Abstract
Alterations in sodium channel expression and function have been suggested as a key molecular event underlying the abnormal processing of pain after peripheral nerve or tissue injury. Although the relative contribution of individual sodium channel subtypes to this process is unclear, the biophysical properties of the tetrodotoxin-resistant current, mediated, at least in part, by the sodium channel PN3 (SNS), suggests that it may play a specialized, pathophysiological role in the sustained, repetitive firing of the peripheral neuron after injury. Moreover, this hypothesis is supported by evidence demonstrating that selective “knock-down” of PN3 protein in the dorsal root ganglion with specific antisense oligodeoxynucleotides prevents hyperalgesia and allodynia caused by either chronic nerve or tissue injury. In contrast, knock-down of NaN/SNS2 protein, a sodium channel that may be a second possible candidate for the tetrodotoxin-resistant current, appears to have no effect on nerve injury-induced behavioral responses. These data suggest that relief from chronic inflammatory or neuropathic pain might be achieved by selective blockade or inhibition of PN3 expression. In light of the restricted distribution of PN3 to sensory neurons, such an approach might offer effective pain relief without a significant side-effect liability.
Spontaneous and/or evoked hyperexcitability of the peripheral nerve after injury is considered to be a principal feature of the underlying pathophysiology associated with many chronic, in particular neuropathic, pain syndromes (1, 2). A prominent molecular basis for this abnormal, repetitive firing of injured primary afferents is an accumulation and increased membrane density of sodium channels at focal sites of injury (3, 4). The resultant membrane remodeling contributes to a lower threshold for action potential generation at these sites and, consequently, precipitates ectopic impulse generation (5, 6). Further, sodium channel blockade with subanesthetic doses of a local anesthetic suppresses ectopic electrogenesis and may account for the analgesic effectiveness of these agents (7). At present, the relative contribution of individual sodium channel subtypes toward this altered processing of sensory input remains unclear. In the dorsal root ganglion (DRG), two main types of sodium currents, termed TTX-sensitive (TTX-S) and TTX-resistant (TTX-R), have been identified on the basis of their kinetics and sensitivity to the neurotoxin, tetrodotoxin (TTX) (8, 9, 10). The fast-inactivating, TTX-S current, found in all types of DRG cells, may be mediated by one or more of several α-subunits known to be expressed in these cells: brain types I, IIA, III (11), PN1 (12, 13), and NaCh6 (14) [also known as either SCN8A (15) or PN4 (16)]. In contrast, in normal adult DRG neurons, the more slowly inactivating TTX-R current appears to be preferentially expressed in a subpopulation of small diameter, unmyelinated, capsaicin-sensitive neurons, otherwise referred to as nociceptors (8, 9, 17). Until recently, only a single sodium channel α-subunit, PN3 (18), also known as sensory neuron specific or SNS (19), had been identified that displayed the biophysical properties, resistance to TTX, and anatomical distribution of the TTX-R current (18, 19, 20). However, multiple types of TTX-R current, termed TTX-R1, R2, and R3, have now been suggested to be present in the small diameter neurons of the adult rat DRG (21). Although the biophysical properties of PN3 make it a likely candidate for TTX-R1, the most abundant form of TTX-R current, a second type of novel sodium channel was recently cloned from rat DRG, termed NaN (22). NaN, also referred to as SNS2 (23), appears to be preferentially localized to an even more restricted subpopulation of small diameter sensory neurons within the DRG (22, 23). In comparison with PN3/SNS, it has intermediate resistance to TTX (1 μM), and its biophysical properties (23) suggest it may be a possible candidate for the TTX-R3 current (21). The physiological and/or pathophysiological role of NaN/SNS2 remains to be elucidated, but it is possible that changes in the expression and function of this channel, in addition to PN3/SNS, may make an important contribution to the establishment of certain chronic pain states. Further, implication of either channel has the additional importance that selective blockade may produce pain relief in these states without many of the limiting central nervous system and other side-effects associated with current therapies.
Evidence for a Role for PN3 in the Mediation of Abnormal Pain Behaviors After Nerve and Tissue Injury
The selective expression of TTX-R INa, as well as PN3/SNS and NaN/SNS2, in a specific subpopulation of capsaicin-sensitive, primary afferent neurons suggests that these channels may play a crucial role in the regulation of sensory, nociceptive function. Moreover, the rapid repriming properties, in addition to the higher threshold and slower rate of inactivation, of TTX-R INa (i.e., TTX-R1) and PN3/SNS, further suggest that cells expressing a large proportion of TTX-R1 sodium channels should be ideally suited to sustain repetitive firing at the depolarized potentials characteristic of an injured peripheral nerve; that is, they will be slowly adapting in response to a persistent, depolarizing stimulus (10, 21, 24).
Immunohistochemical Studies.
Such a potentially specialized, pathophysiological role for TTX-R INa/PN3 has been supported by alterations in channel distribution observed after sustained injury to the peripheral nerve. The effect, however, is complex and appears dependent on the nature and degree of the injury. After axotomy, TTX-R INa is substantially reduced in the small cells of the DRG (25, 26) with the level of PN3/SNS mRNA (27) and protein (20) expression reduced in parallel. In contrast, in the chronic constriction injury model of neuropathic pain in the rat, there was no significant change in the amplitude or I–V relationship of either TTX-R or TTX-S INa recorded from small-diameter DRG neurons at the maximum time of injury, i.e., 14 days post-surgery (20). This would suggest that the chronic constriction injury type of injury has had little impact on the number of sodium channels present in the somal membrane at any given time. However, an initial loss of PN3/SNS immunolabeling was observed from the DRG at all levels of the lumbar enlargement (L4-L6), presumably from the predominantly intracellular pool, and correlated closely with a subsequent redistribution and accumulation of channel protein within the peripheral nerve just proximal to the site of injury (20). The onset and subsequent reversal of PN3/SNS channel redistribution appeared to correlate closely with temporal changes in behavioral thermal hyperalgesia and, morphologically, with the damage and recovery of primary afferent fibers after this type of nerve ligation (28). A similar observation has been made in another rat model of peripheral nerve injury in which the L5 and L6 dorsal spinal roots are tightly ligated, evoking behavioral signs of hyperalgesia and allodynia (29). A loss of PN3/SNS protein was observed in the DRG at the level of L5 and L6 with a subsequent accumulation at the injury site proximal to the ligatures (P. Mantyh, personal communication). However, although the expression of PN3 protein in the L5 and L6 DRG decreases after spinal nerve ligation (SNL) injury, PN3 protein levels in the uninjured L4 DRG were preserved in small diameter cells and significantly increased in the large diameter cells (Fig. 1). It is possible to speculate that the increased number of large diameter cells expressing PN3 protein after SNL may account, in part, for the observed tactile allodynia. Such observations provide additional support for the involvement of uninjured primary afferents in adjacent segments of the sciatic nerve in mediating certain types of neuropathic pain behaviors (30). The reasons for increased PN3 expression in large diameter cells in the non-injured L4 DRG after SNL and decreases in PN3 expression in cell bodies of the injured L5/L6 DRG are unclear but may reflect the differential availability of factors such as nerve growth factor, which is known to be released from peripheral tissues and to be retrogradely transported to the ganglion, where it regulates mRNA expression of PN3 (31) as well as other sodium channels (32, 33). However, it should be emphasized that the extent to which the immunolabeling pattern observed in the L4 ganglion translates into axonal accumulation of the channel protein and/or changes in the response thresholds to non-noxious sensory stimulation after nerve injury is unknown. In this regard, the possible accumulation of the PN3 channel at the site of the injured nerve in the L5 or L6 fibers may be sufficient to generate sustained ectopic discharge necessary to maintain the spinal cord in a “sensitized” state and to produce the well known changes in expression of central proteins characteristic of the nerve-injured state. The importance of the contribution of the input from the injured fibers is well documented as rhizotomy of the L5 and L6 dorsal roots blocks established SNL induced allodynia/hyperalgesia (34).
Figure 1.
Immunohistochemical (peroxidase-diaminobenzidine) analysis of PN3 antibody labeling of L4 DRG cells from normal and SNL animals, 7 days post-surgery. (A) Naive animals show a predominant labeling of the small diameter cells. In contrast, after SNL injury (B), in animals that had received saline for 48 hr, a marked increase was observed in the number of large diameter cells expressing PN3 in the L4 DRG ipsilateral to the side of injury. Animals treated with mismatch (MM) ODN (C) for 48 hr (day 7 post-surgery) exhibited a similar pattern of labeling to the saline controls. In contrast, animals that had received antisense (AS) ODN (D) for a similar time period to the MM ODN demonstrated a marked loss of PN3 immunolabeling in both small and large cells of the L4 DRG ipsilateral to the side of injury. (Bar = 50 μm.)
PN3 also has been implicated in the hyperesthesias that result from tissue injury. Thus, modulation of TTX-R INa, by a number of naturally occurring hyperalgesic substances, e.g., prostaglandin E2 (PGE2), adenosine and serotonin, has been suggested to be a mechanism that could underlie the subsequent increase in excitability and sensitization of sensory neurons mediated by these agents after a peripheral nerve or tissue injury (35, 36, 37). Immunohistochemical studies have found that PN3 protein expression appears to increase in the small-diameter DRG cells of the lumbar enlargement (L4-L6) after chronic inflammation induced by complete Freund’s adjuvant (CFA). In contrast, little evidence has been found for changes in PN3 protein levels after acute inflammation induced by either carrageenan or formalin (P. Mantyh, personal communication).
Antisense Studies.
In support of the immunohistochemical observations, evidence shows that spinal administration (45 μg, intrathecal, twice a day) of an antisense (5′-TCC-TCT-GTG-CTT-GGT-TCT-GGC-CT-3′), but not mismatch (5′-TCC-TTC-GTG-CTG-TGT-TCG-TGC-CT-3′), oligodeoxynucleotide (ODN) to a unique sequence of PN3/SNS produces a selective and reversible block of channel protein expression (Fig. 1) in rats with spinal nerve (L5/L6) ligation and can prevent the behavioral thermal hyperalgesia and tactile allodynia (Fig. 2) evoked by this type of injury. Cessation of the antisense ODN treatment at any time after the SNL injury results in the reoccurrence of tactile allodynia and thermal hyperalgesia within 48 hr, demonstrating the reversibility of the ODN effect. The equivalent time course for the onset and reversibility of the antisense ODN effect is consistent with the reported 26-hr half life for the rate of sodium channel turnover and biosynthesis (38). The lack of effect of the corresponding PN3 mismatch ODN, together with the lack of effect on baseline (i.e., uninjured) responses to non-noxious or noxious stimuli, also suggests that this was not a nonspecific artifact caused by repeated injections of an ODN. Intrathecal administration of ODNs previously have been demonstrated to alter the expression of proteins in the spinal cord, the DRG, and peripheral nerve terminals (39, 40). Further, the PN3 antisense, but not mismatch, ODN affected the expression level of the PN3 channel protein in the DRG, but neither ODN had an effect (Fig. 3) on the TTX-S sodium channels PN1 (12, 13) and NaCh6/SCN8A/PN4 (14, 15, 16). Pretreatment with PN3 antisense also appears to block the development of both tactile allodynia and thermal hyperalgesia in rats treated with CFA but has no effect on the hyperesthesias evoked acutely by carrageenan (Fig. 4). In each case, the corresponding mismatch ODN was completely ineffective.
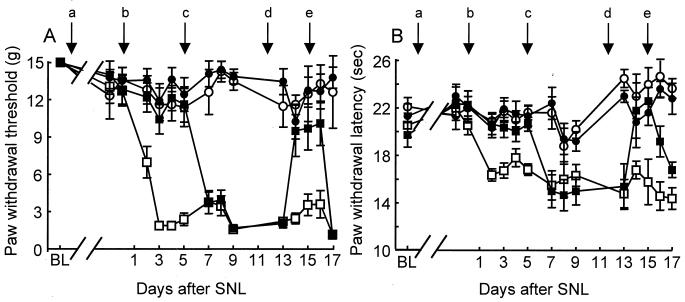
Figure 2.
Antisense (AS), but not mismatch (MM), ODN to PN3 prevents and reverses tactile allodynia (A) and thermal hyperalgesia (B) after SNL. After determination of baseline (BL) responses, the rats received twice daily injections (45 μg, intrathecal in a 5-μl volume) of either MM (open symbols) or AS (filled symbols) (arrow “a”). After 5 days of ODN pretreatment, rats were subjected to either SNL (squares) or sham surgery (circles) (arrow “b”). The ODN injections were terminated after the afternoon injection on day 5 (arrow “c”), recommenced on the morning of day 12 (arrow “d”), and were terminated again after the afternoon injection on day 15 (arrow “e”). Tactile allodynia was indicated by a significant (P ≤ 0.05) reduction in paw withdrawal threshold to application of a series of calibrated (0.4–15 g) von Frey filaments to the plantar surface of the hindpaw. Thermal hyperalgesia was indicated by a significant (P ≤ 0.05) reduction in paw withdrawal latency to application of noxious radiant heat to the plantar surface of the affected hindpaw of the nerve or sham-operated rats. A maximal cut-off of 40 sec was used to prevent tissue damage. n = 6 rats per group.
Figure 3.
Immunohistochemical (peroxidase-diaminobenzidine) analysis of PN1 (A–D) and PN4 (E–H) antibody labeling of L4 DRG cells from normal and SNL animals, 7 days post-surgery. (A) Naive animals show labeling of all cell types but with an increased intensity observed in small diameter cells. (B) The pattern of PN1 immunolabeling remains unchanged by SNL injury in animals that also received saline for 48 hr. SNL animals treated with either PN3 MM (C) or AS (D) ODNs exhibited a similar pattern of PN1 immunolabeling to the saline treated controls. Immunolabeling of L4 DRG cells with PN4 antibody followed a similar pattern to that observed with the PN1 antibody. (E) PN4 immunolabeling of all cell types, in L4 DRGs taken from naïve animals, but with an increased intensity observed in small diameter cells. (F) Animals receiving a SNL injury and treated with saline showed a similar PN4 immunolabeling pattern to the naïve animals. SNL animals treated with either PN3 MM (G) or AS (H) ODNs exhibited a similar pattern of PN4 immunolabeling to the saline treated controls. (Bar = 50 μm.)
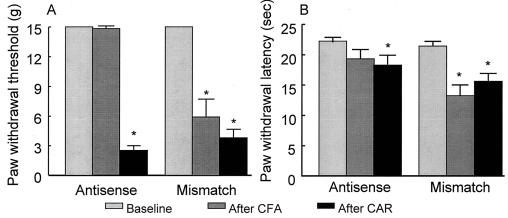
Figure 4.
Prevention of the development of tactile allodynia (A) or thermal hyperalgesia (B) after CFA, but not carrageenan (CAR)-induced, inflammation by PN3 antisense (AS) ODN. Rats received twice-daily injections (45 μg, intrathecal) of either mismatch (MM) or AS for 2 days and again on the morning of day 3. On the afternoon of day 3, the rats received an injection of CFA (150 μl) or of CAR (200 μl of 2%) to the hindpaw. Animals receiving CFA were tested 4 days afterward while receiving twice-daily administration of AS and MM ODNs throughout this period. Rats receiving CAR were tested on the afternoon of day 3, 3 hr after CAR injection. Tactile allodynia was indicated by a significant (∗, P ≤ 0.05) reduction in paw withdrawal threshold in rats receiving MM, but not AS, on the afternoon of the fourth day after CFA injection. Thermal hyperalgesia was indicated by a significant (*, P ≤ 0.05) reduction in paw withdrawal latency in rats receiving MM, but not AS, on the afternoon of the fourth day after CFA. All treatment groups receiving CAR demonstrated significant (*, P ≤ 0.05) reductions in response thresholds to tactile stimuli (A) or response latencies to thermal stimuli (B), and these responses were unaffected by either AS or MM ODNs. n = 6 rats per group.
These data suggest that the behavioral consequences of SNL injury and chronic inflammation require de novo PN3 protein synthesis. This view is borne out by noting that inhibition of PN3 protein expression by the antisense ODN does not affect normal noxious and non-noxious sensory thresholds on the contralateral side to the nerve injury and fails to affect the allodynia and hyperalgesia seen after carrageenan, an acute inflammation with a time-course that would not likely be associated with new channel protein synthesis. However, prevention of protein synthesis by pretreatment with antisense ODN before, or during, chronic injuries, such as SNL or CFA, results in a complete inhibition of the development of the behavioral consequences of the injury. It should be noted that the lack of effect of PN3 antisense ODN on the behavioral consequences of carrageenan-induced inflammation was studied only at the 3-hr time point after the initiation of the inflammation. This was most probably insufficient time for PN3 gene transcription and translation to occur to a level that would have had an impact on the inflammatory process. It is entirely possible that expression of PN3 might be regulated at a later time point and that PN3 antisense ODN might be active under such circumstances, in a similar manner to the effect observed after CFA treatment. In apparent contrast to these effects observed in vivo, it has been shown that the TTX-R current recorded from small diameter cells of the DRG appears to be modulated rapidly in cell culture by inflammatory agents such as PGE2 (36). This may simply be related to the much more rapid and sustainable local concentrations of the prostanoid achieved in vitro; that is, the time course remains to be determined for such inflammatory-mediated increases in TTX-R current in vivo. Nevertheless, the PGE2 effect on the TTX-R INa in vitro was partially reversed by application of an antisense ODN 21-mer, synthesized against a unique sequence of the PN3/SNS cDNA. Moreover, the PN3 antisense ODN also reversed the behavioral mechanical hyperalgesia evoked by PGE2 intradermal administration into the rat hindpaw (41).
The Pathophysiological Contribution of NaN/SNS2 in Peripheral Nerve Injury?
The novel sodium channel NaN/SNS2, recently cloned from rat DRG (22, 23), has an even more restricted distribution within the small diameter cell population of the DRG. This has led to its consideration as a second potential candidate for the TTX-R INa found in these cells. However, in contrast to PN3, the low threshold for activation, fast rate of inactivation, and intermediate TTX sensitivity of NaN/SNS2 resemble most closely the previously described properties of the cardiac channel and, more specifically, the TTX-R3 subtype of INa (21). In chronic pain states, a potential role for TTX-R3 INa and, for that matter, NaN/SNS2 has not yet been elucidated, but, like PN3/SNS, NaN/SNS2 mRNA levels in the DRG are markedly reduced after a peripheral nerve (sciatic) axotomy (23) and are elevated in the small diameter DRG cells after persistent inflammation evoked by CFA (23).
In contrast to the findings with PN3 antisense, spinal administration of antisense (5′ GCC TTG TCT TTG GAC TTC TTC 3′) and mismatch (5′ GCT CTG TTC TTG AGC TTT CTC 3′) ODN to NaN/SNS2 failed to produce any change in sensory thresholds after spinal nerve (L5/L6) ligation injury (Fig. 5) and did not alter gross behavior, as demonstrated by normal food intake, weight gain, and/or motor performance, in spite of a significant “knock-down” of the NaN/SNS2 protein (Fig. 6). This observation suggests, therefore, that the NaN/SNS2 subtype of TTX-insensitive sodium channel appears unlikely to play a prominent role in the alterations in the sensory phenotype that have been proposed to contribute to the ongoing paresthesias and pain after peripheral nerve injury. NaN/SNS2 channels, like TTX-R3 INa, appear to be activated, and possibly also inactivated, at much more hyperpolarized potentials than, for example, TTX-R1 INa/PN3. It is therefore possible that the ineffectiveness of the NaN/SNS2 antisense may reflect the unavailability of NaN/SNS2 channels to contribute to repetitive firing at the sustained level of membrane depolarization associated with injury to a peripheral nerve; that is, most will be in the inactivated state (21).
Figure 5.
Antisense (AS) to NaN/SNS2 administered to rats with SNL injury produced no effect on either tactile allodynia (A) or thermal hyperalgesia (B). Groups of six rats were used for each of the ODN or saline treatments and were monitored daily for tactile (von Frey) and thermal nociceptive (radiant heat) responses of the ipsilateral hindpaw. Mismatch (MM) or AS ODN (45 μg, intrathecal) were given twice daily to sham-operated rats and rats with SNL injury. Neither MM nor AS reversed tactile allodynia or thermal hyperalgesia in the ligated groups, and, likewise, neither treatment altered baseline values in the sham-operated groups. (●), sham-operated, AS; (■), SNL, AS; (▵), sham-operated, MM; (♦), SNL, MM.
Figure 6.
Immunohistochemical (peroxidase-diaminobenzidine) analysis of NaN/SNS2 antibody labeling of L4 DRG cells from normal and SNL animals. (A) Naive animals show labeling of small diameter neurons with NaN/SNS2 antibody. No labeling is seen in the preabsorbed control group (B). In contrast, sham-operated (C) or SNL (D) animals that had received AS ODN to NaN/SNS2 for 48 hr (a total of four injections) demonstrated a marked loss of NaN/SNS2 immunolabeling in small cells of the L4 DRG ipsilateral to the side of surgery. Labeling for NaN/SNS2 returned in both sham-operated (E) or SNL rats (F) that were perfused 4 days after the last AS ODN to NaN/SNS2 injection. (Bar = 50 μm.)
The physiological or, indeed, pathophysiological role of NaN/SNS2 therefore remains to be elucidated, particularly in persistent inflammatory conditions, where an increased expression level of NaN/SNS2 protein in the small diameter cells of the DRG has been recently reported (23). The lack of behavioral effects of NaN/SNS2 knock-down serves to validate that ODN administration does not elicit changes in behaviorally determined thresholds to noxious and non-noxious sensory stimuli in normal or nerve-injured animals non-specifically but, rather, that knock-down of a functionally important protein (i.e., PN3) is required for these effects to occur.
Conclusions
Collectively, therefore, the data suggest that the primary symptoms of neuropathic pain may be significantly attenuated by interfering with the expression and, consequently, the function of PN3 but not of other sodium channels, which are mainly distributed within the DRG such as NaN/SNS2. The PN3 antisense-mediated prevention of thermal hyperalgesia induced by CFA, but not by carrageenan, treatment indicates that the clinical potential for a selective inhibitor of PN3 may extend to pain resulting from chronic tissue, as well as nerve, injury. Such an effect is also consistent with recent evidence indicating that hyperalgesic substances associated with tissue injury can alter the function of PN3 (35, 36). The lack of an effect of the PN3 antisense on the noxious/non-noxious response thresholds of the contralateral side to nerve and tissue injury and on the consequences of an acute inflammation (i.e., carrageenan), where the time-course of the response makes new expression of the PN3 protein unlikely, suggests that this channel does not play a role in normal nociceptive function. Consequently, these data strongly suggest that selective inhibition of PN3 will not result in changes in normal, nociceptive function, although this clearly needs to be confirmed. The collective profile of the antisense ODNs against PN3 therefore implicates this channel in the pathophysiology of pain after nerve and tissue injury. However, it will be important to determine whether PN3 antisense administration will reverse an established injury in the same way that it has been found to prevent the injury. Moreover, although a role for PN3 appears to be emerging in peripheral nerve injury, it will be intriguing to see whether this profile might expand to encompass some of the other types of common neuropathies, e.g., metabolic and chemotherapy. The lack of any overt, particularly central nervous system, adverse events with the antisense ODN was consistent with the previously described discrete localization of the channel to sensory nerve fibers with an absence of staining in the central nervous system and cardiac tissue (20). It also reaffirms the potential that a selective inhibitor of PN3 may be clinically analgesic, providing not only an improved therapeutic window over existing therapies, but offering relief from neuropathic pain that is normally resistant to current therapies.
Acknowledgments
We thank Elda Tzoumaka, Dan Waligora, and Kimberly Wong for their expert technical assistance.
ABBREVIATIONS
- TTX-R
tetrodotoxin-resistant
- TTX-S
TTX-sensitive
- DRG
dorsal root ganglion
- SNL
spinal nerve ligation
- CFA
complete Freund’s adjuvant
- ODN
oligodeoxynucleotide
- MM
mismatch
- AS
antisense
- CAR
carrageenan
- PGE2
prostaglandin E2
References
- 1.Devor M. In: Textbook of Pain. Wall P D, Melzack R, editors. Edinburgh: Churchill Livingstone; 1994. pp. 79–100. [Google Scholar]
- 2.Woolf C J. In: Textbook of Pain. Wall P D, Melzack R, editors. Edinburgh: Churchill Livingstone; 1994. pp. 101–112. [Google Scholar]
- 3.Devor M, Govrin-Lippmann R, Angelides K. J Neurosci. 1993;13:1976–1992. doi: 10.1523/JNEUROSCI.13-05-01976.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.England J D, Happel L T, Kline D G, Gamboni F, Thouron C L, Liu Z P, Levinson S R. Neurology. 1996;47:272–276. doi: 10.1212/wnl.47.1.272. [DOI] [PubMed] [Google Scholar]
- 5.Wall P D, Gutnick M. Nature (London) 1974;248:740–743. doi: 10.1038/248740a0. [DOI] [PubMed] [Google Scholar]
- 6.Matzner O, Devor M J. J Neurophysiol. 1994;72:349–359. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- 7.Devor M, Wall P D, Catalan N. Pain. 1992;48:261–268. doi: 10.1016/0304-3959(92)90067-L. [DOI] [PubMed] [Google Scholar]
- 8.Ogata N, Tatebayashi H. J Physiol. 1993;466:9–37. [PMC free article] [PubMed] [Google Scholar]
- 9.Roy M L, Narahashi T. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott A A, Elliott J R. J Physiol. 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black J A, Dib-Hajj S, McNabola K, Jeste S, Rizzo M A, Kocsis J D, Waxman S G. Mol Brain Res. 1996;43:117–132. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 12.Toledo-Aral J J, Moss B L, He Z J, Koszowski A G, Whisenand T, Levinson S R, Wolf J J, Silos-Santiago I, Halegoua S, Mandel G. Proc Natl Acad Sci USA. 1997;94:1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangameswaran L, Fish L M, Koch B D, Rabert D K, Delgado S G, Ilnicka M, Jakeman L B, Novakovic S, Wong K, Sze P, et al. J Biol Chem. 1997;272:14805–14809. doi: 10.1074/jbc.272.23.14805. [DOI] [PubMed] [Google Scholar]
- 14.Schaller K L, Krzemien D M, Yarowsky P J, Krueger B K, Caldwell J H. J Neurosci. 1995;15:3231–3242. doi: 10.1523/JNEUROSCI.15-05-03231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess D L, Kohrman D C, Galt J, Plummer N W, Jones J M, Spear B, Meisler M H. Nat Genet. 1995;10:461–465. doi: 10.1038/ng0895-461. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich P S, McGivern J G, Delgado S G, Koch B D, Eglen R M, Hunter J C, Sangameswaran L. J Neurochem. 1998;70:2262–2272. doi: 10.1046/j.1471-4159.1998.70062262.x. [DOI] [PubMed] [Google Scholar]
- 17.Arbuckle J B, Docherty R J. Neurosci Lett. 1995;185:70–73. doi: 10.1016/0304-3940(94)11227-a. [DOI] [PubMed] [Google Scholar]
- 18.Sangameswaran L, Delgado S G, Fish L M, Koch B D, Jakeman L B, Stewart G R, Sze P, Hunter J C, Eglen R M, Herman R C. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 19.Akopian A N, Sivilotti L, Wood J N. Nature (London) 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 20.Novakovic S D, Tzoumaka E, McGivern J G, Haraguchi M, Sangameswaran L, Gogas K R, Eglen R M, Hunter J C. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rush A M, Brau M E, Elliott A A, Elliott J R. J Physiol. 1998;551:771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dib-Hajj S D, Tyrrell L, Black J A, Waxman S G. Proc Natl Acad Sci USA. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tate S, Benn S, Hick C, Trezise D, John V, Mannion R J, Costigan M, Plumpton C, Grose D, Gladwell Z, et al. Nat Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- 24.Elliott J R. Brain Res. 1997;754:221–226. doi: 10.1016/s0006-8993(97)00072-3. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo M A, Kocsis J D, Waxman S G. Neurobiol Disease. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 26.Cummins T R, Waxman S G. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dib-Hajj S, Black J A, Felts P, Waxman S G. Proc Natl Acad Sci USA. 1996;93:14950–14954. doi: 10.1073/pnas.93.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coggeshall R E, Dougherty P M, Pover C M, Carlton S M. Pain. 1993;52:233–242. doi: 10.1016/0304-3959(93)90136-D. [DOI] [PubMed] [Google Scholar]
- 29.Kim S H, Chung J M. Pain. 1992;50:355–363. [Google Scholar]
- 30.Yoon Y W, Na H S, Chung J M. Pain. 1996;64:27–36. doi: 10.1016/0304-3959(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 31.Black J A, Langworthy K, Hinson A W, Dib-Hajj S D, Waxman S G. NeuroReport. 1997;8:2331–2335. doi: 10.1097/00001756-199707070-00046. [DOI] [PubMed] [Google Scholar]
- 32.D’Arcangelo G, Paradiso K, Shepherd D, Brehm P, Halegoua S, Mandel G. J Cell Biol. 1993;122:915–921. doi: 10.1083/jcb.122.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zur K B, Oh Y, Waxman S G, Black J A. Mol Brain Res. 1995;30:97–103. doi: 10.1016/0169-328x(94)00283-k. [DOI] [PubMed] [Google Scholar]
- 34.Sheen K, Chung J M. Brain Res. 1993;610:62–68. doi: 10.1016/0006-8993(93)91217-g. [DOI] [PubMed] [Google Scholar]
- 35.England S, Bevan S, Docherty R J. J Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gold M S, Reichling D B, Shuster M J, Levine J D. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardenas C G, Del Mar L P, Scroggs R S. J Neurosci. 1997;17:7181–7189. doi: 10.1523/JNEUROSCI.17-19-07181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waechter C J, Schmidt J W, Catterall W A. J Biol Chem. 1983;258:5117–5123. [PubMed] [Google Scholar]
- 39.Bilsky E J, Wang T, Lai J, Porreca F. Neurosci Lett. 1996;220:155–158. doi: 10.1016/s0304-3940(96)13262-6. [DOI] [PubMed] [Google Scholar]
- 40.Khasar S G, Gold M S, Dastmalchi S, Levine J D. Neurosci Lett. 1996;218:17–20. doi: 10.1016/0304-3940(96)13111-6. [DOI] [PubMed] [Google Scholar]
- 41.Khasar S G, Gold M S, Levine J D. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]