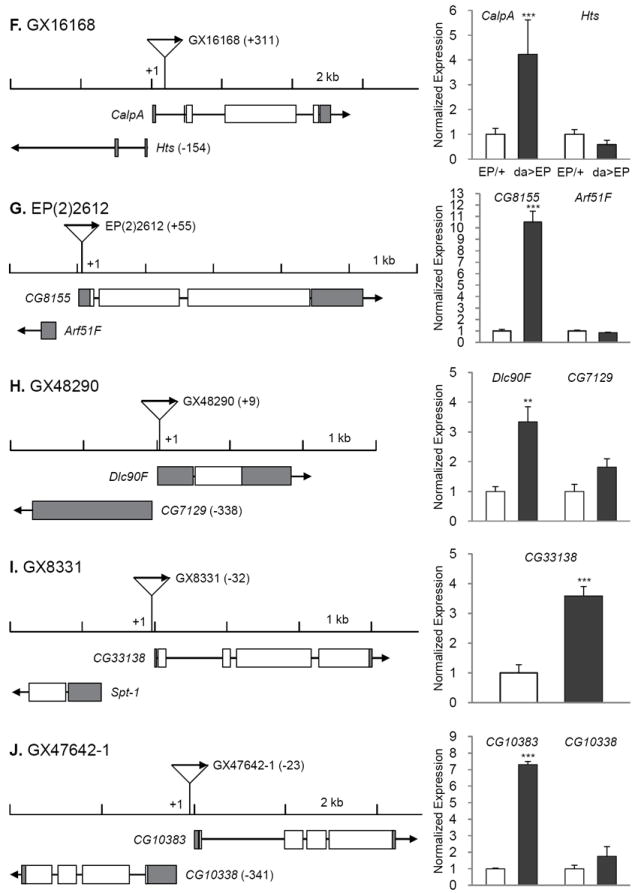
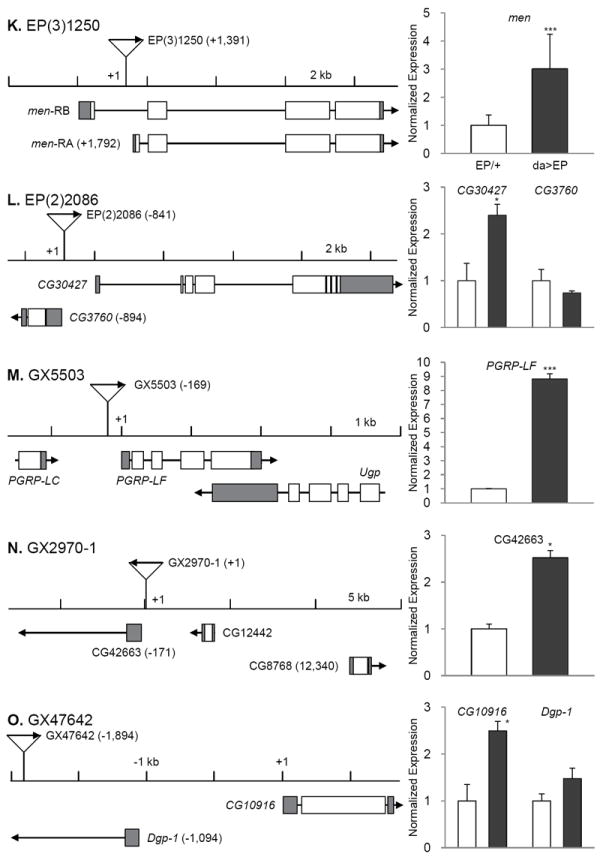
The transcription start sites are set as +1 and the relative positions of P-element are indicated in parentheses. Direction of transcription from EP and that of adjacent gene are marked with arrows. Induction levels of the target genes were measured by real time RT-PCR in EPCS10/+CS10 and da-Gal4CS10 >EPCS10 flies and normalized to those of each EPCS10/+CS10. Statistical significance in induction levels between EPCS10/+CS10 and da-Gal4CS10 >EPCS10 was obtained by chi-square test. In cases an EP is inserted in the region where it could affect two genes transcribed to the opposite directions, we measured expression levels of both genes (B, C, D, E, F, G, H, J, L, and O). Data are presented with mean and standard error bars. Asterisks indicate significant changes by p < 0.05-0.001.
The EP line GX4499 contains an EP adjacent to transcription start site of ImpL2-RB and causes significant induction of ImpL2 mRNA when driven by da-Gal4 (Fig. 3D). ImpL2 has 3 transcriptional isoforms, which are likely to produce similar proteins after post-translational modification (Flybase; http://flybase.org/). Lifespan was reduced when ImpL2 was strongly over-expressed throughout the adult by the conditional GS drivers, act-GS-Gal4 or da-GS-Gal4 (data not shown). However, restricted over-expression of the ImpL2 in fat cells by using S106-GS-Gal4 significantly extended lifespan in both sexes (Fig. 2D, 2E), and in repeated trials (Supplementary Table 2). S106-GS-Gal4>UAS-ImpL2 in fat cells increased ImpL2 mRNA about 6-fold (Fig. 2F). ImpL2 was originally described as a gene induced by ecdysone (Natzle et al., 1986) and has more recently been recognized as an inhibitory insulin-like peptide binding protein (Honegger et al., 2008; Leopold and Perrimon, 2007; Sloth Andersen et al., 2000). A role for ImpL2 in aging was previously suggested in the context of extended longevity when germline stems cells were genetically reduced (Flatt et al., 2008). Message of Drosophila insulin-like peptides (DILPs) was increased in those sterile, long-lived flies but insulin signaling at peripheral tissues appeared to be repressed. At the same time, mRNA for ImpL2 was strongly elevated, suggesting that this binding protein might counteract the overproduction of DILPs and thus extend lifespan. Recently, over-expression of ImpL2 was reported to extend Drosophila lifespan, including through some broadly expressed drivers that differed from our negative result with tubulin-GS-Gal4 and, importantly, by S106-GS-Gal4, which concurs with our current report (Alic et al., 2011).
The remaining 13 longevity genes are distributed among functional categories (Table 1). The category ‘transcription and translation’ includes kis, Sin3A, and Smooth (sm). The Kismet protein is a DNA helicase containing an SNF2-like ATPase domain, and functions in trithorax mediated chromatin remodeling (Daubresse et al., 1999). Mutations of its human homologue, chromodomain helicase DNA binding protein 7 cause the CHARGE syndrome, of which clinical symptoms include developmental retardation, heart malformation, and coloboma (Lin et al., 1990). Reduced expression of Drosophila kis yields similar pathogenic phenotypes including defects in motor ability, neuronal development, and learning/memory (Melicharek et al., 2010). Kismet also functions in circadian photo-response and control of hedgehog expression (Dubruille et al., 2009; Terriente-Felix et al., 2011). Over-expression of Kismet may increase longevity by increasing chromatin silencing and homeostasis (Oberdoerffer and Sinclair, 2007). Likewise, Sin3 is a scaffolding protein that complexes with histone deacetylases (HDAC1/2) where it interacts with corepressors to control transcriptional silencing of genes (Grzenda et al., 2009). Notably, rpd3, which encode HDAC1 in Drosophila, is required for Sir2 expression to increase lifespan (Rogina et al., 2002). Sm, a homolog of the human heterogeneous nuclear ribonucleoprotein L (hnRNP L), is involved in mRNA synthesis and maturation. Sm is primarily expressed in chemosensory neurons and homozygous sm mutants show defects in axonal arborization of chemosensory neurons and in feeding behavior. These defects may be related to their early death after eclosion (Layalle et al., 2005). Interestingly, hnRNP L and hnRNP A2 are known to bind to the 3’ UTR of glucose transporter-1 mRNA to repress its translation in the glioblastoma cells (Hamilton et al., 1999). Functions of sm in longevity control are unknown.
‘Signal transduction’ includes ImpL2 as described above, SIFamide receptor (SIFR), Calpain A (CalpA) and CG8155. SIFR is a G protein-coupled neuropeptide receptor expressed in the intestines, brain and thoracicoabdominal ganglion of adult flies (Jorgensen et al., 2006; Veenstra et al., 2008). Its ligand, SIFamide is produced in pars intercerebralis (PI) of the brain. Reduced levels of the ligand causes hyperactive courtship behaviors in both sexes (Terhzaz et al., 2007). Increased SIFR may reduce reproductive behaviors and consequently extend lifespan. On the other hand, the PI secretes DILPs, and reducing secretion of these DILPs or ablation of PI increases lifespan (Broughton et al., 2005; Wessells et al., 2004). It remains to be investigated if SIFamide signaling influences the synthesis or secretion of DILPs in the PI. Calpains, calcium-dependent cysteine proteases, are implicated in protein turnover, intracellular cell signaling, cell cycle, apoptosis, and cell motility (Nixon, 2003). Calpain activity increases in normal brains with age (Benuck et al., 1996). Calpain is also elevated in the postmortem brain tissues of patients of Alzheimer’s and Parkinson’s diseases (AD and PD) (Crocker et al., 2003; Saito et al., 1993). Conversely, calpain activity is low in long-living bats (Baudry et al., 1986) and inhibition of calpain improves symptoms of AD (Trinchese et al., 2008) and PD (Crocker et al., 2003). Thus, while an increase of calpain activity should be adverse to longevity, our results suggest the opposite, and further investigation is needed to understand the effect of calpain function in aging. CG8155 is a Rab GTPase activator and homologue of human TBC1 domain family member 25. It functions in intracellular trafficking of vesicles and signaling with phosphoinositides and growth factor receptors (Stenmark, 2009). High expression of Rab25, one of Rab GTPases, is found in ovarian and breast cancer (Cheng et al., 2004). Another subtype of Rab GTPase, Rab27B, is enhanced in senescent human fibroblast (Fujii et al., 2006). It is unclear whether increases of theses Rab GTPases are causes or consequences of aging. Our results suggest that certain types of Rab GTPases could be beneficial in longevity.
Dynein light chain 90F (Dlc90F) in ‘cellular component movement’ is a member of the dynein light-chain family (Davis and Smith, 2005). In aged monkey brain, dynein accumulates at the nerve endings and less dynein interacts with dynactin, causing accumulation of endogenous Tau and amyloid precursor proteins (Kimura et al., 2007). Mutations of Dynein are thought to reduce autophagic clearance of aggregate-prone proteins, leading to aggregation of GpC rich proteins such as Huntingtin (Ravikumar et al., 2005). Mutants of Drosophila dynein light chain 1 likewise reduce of autophagy in neurons and affect larval motility (Batlevi et al., 2010). We might predict, therefore, that over-expression of Dlc90F increases neuronal autophagic activity, and this might be sufficient to increase lifespan as has been observed for over-expression of Atg8a (Simonsen et al., 2008).
The category ‘cellular metabolism’ includes: aforementioned CG33138, CG10383, NADP-dependent malate dehydrogenase (men) and CG30427. CG10383 is the homologue of human serine active site containing 1 (SERAC1) and is inferred to play a role in glycosylphosphatidylinositol metabolism, and has been associated with male sterility (Schimenti et al., 2005). No information is available about CG10383. CG30427 is a homologue of human fatty acyl-CoA reductase, which converts long-chain aldehyde to long-chain acyl CoA, in the process producing NADPH (Riendeau et al., 1982). men, a key enzyme of the malate-pyruvate shuttle, converts malate to pyruvate to produce NADPH in the cytosol (Geer et al., 1979; MacDonald, 1995). The production of NADPH from over-expression of men and CG30427 may assist enzymes that scavenge cellular reactive oxygen species.
In the category ‘immunity’, peptidoglycan recognition protein LF (PGRP-LF) was identified from our screen. Unlike PGRP-LC and -LE that activate immune deficiency (IMD) signaling pathway, PGRP-LF inhibits immunity by sequestering circulating peptidoglycans (Aggarwal and Silverman, 2008; Maillet et al., 2008). While over-expression of PGRP-LE in Drosophila fat body enhanced pathogen resistance, this also shortened lifespan (Libert et al., 2006). Considered with our current results, activated immunity appears to represses lifespan, while suppressed immunity, all else being equal, slows Drosophila aging.
Two genes, CG42663 and CG10916, are uncategorized. CG10916, a zinc ion binding protein, was identified as one of the genes that were significantly up-regulated under hyperoxia in Drosophila heads (Gruenewald et al., 2009). Taken together with our results, such increase may be protective from hyperoxic stress.
The wild-type CS10 is established by backcrossing Canton-S to w1118 (Simon et al., 2003). The MLS of CS10 (33 days) measured in the present study is similar to the other previous report (Yamazaki et al., 2007), but is much shorter than Canton-S and w1118 (Grandison et al., 2009; Lin et al., 1998). So, we compared fecundity, feeding behavior and locomotor activity of CS10 with those of Canton-S and w1118 flies (Supplementary Fig. 1). But no difference in these criteria was found among these wild-type flies. The cause of shorter lifespan in CS10 remains unclear.
One of the strongest assets of invertebrate genetic models of aging is their capacity for forward genetic screening. In this way, one can efficiently discover novel genes and pathways that assure longevity and thus lead to insights on the mechanism underlying senescence. This approach has been used several times with the nematode C. elegans where gene knockdown is rapidly induced by feeding E. coli engineered to produce specific dsRNA (Timmons and Fire, 1998). Screening with Drosophila is conducted by chemical mutagenesis or by the random insertion of engineered transposons, which have the capacity to produce both loss- or gain-of-function mutations (Ashburner et al., 2005; Rørth et al., 1998). Several previous studies have reported results from transposon screens. The first gene described to affect Drosophila lifespan was methusulah, which was identified from a collection of P-element insertion mutants (Lin et al., 1998). Likewise, indy (I am not dead yet) was found in a collection of P-element lac-z strains (Rogina et al., 2000). Seong et al. conducted a systematic gain-of-function screen for longevity benefits among 646 P-element insertions and reported 23 genes extending the lifespan (Seong et al., 2001). In an important experimental design, Landis et al. developed a system of doxycycline-inducible P-element insertion to make perfect genetic controls for each individual insertion genotype (Landis et al., 2003). They reported 6 longevity genes from a screen of approximately 10,000 mutants. Despite these collected efforts, it is clear that screening in Drosophila has not yet reached saturation for the aging phenotype because to date there is little to no overlap in the candidates so far described.
4. Conclusions
We studied the lifespan of 45 isogenic Drosophila EP lines to find novel genes that extend the lifespan, and confirmed 15 genes as longevity genes. Among these longevity genes, we also verified that gene-specific over-expression of ImpL2 (ecdysone-inducible gene L2) and CG33138 (1,4-alpha-glucan branching enzyme) by ubiquitous or tissue-specific GS-Gal4 drivers is sufficient to extend the lifespan. Extensive investigation of these longevity genes would fit some genes in current aging mechanisms, and offer opportunities to identify new systems that control aging processes.
Supplementary Material
Note:
Bold indicates significant tests where induced expression of CG33138 by different doses of RU486 enhances survival relative to the matched, un-induced control.
- Honegger: UAS-ImpL2-L in the balanced w1118 genetic background provided by the originating laboratory of H. Stocker and E. Hafen (Honegger et al., 2008). wDah: the UAS-ImpL2-L transgene backcrossed into a white-Dahomomey stock for 10 generations.
- Median lifespan with 95% confidence intervals, and values for the 25th and 75th qurtiles.
- Bold indicates significant tests where induced expression of ImpL2 increases survival relative to the matched, un-induced control.
The expression levels of EP target genes in da-Gal4CS10/+CS10 and EPCS10/+CS10 flies were measured by real time RT-PCR and normalized to those of +CS10/+CS10 wild-type flies. Data of da-Gal4CS10/+CS10 or EPCS10/+CS10 for each target gene were compared with those of +CS10/+CS10 by chi-square test. EP heterozygotes of GX21702-1 (sm), GX47280-1 (SIFR), EP(3)1250 (men) and EP(2)2086 (CG30427) show strong increase in mRNA without the Gal4 driver. In GX8304 (Sin3A) and GX8331 (CG33138), moderate overexpression is observed with EPCS10/+CS10 alone. Data are described with mean and standard error (SE).
Fecundity, feeding behavior and locomotor activity were measured in CS10, Canton-S, and w1118 wild-type flies. Data were analyzed repeated-measures of analysis of variance (ANOVA) with genotype and time for fecundity test as main effects. Fecundity of CS10 were compared with Canton-S and w1118 wild-type flies, following the previously reported methods (Rogina et al., 2000). No significant effects of genotype on fecundity was detected for 20 days (A; F(4,478) = 0.703, p = 0.59). Generally, these wild-type flies lay around 50 eggs a day from 3 days after eclosion, then the number of eggs declines gradually with age (A). Feeding behavior was estimated in males (B) and females (C) among these wild-type flies, as previously described (Xu et al., 2008). No significant effects of genotype (F(4,166) = 2.19, p = 0.07) was found on the relative amount of food taken by these wild-type flies. In addition, climbing behavior was estimated in males (D) and females (E) by the negative geotaxis assay (Rhodenizer et al., 2008). Though w1118 males showed hyperactivity at 20 days (D), but no genotype effect was found among wild-type flies (F(4,166) = 1.44, p = 0.22).
Acknowledgments
We thank Dr. Saitoe for CS10 flies, Dr. Monnier for da-GS-Gal4 flies, Szeged Stock Center for some EP lines, and Bloomington Stock Center for Hsp70-Gal4 and S106-GS-Gal4 flies. We also thank Dr. Silverman for his comments on this manuscript. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 20100028414). Work from the laboratory of M. Tatar was supported through the National Institute of Aging, NIH (USA) by R01AG024360 and R01AG031152, and by the Glenn Medical Foundation and the Ellison Medical Foundation.
Abbreviations
- AD
Alzheimer’s disease
- CalpA
Calpain A
- da
daughterless
- DILPs
Drosophila insulin-like peptides
- Dlc90F
Dynein light chain 90F
- GBE1
1,4-alpha-glucan branching enzyme 1
- GS
GeneSwitch
- hnRNP L
heterogeneous nuclear ribonucleoprotein L
- hsp70
heat shock 70
- IMD
immune deficiency
- kis
kismet
- MaxLS
maximum lifespan
- men
NADP-dependent malate dehydrogenase
- MLS
mean lifespan
- PD
Parkinson’s diseases
- PGRP-LF
peptidoglycan recognition protein
- PI
pars intercerebralis
- SERAC1
serine active site containing 1
- SIFR
SIFamide receptor
- sm
Smooth
- UAS
Upstream Activator Sequence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal K, Silverman N. Positive and negative regulation of the Drosophila immune response. BMB Rep. 2008;41:267–277. doi: 10.5483/bmbrep.2008.41.4.267. [DOI] [PubMed] [Google Scholar]
- Alic N, Hoddinott MP, Vinti G, Partridge L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell. 2011;10:137–147. doi: 10.1111/j.1474-9726.2010.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Golic KG, Hawley RS. Drosophila: A Laboratory Handbook and Manual. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2005. [Google Scholar]
- Batlevi Y, Martin DN, Pandey UB, Simon CR, Powers CM, Taylor JP, Baehrecke EH. Dynein light chain 1 is required for autophagy, protein clearance, and cell death in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:742–747. doi: 10.1073/pnas.0907967107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, DuBrin R, Beasley L, Leon M, Lynch G. Low levels of calpain activity in Chiroptera brain: implications for mechanisms of aging. Neurobiology of aging. 1986;7:255–258. doi: 10.1016/0197-4580(86)90004-7. [DOI] [PubMed] [Google Scholar]
- Benuck M, Banay-Schwartz M, DeGuzman T, Lajtha A. Changes in brain protease activity in aging. Journal of neurochemistry. 1996;67:2019–2029. doi: 10.1046/j.1471-4159.1996.67052019.x. [DOI] [PubMed] [Google Scholar]
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno C, van Diggelen OP, Cassandrini D, Gimpelev M, Giuffre B, Donati MA, Introvini P, Alegria A, Assereto S, Morandi L, Mora M, Tonoli E, Mascelli S, Traverso M, Pasquini E, Bado M, Vilarinho L, van Noort G, Mosca F, DiMauro S, Zara F, Minetti C. Clinical and genetic heterogeneity of branching enzyme deficiency (glycogenosis type IV) Neurology. 2004;63:1053–1058. doi: 10.1212/01.wnl.0000138429.11433.0d. [DOI] [PubMed] [Google Scholar]
- Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, Gray JW, Mills GB. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nature medicine. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S, Robertson GS, Anisman H, Merali Z, Park DS. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvejic S, Zhu Z, Felice SJ, Berman Y, Huang XY. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nature cell biology. 2004;6:540–546. doi: 10.1038/ncb1133. [DOI] [PubMed] [Google Scholar]
- Daubresse G, Deuring R, Moore L, Papoulas O, Zakrajsek I, Waldrip WR, Scott MP, Kennison JA, Tamkun JW. The Drosophila kismet gene is related to chromatin-remodeling factors and is required for both segmentation and segment identity. Development. 1999;126:1175–1187. doi: 10.1242/dev.126.6.1175. [DOI] [PubMed] [Google Scholar]
- Davis L, Smith GR. Dynein promotes achiasmate segregation in Schizosaccharomyces pombe. Genetics. 2005;170:581–590. doi: 10.1534/genetics.104.040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Roshina NV, Geiger-Thornsberry GL, Lyman RF, Pasyukova EG, Mackay TF. Dopa decarboxylase (Ddc) affects variation in Drosophila longevity. Nature genetics. 2003;34:429–433. doi: 10.1038/ng1218. [DOI] [PubMed] [Google Scholar]
- Dubruille R, Murad A, Rosbash M, Emery P. A constant light-genetic screen identifies KISMET as a regulator of circadian photoresponses. PLoS Genet. 2009;5:e1000787. doi: 10.1371/journal.pgen.1000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Tanzi RE. Genetics of aging. Science. 1997;278:407–411. doi: 10.1126/science.278.5337.407. [DOI] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Kawai Y, Endoh M, Hossain MN, Nakabayashi K, Ayusawa D. Expression of RAB27B is up-regulated in senescent human cells. Mechanisms of ageing and development. 2006;127:639–642. doi: 10.1016/j.mad.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Geer BW, Krochko D, Williamson JH. Ontogeny, cell distribution, and the physiological role of NADP-malic enzyme in Drosophila melanogaster. Biochemical genetics. 1979;17:867–879. doi: 10.1007/BF00504309. [DOI] [PubMed] [Google Scholar]
- Grandison RC, Wong R, Bass TM, Partridge L, Piper MD. Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS ONE. 2009;4:e4067. doi: 10.1371/journal.pone.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald C, Botella JA, Bayersdorfer F, Navarro JA, Schneuwly S. Hyperoxia-induced neurodegeneration as a tool to identify neuroprotective genes in Drosophila melanogaster. Free radical biology & medicine. 2009;46:1668–1676. doi: 10.1016/j.freeradbiomed.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Grzenda A, Lomberk G, Zhang JS, Urrutia R. Sin3: Master scaffold and transcriptional corepressor. Biochim Biophys Acta. 2009;1789:443–450. doi: 10.1016/j.bbagrm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Hamilton BJ, Nichols RC, Tsukamoto H, Boado RJ, Pardridge WM, Rigby WF. hnRNP A2 and hnRNP L bind the 3’UTR of glucose transporter 1 mRNA and exist as a complex in vivo. Biochemical and biophysical research communications. 1999;261:646–651. doi: 10.1006/bbrc.1999.1040. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Jorgensen LM, Hauser F, Cazzamali G, Williamson M, Grimmelikhuijzen CJ. Molecular identification of the first SIFamide receptor. Biochemical and biophysical research communications. 2006;340:696–701. doi: 10.1016/j.bbrc.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura N, Imamura O, Ono F, Terao K. Aging attenuates dynactin-dynein interaction: down-regulation of dynein causes accumulation of endogenous tau and amyloid precursor protein in human neuroblastoma cells. Journal of neuroscience research. 2007;85:2909–2916. doi: 10.1002/jnr.21408. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Landis GN, Bhole D, Tower J. A search for doxycycline-dependent mutations that increase Drosophila melanogaster life span identifies the VhaSFD, Sugar baby, filamin, fwd and Cctl genes. Genome Biol. 2003;4:R8. doi: 10.1186/gb-2003-4-2-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layalle S, Coessens E, Ghysen A, Dambly-Chaudiere C. Smooth, a hnRNP encoding gene, controls axonal navigation in Drosophila. Genes Cells. 2005;10:119–125. doi: 10.1111/j.1365-2443.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010a;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010b;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- Libert S, Chao Y, Chu X, Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell. 2006;5:533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Lin AE, Siebert JR, Graham JM., Jr Central nervous system malformations in the CHARGE association. Am J Med Genet. 1990;37:304–310. doi: 10.1002/ajmg.1320370303. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell metabolism. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. The Journal of biological chemistry. 1995;270:20051–20058. [PubMed] [Google Scholar]
- Maillet F, Bischoff V, Vignal C, Hoffmann J, Royet J. The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation. Cell Host Microbe. 2008;3:293–303. doi: 10.1016/j.chom.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Vina J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- Melicharek DJ, Ramirez LC, Singh S, Thompson R, Marenda DR. Kismet/CHD7 regulates axon morphology, memory and locomotion in a Drosophila model of CHARGE syndrome. Hum Mol Genet. 2010;19:4253–4264. doi: 10.1093/hmg/ddq348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natzle JE, Hammonds AS, Fristrom JW. Isolation of genes active during hormone-induced morphogenesis in Drosophila imaginal discs. The Journal of biological chemistry. 1986;261:5575–5583. [PubMed] [Google Scholar]
- Nixon RA. The calpains in aging and aging-related diseases. Ageing research reviews. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007;8:692–702. doi: 10.1038/nrm2238. [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Rørth P. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12418–12422. doi: 10.1073/pnas.93.22.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rørth P, Szabo K, Bailey A, Laverty T, Rehm J, Rubin GM, Weigmann K, Milan M, Benes V, Ansorge W, Cohen SM. Systematic gain-of-function genetics in Drosophila. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O’Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nature genetics. 2005;37:771–776. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol. 2008;43:739–748. doi: 10.1016/j.exger.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau D, Rodriguez A, Meighen E. Resolution of the fatty acid reductase from Photobacterium phosphoreum into acyl protein synthetase and acyl-CoA reductase activities. Evidence for an enzyme complex. The Journal of biological chemistry. 1982;257:6908–6915. [PubMed] [Google Scholar]
- Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- Rogina B, Reenan RA, Nilsen SP, Helfand SL. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science. 2000;290:2137–2140. doi: 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MI, Parkhurst SM. Drosophila Sir2 is required for heterochromatic silencing and by euchromatic Hairy/E(Spl) bHLH repressors in segmentation and sex determination. Cell. 2002;109:447–458. doi: 10.1016/s0092-8674(02)00732-8. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 4. Wadsworth Publishing Company; Belmont: 1995. [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimenti JC, Reynolds JL, Planchart A. Mutations in Serac1 or Synj2 cause proximal t haplotype-mediated male mouse sterility but not transmission ratio distortion. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3342–3347. doi: 10.1073/pnas.0407970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong KH, Ogashiwa T, Matsuo T, Fuyama Y, Aigaki T. Application of the gene search system to screen for longevity genes in Drosophila. Biogerontology. 2001;2:209–217. doi: 10.1023/a:1011517325711. [DOI] [PubMed] [Google Scholar]
- Simon AF, Shih C, Mack A, Benzer S. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- Sloth Andersen A, Hertz Hansen P, Schaffer L, Kristensen C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. The Journal of biological chemistry. 2000;275:16948–16953. doi: 10.1074/jbc.M001578200. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell. 1999;99:735–745. doi: 10.1016/s0092-8674(00)81671-2. [DOI] [PubMed] [Google Scholar]
- Tatar M, Khazaeli AA, Curtsinger JW. Chaperoning extended life. Nature. 1997;390:30. doi: 10.1038/36237. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tay SK, Akman HO, Chung WK, Pike MG, Muntoni F, Hays AP, Shanske S, Valberg SJ, Mickelson JR, Tanji K, DiMauro S. Fatal infantile neuromuscular presentation of glycogen storage disease type IV. Neuromuscul Disord. 2004;14:253–260. doi: 10.1016/j.nmd.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Terhzaz S, Rosay P, Goodwin SF, Veenstra JA. The neuropeptide SIFamide modulates sexual behavior in Drosophila. Biochemical and biophysical research communications. 2007;352:305–310. doi: 10.1016/j.bbrc.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Terriente-Felix A, Molnar C, Gomez-Skarmeta JL, de Celis JF. A conserved function of the chromatin ATPase Kismet in the regulation of hedgehog expression. Developmental biology. 2011;350:382–392. doi: 10.1016/j.ydbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Tricoire H, Battisti V, Trannoy S, Lasbleiz C, Pret AM, Monnier V. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mechanisms of ageing and development. 2009;130:547–552. doi: 10.1016/j.mad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Fa M, Liu S, Zhang H, Hidalgo A, Schmidt SD, Yamaguchi H, Yoshii N, Mathews PM, Nixon RA, Arancio O. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. The Journal of clinical investigation. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra JA, Agricola HJ, Sellami A. Regulatory peptides in fruit fly midgut. Cell and tissue research. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Developmental cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nature genetics. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell metabolism. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Horiuchi J, Nakagami Y, Nagano S, Tamura T, Saitoe M. The Drosophila DCO mutation suppresses age-related memory impairment without affecting lifespan. Nature neuroscience. 2007;10:478–484. doi: 10.1038/nn1863. [DOI] [PubMed] [Google Scholar]
- Zhao J, Chen H, Davidson T, Kluz T, Zhang Q, Costa M. Nickel-induced 1,4-alpha-glucan branching enzyme 1 up-regulation via the hypoxic signaling pathway. Toxicology and applied pharmacology. 2004;196:404–409. doi: 10.1016/j.taap.2004.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note:
Bold indicates significant tests where induced expression of CG33138 by different doses of RU486 enhances survival relative to the matched, un-induced control.
- Honegger: UAS-ImpL2-L in the balanced w1118 genetic background provided by the originating laboratory of H. Stocker and E. Hafen (Honegger et al., 2008). wDah: the UAS-ImpL2-L transgene backcrossed into a white-Dahomomey stock for 10 generations.
- Median lifespan with 95% confidence intervals, and values for the 25th and 75th qurtiles.
- Bold indicates significant tests where induced expression of ImpL2 increases survival relative to the matched, un-induced control.
The expression levels of EP target genes in da-Gal4CS10/+CS10 and EPCS10/+CS10 flies were measured by real time RT-PCR and normalized to those of +CS10/+CS10 wild-type flies. Data of da-Gal4CS10/+CS10 or EPCS10/+CS10 for each target gene were compared with those of +CS10/+CS10 by chi-square test. EP heterozygotes of GX21702-1 (sm), GX47280-1 (SIFR), EP(3)1250 (men) and EP(2)2086 (CG30427) show strong increase in mRNA without the Gal4 driver. In GX8304 (Sin3A) and GX8331 (CG33138), moderate overexpression is observed with EPCS10/+CS10 alone. Data are described with mean and standard error (SE).
Fecundity, feeding behavior and locomotor activity were measured in CS10, Canton-S, and w1118 wild-type flies. Data were analyzed repeated-measures of analysis of variance (ANOVA) with genotype and time for fecundity test as main effects. Fecundity of CS10 were compared with Canton-S and w1118 wild-type flies, following the previously reported methods (Rogina et al., 2000). No significant effects of genotype on fecundity was detected for 20 days (A; F(4,478) = 0.703, p = 0.59). Generally, these wild-type flies lay around 50 eggs a day from 3 days after eclosion, then the number of eggs declines gradually with age (A). Feeding behavior was estimated in males (B) and females (C) among these wild-type flies, as previously described (Xu et al., 2008). No significant effects of genotype (F(4,166) = 2.19, p = 0.07) was found on the relative amount of food taken by these wild-type flies. In addition, climbing behavior was estimated in males (D) and females (E) by the negative geotaxis assay (Rhodenizer et al., 2008). Though w1118 males showed hyperactivity at 20 days (D), but no genotype effect was found among wild-type flies (F(4,166) = 1.44, p = 0.22).


