Abstract
Puberty onset in female sheep is marked by a decrease in estradiol-negative feedback, allowing for the increase in GnRH and LH pulses that heralds the first ovulation. Based on recent genetic studies in humans, two possible neuropeptides that could promote puberty onset are kisspeptin and neurokinin B (NKB). Our first experiment determined whether the NKB agonist, senktide, could stimulate LH secretion in prepubertal ewes. A second study used prepubertal and postpubertal ewes that were intact or ovariectomized (OVX) to test the hypothesis that expression of kisspeptin and NKB in the arcuate nucleus increased postpubertally. For comparison, kisspeptin and NKB expression in age-matched intact, and castrated males were also examined. In experiment 1, the percentage of ewes showing an LH pulse immediately after injection of senktide (100 μg, 60%; 500 μg, 100%) was greater than that for water-injected controls (experiment 1a, 25%; experiment 1b, 20%). In experiment 2, kisspeptin-positive cell numbers in the arcuate nucleus increased after puberty in intact females and were increased by OVX in prepubertal but not postpubertal ewes. Changes in kisspeptin cell numbers were paralleled by changes in kisspeptin-close contacts onto GnRH neurons in the medial preoptic area. NKB cell numbers did not differ significantly between intact prepubertal and postpubertal ewes but increased with OVX in both age groups. NKB fiber immunoreactivity was greater in postpubertal than in prepubertal intact ewes. In age-matched males, kisspeptin and NKB cell numbers increased with castration, but decreased with age. These results support the hypothesis that kisspeptin is a gatekeeper to female ovine puberty and raise the possibility that NKB may also play a role, albeit through different means.
Puberty is defined as the time when an individual gains the ability to reproduce. In sheep, achievement of puberty is caused by a decrease in response to estradiol-negative feedback, resulting in an increased frequency of GnRH, and subsequently LH, pulses (1). In ewes, this increase in LH pulse frequency leads to increased estradiol production that in turn induces the subsequent GnRH/LH surge and first ovulation (2). An escape from estradiol-negative feedback and the resultant increase in LH secretion occurs around 25–32 wk of age in ewes (2–4), whereas in rams, this occurs earlier at 10–15 wk of age (5). Because GnRH neurons are devoid of estrogen receptor-α (6, 7), this neuroendocrine change in sensitivity to estradiol most likely occurs through interneurons that have yet to be identified. Kisspeptin and neurokinin B (NKB) are two likely neuropeptide candidates that could mediate such interneuronal communication.
Kisspeptin has received considerable attention due to reports that mutations in the kisspeptin receptor, G-protein coupled receptor 54, block pubertal development, and lead to hypogonadotropic hypogonadism in humans (8, 9) and mice (9). In subsequent studies across several species, it has been demonstrated that kisspeptin potently stimulates the GnRH/LH axis both before (10–13) and after puberty (14). Moreover, kisspeptin may drive the awakening of reproductive function at puberty because an increase in hypothalamic kisspeptin mRNA abundance is observed after puberty onset in rats (10) and monkeys (15). It has also been shown that kisspeptin mRNA in the hypothalamus increases after ovariectomy (OVX) and is inhibited by steroid replacement (10, 16–18). These data are consistent with the possibility that the kisspeptin/GnRH network is intact before puberty but merely inhibited by heightened sensitivity to estradiol negative feedback. One recent study has challenged the hypothesis that kisspeptin is essential for puberty and normal reproduction, because congenital ablation of kisspeptin neurons did not alter puberty or prevent ovulatory cycles in mice (19). However, they also reported that ablation of these neurons at 20 d of age in prepubertal mice completely abolished ovulatory cycles, suggesting that kisspeptin neurons are essential for reproduction in mice that have developed normally.
More recently, NKB has also been implicated in puberty, because mutations in the gene that encodes for NKB or its receptor, neurokinin 3 receptor (NK3R), block pubertal development in humans (20). Interestingly, within the arcuate nucleus (ARC) of the hypothalamus, NKB is found in the same neurons as kisspeptin in mice (21), sheep (22), goats (23), monkeys (24), and humans (25). Much like kisspeptin, ARC NKB mRNA abundance is inhibited by estradiol in adult female mice (26), rats (27), sheep (28), and monkeys (29). However, little information is available on changes in NKB expression across puberty. Although NKB action on the GnRH/LH axis in adult females varies depending on endocrine status (26, 30), recent reports in intact adult female rats (26) and sheep (31) show that an NKB receptor agonist, senktide, stimulates LH release. Furthermore, although NKB and senktide increase GnRH/LH secretion before puberty in the male monkey (24), the question still remains as to whether NKB can stimulate LH secretion in prepubertal females. Thus, we hypothesized that kisspeptin and NKB play critical roles in puberty onset in female sheep. This hypothesis leads to three testable predictions. First, we predict that the NKB network is intact before puberty so that senktide will stimulate LH release in prepubertal ewes. Second, because both appear to have stimulatory effects on GnRH/LH, we predict that kisspeptin and NKB expression will be greater in postpubertal than in prepubertal ovary-intact ewes. Third, because puberty is driven by a decreased response to estradiol-negative feedback, we predict that OVX will produce a larger increase in kisspeptin and NKB expression in pre- than postpubertal ewes. Because male sheep show sperm production (3) and a decreased sensitivity to estradiol-negative feedback (5) at 10–15 wk of age, we used age-matched males that were presumed to be postpubertal as a control for any effect of age that was independent of reproductive function.
Materials and Methods
Animals
For experiments 1a and 1b, prepubertal ewes (5–6 months old) of mixed breeding were housed and studied in an open barn. They received a daily diet of hay and water ad libitum. For experiment 2, a different group of mixed-breed prepubertal female sheep (5–6 months old), postpubertal female sheep (>9 months old), and age-matched males was used during the breeding season (October to February). As mentioned above, all males were presumed to be postpubertal. Sheep were housed in an open barn until 3–14 d before the study, when they were moved indoors. While indoors, they received an alfalfa pellet food ration and had open access to water and mineral supplement. Indoor lighting simulated the natural changes in day length. OVX were performed by midlateral laparotomy under gas anesthesia (oxygen + nitric oxide + 3% halothane) 2 wk before tissue collection. Blood samples were collected via jugular venipuncture into heparinized tubes, and plasma was stored at −20 C. All procedures were approved by the West Virginia University Animal Care and Use Committee and followed National Institutes of Health guidelines for use of animals in research.
Experimental design
Experiment 1a
Fourteen ovary-intact prepubertal ewes were weighed and placed into one of three treatment groups: sterile water (n = 4), senktide (n = 5; Tocris Bioscience, Ellisville, MO), or senktide + acyline (n = 5) (National Institute of Child Health and Human Development, Rockville, MD). Acyline (60 μg/kg), a GnRH receptor antagonist, was administered im immediately before blood collection. Blood samples were collected at 12-min intervals from ewes for 6 h with senktide (100 μg) or water (2 ml) administered iv at 3 h of sampling. Mean body weight of ewes injected with water (36.6 ± 5.2 kg), senktide (35.3 ± 3.4 kg), or senktide + acyline (36.6 ± 3.6 kg) did not differ among treatment groups.
Experiment 1b
Based on the results from experiment 1a, a second experiment was performed to examine a higher dose of senktide (500 μg). Ten ovary-intact prepubertal ewes from experiment 1a were randomly selected and received either water (n = 5) or senktide (n = 5). Blood samples were collected every 12 min for 6 h with injection of water (3 ml) or senktide (500 μg) administered iv at 3 h of sampling.
Experiment 2
Four groups (five per group) of females (prepubertal intact, prepubertal OVX, postpubertal intact-early follicular, and postpubertal OVX) were used for experiment 2. Early follicular (EF) phase ewes were used, because at this time in the ovarian cycle, the dominant steroid is estradiol and as such are similar in steroid environment to prepubertal intact ewes. The ovarian cycles of EF ewes were synchronized by two im injections of prostaglandin-F2α (6 mg/injection; Lutalyse; Henry Schein, Melville, NY) given 3 h apart and followed 7 d later by another two im injections given 3 h apart (32). Killing of ewes in this group occurred 24 h after the fourth injection of prostaglandin-F2α. Early follicular endocrine status was confirmed by absence of corpora lutea and low plasma progesterone (0.32 ± 0.1 ng/ml) on the day of killing. Four groups of age-matched males were either intact (rams) or castrated (wether) (young ram, young wether, older ram, and older wether). Blood samples (3 ml) were taken every 12 min for 4 h from all sheep immediately before killing.
Tissue was collected as described previously (33). Briefly, all sheep were heparinized (20,000 U) and killed using an iv overdose of sodium pentobarbital (Euthasol; Webster Veterinary, Devens, MA). Heads were removed and perfused with four liters of 4% paraformaldehyde in 0.1 m phosphate buffer (PB) (pH 7.4) containing 0.1% sodium nitrite via the carotid arteries. Blocks of tissue containing the preoptic area (POA) and the hypothalamus were then removed and stored in 4% paraformaldehyde for 24 h at 4 C and transferred to 20% sucrose until sectioned. Frozen coronal sections (50 μm) were cut with a freezing microtome and stored in cryopreservative until the time of immunocytochemical staining.
Immunocytochemistry for kisspeptin or NKB
On d 1, sections were washed 4 × 5 min in 0.1 m PBS and stored overnight at 4 C. On d 2, sections were washed 4 × 5 min in PBS then placed in 10% H2O2 for 10 min followed by 4 × 5-min washes in PBS. Tissue was then incubated for 1 h with 0.4% Triton X (Sigma-Aldrich, St. Louis, MO) in 20% normal goat serum for kisspeptin or 4% normal goat serum for NKB, both made in PBS. Kisspeptin and NKB neurons were identified using primary antibodies for kisspeptin (gift from A. Caraty) and NKB (Peninsula Laboratories, San Carlos, CA) that have been validated for use in sheep (22, 33). Five to six sections of the middle to caudal ARC from each animal were incubated with 1:50,000 kisspeptin antiserum raised in rabbit or 1:100,000 NKB antiserum raised in rabbit for 18 h at room temperature; one to two sections from the POA were also analyzed immunocytochemically for kisspeptin (1:50,000). On d 3, biotinylated goat antirabbit antibody (Vector Laboratories, Burlingame, CA) at 1:500 and Vectastain ABC-elite (Vector Laboratories) at 1:500 were applied sequentially for 1 h each with 4 × 5-min washes of PBS between incubations. Sections were then placed in a 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution (10 mg of DAB; Sigma-Aldrich) in 50 ml of PB with 20 μl of 30% H2O2 added just before incubation for 10 min. After 3 × 5-min washes in PB, sections were mounted on Superfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA), dehydrated using a series of increasing alcohol baths, and coverslipped using DPX Mounting Medium (Electron Microscopy Sciences, Hatfield, PA).
Dual immunocytochemistry for kisspeptin and GnRH
One section from the medial POA, which included the organum vasculosum of the lamina terminalis for each female ewe used in experiment 2 was chosen to analyze kisspeptin-positive close contacts on GnRH neurons. Kisspeptin staining was the same as described above through the incubation with ABC solution, except that the kisspeptin antibody was used at a 1:75,000 dilution. After the 1-h incubation with ABC solution, tissue was incubated for 30 min with Alexa Fluor 555 streptavidin (Invitrogen, Carlsbad, CA) at 1:300 in PBS followed by 4 × 5 min in PBS; sections and reagents with fluorescent substrates were covered to prevent light exposure from here on. Sections were incubated in 0.4% Triton X in PBS containing 4% normal goat serum for 1 h followed by overnight incubation in PBS with 4% normal goat serum containing mouse anti-GnRH antibody (1:3000, lot no. 3; Sternberger Monoclonal, Inc., Lutherville, MD) at room temperature. On d 4, sections were washed 4 × 5 min in PBS and then incubated in Alexa Fluor 488 conjugated goat antimouse antibody (Invitrogen) at 1:200 in PBS for 30 min. Tissue was washed 4 × 5 min in PB, then coverslipped using gelvatol and stored in the dark at 4 C.
Data analysis
Immunocytochemistry
For single antigen staining, cell bodies, identified by cells containing a brown cytoplasmic staining, were counted manually using an Olympus AZ70 transmitted light microscope (Center Valley, PA) from four (NKB) or five (kisspeptin) sections of middle to caudal ARC, and mean cell numbers/section for each group were averaged, because there was no difference in cell numbers between the middle and caudal ARC. NKB fiber immunoreactivity was assessed for one representative caudal ARC section from each intact and OVX ewe by three individuals blinded to treatment group. Each individual ranked fiber staining from zero to four, with zero being minimal fiber immunoreactivity and four being the most dense.
For kisspeptin/GnRH staining, kisspeptin-positive close contacts (red) on GnRH neurons (green; 9–11 neurons per animal) were acquired from a three-dimensional (3D) reconstruction using a Zeiss LSM 510 laser scanning confocal (Hornwood, NY) on a Zeiss Axio Image Z1 upright microscope with a Plan Apochromat 63×/1.4 oil objective. 3D images presented here were taken at 1-μm increments through each GnRH cell, the image stack was deconvoluted using Auto Quant X2.2 (Media Cybernetics, Bethesda, MD), and 3D rendering was performed using NIS Elements AR 3.2 (Melville, NY).
Assays
LH concentrations were measured in duplicate with a RIA using 100–200 μl of plasma and reagents provided by the National Hormone and Peptide Program (Torrance, CA) as previously described (34). LH assay sensitivity averaged 0.07 ng/ml (NIH S24) with intra- and interassay coefficients of variation being 12.7 and 18.2%, respectively. Progesterone concentrations were measured in duplicate 150-μl aliquots of two plasma samples from each EF ewe using a RIA that has been validated for use in sheep (35); assay sensitivity was 0.01 ng/ml.
Statistics
Pulses were identified using previously described criteria (36). For experiment 1, the comparison of percentages of animals that displayed an LH pulse within 24 min (two samples) after iv administration of water or senktide was analyzed using the χ2 test. Also for experiment 1, mean LH for two samples immediately before iv injection was averaged and compared with the mean of two samples taken immediately after iv injection using a paired t-test. For experiment 2, mean LH, LH pulse amplitude, mean number of cell bodies between groups within sex, and number of kisspeptin-positive close contacts per GnRH neuron were analyzed using two-way ANOVA. The percentage of GnRH neurons contacted by kisspeptin was transformed using natural log and then analyzed using a two-way ANOVA. The three rankings of NKB fiber immunoreactivity were averaged, and differences between prepubertal and postpubertal intact ewes or prepubertal and postpubertal OVX ewes were analyzed using the nonparametric permutation test for two independent samples. LH pulse frequency was analyzed using the Mann-Whitney U test. Differences were considered to be significant at P < 0.05.
Results
Experiment 1a. Can senktide, an NK3R agonist, stimulate LH secretion in prepubertal lambs?
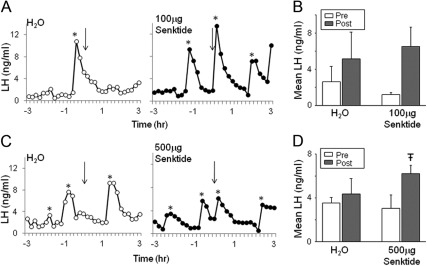
Representative LH pulse profiles from prepubertal ewes receiving an iv injection of water (open circles) or 100 μg of senktide (closed circles) are illustrated in Fig. 1A. Senktide had no significant effect on pulse frequency (preinjection, 1.4 ± 0.3 pulses/3 h vs. postinjection, 1.8 ± 0.4 pulses/3 h). Because it was possible that the effects of the senktide were immediate but transitory, we analyzed the percentage of animals that responded with an LH pulse within 24 min of injection and found a significant difference between senktide and water-injected animals. Senktide stimulated an LH pulse in three of five (60%) prepubertal ewes within 24 min, whereas only one of four (25%) prepubertal ewes receiving water displayed an LH pulse. Mean LH 24 min after injection of water (5.14 ± 2.58 ng/ml) did not significantly differ from mean values 24 min before injection (2.63 ± 1.45 ng/ml) but there was a strong tendency (P = 0.052) (Fig. 1B) for mean LH to be greater 24 min after 100 μg of senktide (6.51 ± 1.93 ng/ml) than 24 min before senktide (1.18 ± 0.21 ng/ml). None of the five prepubertal ewes that received the GnRH receptor antagonist, acyline, just before blood collection showed an LH pulse in response to 100 μg of senktide (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Fig. 1.
A, LH profiles from individual prepubertal ewes that were treated with water (open circles) or 100 μg of senktide (closed circles). B, Mean LH 24 min before (Pre) and 24 min after (Post) iv injection. C, LH profiles from individual prepubertal ewes that were treated with water (open circles) or 500 μg of senktide (closed circles). D, Mean LH 24 min before (Pre) and 24 min after (Post) iv injection. Arrows indicate time of iv injection. *, LH pulses. Significance (P < 0.05) is indicated by [stroke]T.
Experiment 1b
Given the partial response in experiment 1a, we next tested the response to a higher dose (500 μg) of senktide. Representative LH pulse profiles from prepubertal ewes receiving an iv injection of either water (open circles) or 500 μg of senktide (closed circles) is illustrated in Fig. 1C. This dose of senktide stimulated an LH pulse in all five (100%) prepubertal ewes, whereas only one of five (20%) prepubertal ewes receiving an injection of water displayed an LH pulse within 24 min of injection, a difference in response that was significant. Furthermore, mean LH for 24 min after injection of 500 μg of senktide (6.22 ± 0.68 ng/ml) was significantly greater than mean values for 24 min before injection (3.05 ± 1.08 ng/ml), whereas vehicle had no significant effect (preinjection, 3.56 ± 0.44 ng/ml; postinjection, 4.36 ± 1.27 ng/ml) on mean LH concentrations (Fig. 1D).
Experiment 2. Does expression of kisspeptin or NKB protein increase with puberty and/or gonadectomy (GNDX)?
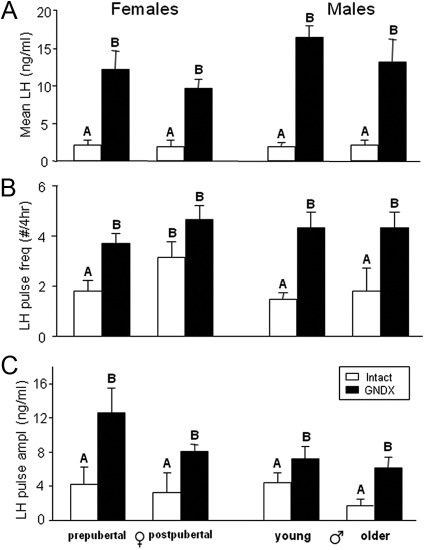
Mean LH (Fig. 2A) and LH pulse amplitude (Fig. 2C) were significantly increased after GNDX in both ewes and rams at all ages, but there was no effect of age and no age by GNDX interaction. LH pulse frequency (Fig. 2B) was also significantly increased after GNDX in prepubertal females and in both young and older males, but not in postpubertal females.
Fig. 2.
Group means (±sem) for mean LH (A), LH pulse frequency (B) and LH pulse amplitude (C) in prepubertal and postpubertal females (left) and age-matched males (right). Open bars represent gonad-intact animals; postpubertal ewes are in the early follicular (EF) phase. Closed bars represent GNDX animals. Significance (P < 0.05) within sex is indicated by differing superscripts.
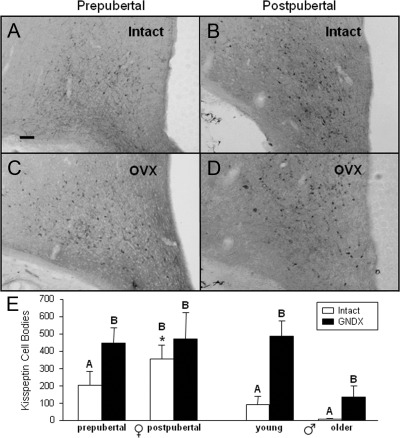
Analysis of kisspeptin cell numbers in the ARC indicated that the number of kisspeptin-positive cells was higher after puberty (358 ± 77 per ewe) than before puberty (204 ± 81 per ewe) in ovary-intact females (Fig. 3, A, B, and E). Furthermore, kisspeptin-positive cell numbers significantly increased after OVX (Fig. 3E) in prepubertal ewes (447 ± 88 per ewe), but not in postpubertal ewes (473 ± 151 per ewe). In males, there was a significant effect of GNDX, because kisspeptin cell numbers were higher in castrated males compared with intact rams (Fig. 3E and Supplemental Fig. 2). There also was a significant effect of age, indicating that cell numbers were lower in both intact and castrated older males compared with younger males (Fig. 3E) but no interaction of age and GNDX. Kisspeptin cell numbers strongly tended (P = 0.068) to be greater in postpubertal OVX ewes (473 ± 96 per ewe) than age-matched GNDX males (138 ± 105 per wether) but were not different between prepubertal OVX ewes (447 ± 89 per ewe) and age-matched GNDX males (486 ± 96 per wether).
Fig. 3.
A–D, Photomicrographs of kisspeptin-positive neurons in the caudal portion of the ARC. Sections are from representative prepubertal and postpubertal females that were intact (A and B) or OVX (C and D). E, Mean (±sem) number of kisspeptin-positive neurons in the ARC of prepubertal and postpubertal females (left) or age-matched males (right). Significance (P < 0.05) within sex is indicated by differing superscripts. *, Significant (P < 0.05) difference between intact female groups. Scale bar, 200 μm.
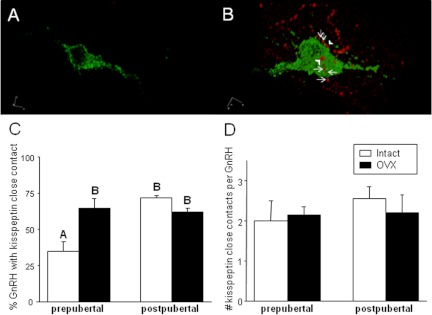
To determine whether puberty-related changes in kisspeptin cell numbers altered kisspeptin synaptic input onto GnRH neurons, we examined kisspeptin-positive close contacts on GnRH neurons in the medial POA in females (Fig. 4). The change in the percentage of GnRH neurons displaying kisspeptin-positive close contacts (Fig. 4C) paralleled the changes that we observed in kisspeptin cell numbers. Specifically, a significantly higher percentage of GnRH neurons received close contacts after puberty in ovary-intact females (prepubertal, 34.9 ± 6.5%; postpubertal, 71.7 ± 1.8%). This percentage significantly increased after OVX in prepubertal ewes (64.9 ± 6.6%), but not in postpubertal ewes (61.8 ± 2.7%). However, the number of kisspeptin-positive close contacts per GnRH neuron (Fig. 4D) did not differ significantly among groups.
Fig. 4.
Top panels, 3D reconstruction of confocal microscopic images of GnRH neurons (green) lacking (A) and with (B) kisspeptin (red) close contacts from a prepubertal and postpubertal ewe, respectively. B, Kisspeptin-positive close contacts (arrowheads) with incoming kisspeptin fiber (arrows) adjacent to the GnRH neuron. Bottom panels, Mean (±sem) percentage of GnRH neurons with at least one kisspeptin close contact (C) and the mean (±sem) number of close contacts per GnRH neuron that received at least one close apposition (D). Open bars represent intact ewes, and closed bars represent OVX ewes. Significance (P < 0.05) is indicated by differing superscripts. Images were visualized using a 63×/1.4 oil immersion objective.
POA sections containing GnRH neurons were also examined at lower magnification to quantify kisspeptin-containing cell bodies in this region with immunofluorescence. In contrast to previous analysis of tissue from adults (22), only one kisspeptin-positive cell body was observed in all sections from these young females. Therefore, we examined kisspeptin in a wider range of POA sections from these same females using single-label immunocytochemistry. Even using DAB as a chromogen, kisspeptin immunoreactivity in the POA was light and inconsistent across ewes (Supplemental Fig. 3), making accurate identification and counting of cells impossible. Therefore, we did not quantify kisspeptin cells in the POA for this study.
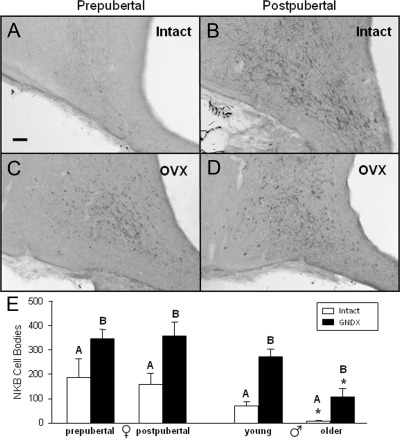
NKB cell numbers were examined only in the ARC, because NKB-containing neurons are largely limited to this nucleus in the ovine hypothalamus (37). The number of NKB cells (Fig. 5) significantly increased after OVX in both prepubertal and postpubertal females (from 188 ± 75 to 347 ± 37 per ewe and from 158 ± 46 to 357 ± 56 per ewe, respectively) (Fig. 5E). However, in contrast to kisspeptin, there was no change in the number of NKB-containing cells between prepubertal (188 ± 75 per ewe) and postpubertal (158 ± 46 per ewe) ovary-intact ewes (Fig. 5E). While counting cell numbers, we noted obvious variation among animals in NKB fiber density. After further assessment, we found that NKB fiber immunoreactivity was significantly greater in EF ewes (rank of 2.7 ± 0.8) compared with prepubertal intact ewes (rank of 1.2 ± 0.4) with no significant difference in fiber density between prepubertal (rank of 1.3 ± 0.8) and postpubertal OVX ewes (rank of 2.8 ± 0.6). In males, GNDX significantly increased NKB cell numbers at both ages (Fig. 5E). There was an interaction of age and GNDX on NKB-positive cell numbers, likely due to the larger absolute decrease in NKB cell numbers observed in GNDX males (δ, −143 per wether) with age compared with intact males (δ, −73 per ram) (Fig. 5E and Supplemental Fig. 4). However, the percent decrease with age appeared to be greater for the intact (89% decrease) than GNDX (60% decrease) males. NKB cell numbers were significantly greater in postpubertal OVX ewes (357 ± 37 per ewe) than age-matched GNDX males (108 ± 45 per wether), but were not different between prepubertal OVX ewes (347 ± 37 per ewe) and age-matched GNDX males (272 ± 37 per wether). In addition, NKB cell numbers decreased with age in GNDX males.
Fig. 5.
A–D, Representative photomicrographs of NKB-positive neurons in the caudal portion of the ARC from ovary-intact (A and B) and OVX (C and D) prepubertal or postpubertal females. E, Mean (±sem) number of NKB-positive neurons in the ARC from prepubertal and postpubertal females (left) and age-matched males (right). Open bars represent gonad-intact animals, and closed bars represent GNDX animals. Significance (P < 0.05) within sex is indicated by difference in superscripts. *, Significant (P < 0.05) age by GNDX interaction. Scale bar, 200 μm.
Discussion
The data herein provide evidence of a role for kisspeptin and NKB in puberty of female sheep. Our observation that kisspeptin cell numbers within the ARC increase after puberty in females, but not in age-matched postpubertal males, is consistent with an important role for this peptide in the onset of puberty. Furthermore, we show an increase in kisspeptin-positive close contacts on GnRH neurons in the medial POA of ewes that closely mirrored the increase in ARC kisspeptin cell numbers after puberty. The stimulatory effect of senktide on LH secretion and the OVX-induced increase in NKB cell number in prepubertal ewes indicate a possible role for NKB in estradiol-negative feedback before puberty. Although there was not an increase in NKB-positive cell bodies after puberty, the increased density of NKB immunoreactive fibers leads us to suggest that NKB expression may also be increased.
It is generally thought that kisspeptin input to GnRH neurons is a limiting factor for induction of the onset of puberty, but whether it is the kisspeptin neurons in the ARC or more rostral areas [anteroventral periventricular nucleus (AVPV) in rodents, POA in other species, (38)] that are involved remains controversial. In female rats, postpubertal increases in kisspeptin mRNA (39, 40) and protein (39) occur in both the AVPV and ARC. In female mice, kisspeptin mRNA and protein increase in the AVPV (41–44), but changes in ARC kisspeptin mRNA expression are inconsistent (41, 42), leading to the hypothesis that AVPV kisspeptin drives puberty onset in mice (43). However, it was recently reported (19) that ablation of ARC kisspeptin neurons at postnatal d 20 disrupted ovarian function in mice, a result consistent with ARC kisspeptin neurons playing a critical role in puberty. The authors argued that these lesions were ARC specific because the time of kisspeptin neuronal ablation occurred before appearance of kisspeptin in the AVPV, but provided no immunocytochemical staining of the AVPV or ARC to support the specificity of the lesion. In estradiol-treated OVX ewes, kisspeptin mRNA increased with age in the POA, but not in the ARC, during pubertal development (45). In the present study, we observed that kisspeptin cell numbers in the ARC are greater in postpubertal than prepubertal ewes, whereas kisspeptin immunoreactivity in the POA was insufficient to accurately quantify cell numbers. Comparison of these two studies in ewes suggests a possible disconnect between mRNA and protein, as has been observed in pubertal mice (41). Notably, both studies observed a positive association between ARC kisspeptin expression and LH pulse frequency during pubertal development, a finding consistent with the hypothesis that an increase in ARC kisspeptin is required for pubertal progression in the ewe.
Interestingly, we also observed that kisspeptin-positive close contacts on GnRH neurons increase after OVX in prepubertal ewes and are significantly greater in postpubertal compared with prepubertal ovary-intact lambs. A similar increase in kisspeptin inputs onto GnRH neurons after puberty has been observed in mice (43), but the origin of this input remains unclear. The coinciding changes in kisspeptin content within the ARC with the changes in kisspeptin-containing close contacts on GnRH neurons strongly suggests that kisspeptin cells in the ARC, and not the POA, are the source of this input in ewes. A similar correlation of kisspeptin in the ARC and kisspeptin input onto GnRH neurons was seen between breeding and nonbreeding seasons in the adult ewe (46). Although it has been reported that only 1–2% of kisspeptin contacts onto GnRH neurons originate from the ARC (47), this tract-tracing study used a small injection volume of Fluoro-Gold, which may have covered only a relatively small area of the POA, in which GnRH neurons reside. A different anatomical approach was used to show that approximately 50–70% of GnRH neurons receive close contacts containing both kisspeptin and dynorphin (48), a percentage consistent with that observed in OVX or intact postpubertal ewes from our study. Because the only set of neurons known to contain both kisspeptin and dynorphin in the hypothalamus are the subset of neurons in the ARC that also contain NKB (22, 38), we conclude that kisspeptin input from within the ARC is primarily responsible for the increased input to GnRH neurons during puberty in the ewe.
Because puberty onset in the ewe is marked by an increase in GnRH/LH secretion due to a decrease in estradiol-negative feedback (1), we predicted that a pubertal increase in kisspeptin production within the ARC would occur in response to a lessening of steroid-negative feedback. The increase in kisspeptin expression after OVX of prepubertal, but not postpubertal, ewes is consistent with this hypothesis. It should also be noted that the proposed negative feedback inhibition of kisspeptin expression in the ARC before puberty is consistent with findings that estradiol exerts negative feedback effects on kisspeptin mRNA and protein expression in the adult ovine ARC during anestrus (46). The lack of an increase after OVX in our postpubertal ewes contrasts with previous data in ewes using in situ hybridization (17). One explanation for these differences is that by using immunocytochemistry, we are unable to detect any increase in kisspeptin production within cells that were already producing immunoreactive peptide. However, this apparent contradiction most likely reflects differences in cell numbers between ovary-intact groups in the luteal (17) and follicular (this study) phases, because the number of kisspeptin cells in the ARC increases after luteolysis in the ewe (49).
As previously mentioned, kisspeptin cells in the ovine ARC of the hypothalamus also contain NKB (22). In humans, NKB and NK3R are crucial for puberty onset (20). We report here that a peripheral injection of senktide, an NK3R agonist, stimulated LH release in the prepubertal ewe. Because acyline abolished LH secretion and blocked any effect of senktide, we suggest that the effect of senktide occurs at the level of the hypothalamus to cause GnRH release. These data fit well with recent reports that intact adult ewes (31) and prepubertal male primates (24) both respond to senktide administration with an increase in LH release. In OVX goats, NKB stimulates multiunit activity within the ARC but decreases LH secretion; no effects of senktide were seen in OVX ewes (31). In rodents, both stimulatory (26, 50, 51) and inhibitory (30, 50) effects of senktide on LH have been observed, a response that seems largely dependent upon steroid milieu.
Unlike kisspeptin, NKB cell numbers did not increase postpubertally in intact ewes, which is similar to a previous report in mice using in situ hybridization to identify these cells (52). It is possible that NKB cell numbers increased in prepubertal ewes before the time analyzed in this study. If so, this increase did not lead to stimulation of LH release or induction of puberty, perhaps due to insufficient kisspeptin. This would be consistent with the hypothesis that NKB acts via kisspeptin to stimulate GnRH/LH release (21, 23, 53, 54) and with the idea that although NKB may be necessary, kisspeptin is the gatekeeper to puberty onset. Our data also indicate that NKB and kisspeptin may be differentially regulated in the same cells, as has been observed previously within kisspeptin/NKB/dynorphin neurons (55) as well as other neurons in the ARC that express more than one neuropeptide (56). Although cell numbers were unaltered, NKB fiber density did increase in EF ewes compared with prepubertal intact ewes. Increases in kisspeptin immunoreactive fibers within the ARC during the pubertal transition have been reported in rodents (43, 44), but this is the first evidence for similar increases in NKB immunoreactive fibers. One simple explanation for this observation is that parallel increases in synthesis and transport of NKB occur in these neurons. Interestingly, NKB cell numbers did increase after GNDX in both prepubertal and postpubertal ewes, suggesting that estradiol was limiting NKB expression at both ages. This is consistent with the report where estradiol failed to suppress NKB gene expression in estrogen receptor-α knockout mice (57), because NKB neurons contain ERα (53).
Sex differences in kisspeptin expression in sheep (55) and humans (25) and NKB expression in sheep (35, 55) have been reported to exist within the ARC. Sex differences in NKB expression are clearly due to organizational effects of testosterone during prenatal development (37, 55), but whether the sexual dimorphism in kisspeptin content is due to organizational or activational effects of gonadal steroids remains unclear (55). The inclusion of OVX ewes and castrated males of a similar age in our study allows us to address this question. Our data show that in the ovine ARC, kisspeptin cell numbers strongly tended to be, and NKB cell numbers were, significantly greater in postpubertal OVX ewes compared with castrated males of a similar age. Because these differences cannot be due to activational actions of gonadal steroids, we conclude that sex differences in both kisspeptin and NKB are caused by organizational actions of steroids. Interestingly, prepubertal OVX ewes did not differ from castrated males of a similar age in kisspeptin and NKB cell numbers. Thus, the organizational effects of steroids on these neurons in sheep may only become evident as they reach adulthood and appear largely to be due to a loss of expression in older males (Figs. 3 and 5).
In conclusion, the data here support the hypothesis that kisspeptin is a gatekeeper to puberty onset in the ewe and raise the possibility that increases in NKB may also contribute to this process. Furthermore, we suggest the increase in kisspeptin input is reflected in the medial POA at the GnRH cell bodies. These data also clearly demonstrate that NKB expression is under the control of steroid negative feedback in both prepubertal and postpubertal ewes and support the proposed stimulatory role for this neuropeptide in the ewe.
Supplementary Material
Acknowledgments
We thank Heather Bungard and Jennifer Lydon (West Virginia University Food Animal Research Facility) for care of animals, Paul Harton for his technical assistance in sectioning tissue, Dr. John Connors for statistical analysis, and Dr. Alain Caraty for the kisspeptin antibody. We also thank Dr. Al Parlow and the National Hormone and Peptide Program for reagents used to measure LH and the Contraception and Reproductive Health Branch of the National Institute of Child Health and Human Development (Rockville, MD) for the gift of acyline.
The work was in part supported by the National Center for Research Resources Grant P20RR16477 awarded to the West Virginia IDeA Network for Biomedical Research Excellence. Imaging experiments and image analysis were performed in the West Virginia University Microscope Imaging Facility, which has been supported by the Mary Babb Randolph Cancer Center and National Institutes of Health (NIH) Grants P20 RR016440 and P30 RR032138. This work was primarily supported by the NIH Grants RO1 HD039916 and HD017864.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- 3D
- three dimensional
- DAB
- 3,3′-diaminobenzidine tetrahydrochloride
- EF
- early follicular
- GNDX
- gonadectomy
- NKB
- neurokinin B
- NK3R
- neurokinin 3 receptor
- OVX
- ovariectomy
- PB
- phosphate buffer
- POA
- preoptic area.
References
- 1. Foster DL, Jackson LM. 2006. Puberty in the sheep. In: Neill JD, ed. Knobil and Neill's physiology of reproduction. 3rd ed Vol 2 Amsterdam: Elsevier; 2127–2176 [Google Scholar]
- 2. Huffman LJ, Inskeep EK, Goodman RL. 1987. Changes in episodic luteinizing hormone secretion leading to puberty in the lamb. Biol Reprod 37:755–761 [DOI] [PubMed] [Google Scholar]
- 3. Claypool LE, Foster DL. 1990. Sexual differentiation of the mechanism controlling pulsatile secretion of luteinizing hormone contributes to sexual differences in the timing of puberty in sheep. Endocrinology 126:1206–1215 [DOI] [PubMed] [Google Scholar]
- 4. Foster DL, Karsch FJ, Olster DH, Ryan KD, Yellon SM. 1986. Determinants of puberty in a seasonal breeder. Recent Prog Horm Res 42:331–384 [DOI] [PubMed] [Google Scholar]
- 5. Olster DH, Foster DL. 1986. Control of gonadotropin secretion in the male during puberty: a decrease in response to steroid inhibitory feedback in the absence of an increase in steroid-independent drive in the sheep. Endocrinology 118:2225–2234 [DOI] [PubMed] [Google Scholar]
- 6. Herbison AE, Robinson JE, Skinner DC. 1993. Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology 57:751–759 [DOI] [PubMed] [Google Scholar]
- 7. Lehman MN, Karsch FJ. 1993. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133:887–895 [DOI] [PubMed] [Google Scholar]
- 8. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 10. Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- 11. Plant TM, Ramaswamy S, Dipietro MJ. 2006. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 147:1007–1013 [DOI] [PubMed] [Google Scholar]
- 12. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Redmond JS, Macedo GG, Velez IC, Caraty A, Williams GL, Amstalden M. 2011. Kisspeptin activates the hypothalamic-adenohypophyseal-gonadal axis in prepubertal ewe lambs. Reproduction 141:541–548 [DOI] [PubMed] [Google Scholar]
- 14. Roseweir AK, Millar RP. 2009. The role of kisspeptin in the control of gonadotrophin secretion. Hum Reprod Update 15:203–212 [DOI] [PubMed] [Google Scholar]
- 15. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. 2005. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 17. Smith JT, Clay CM, Caraty A, Clarke IJ. 2007. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- 18. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. 2007. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 19. Mayer C, Boehm U. 2011. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci 14:704–710 [DOI] [PubMed] [Google Scholar]
- 20. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 23. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. 2010. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci 31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 26. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rance NE, Bruce TR. 1994. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology 60:337–345 [DOI] [PubMed] [Google Scholar]
- 28. Pillon D, Caraty A, Fabre-Nys C, Bruneau G. 2003. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol 15:749–753 [DOI] [PubMed] [Google Scholar]
- 29. Abel TW, Voytko ML, Rance NE. 1999. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab 84:2111–2118 [DOI] [PubMed] [Google Scholar]
- 30. Sandoval-Guzmán T, Rance NE. 2004. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- 31. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. 2010. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deaver DR, Stilley NJ, Dailey RA, Inskeep EK, Lewis PE. 1986. Concentrations of ovarian and pituitary hormones following prostaglandin F2α-induced luteal regression in ewes varies with day of the estrous cycle at treatment. J Anim Sci 62:422–427 [DOI] [PubMed] [Google Scholar]
- 33. Foradori CD, Amstalden M, Goodman RL, Lehman MN. 2006. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18:534–541 [DOI] [PubMed] [Google Scholar]
- 34. Whisnant CS, Goodman RL. 1988. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod 39:1032–1038 [DOI] [PubMed] [Google Scholar]
- 35. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. 2004. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- 36. Goodman RL, Karsch FJ. 1980. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107:1286–1290 [DOI] [PubMed] [Google Scholar]
- 37. Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. 2000. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- 38. Lehman MN, Merkley CM, Coolen LM, Goodman RL. 2010. Anatomy of the kisspeptin neural network in mammals. Brain Res 1364:90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. 2009. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 21:527–537 [DOI] [PubMed] [Google Scholar]
- 40. Takumi K, Iijima N, Ozawa H. 2011. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci 43:138–145 [DOI] [PubMed] [Google Scholar]
- 41. Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. 2010. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One 5:e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. 2010. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107:22693–22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Redmond JS, Baez-Sandoval GM, Spell KM, Spencer TE, Lents CA, Williams GL, Amstalden M. 2011. Developmental changes in hypothalamic Kiss1 expression during activation of the pulsatile release of luteinising hormone in maturing ewe lambs. J Neuroendocrinol 23:815–822 [DOI] [PubMed] [Google Scholar]
- 46. Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. 2008. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Backholer K, Smith J, Clarke IJ. 2009. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology 150:5488–5497 [DOI] [PubMed] [Google Scholar]
- 48. Merkley CM, Coolen LM, Goodman RL, Lehman MN. 2011. Direct projections of arcuate kndy (kisspeptin/neurokinin b/dynorphin) neurons to gnrh neurons in the sheep. Washington, DC: Society for Neuroscience [Google Scholar]
- 49. Smith JT, Li Q, Pereira A, Clarke IJ. 2009. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150:5530–5538 [DOI] [PubMed] [Google Scholar]
- 50. Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O'Byrne KT. 2012. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 153:307–315 [DOI] [PubMed] [Google Scholar]
- 51. Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. 2011. Regulation of NKB pathways and their roles in control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. 2009. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:E1212–E1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lehman MN, Coolen LM, Goodman RL. 2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. 2012. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 153:316–328 [DOI] [PubMed] [Google Scholar]
- 55. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. 2010. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 151:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kas MJ, Bruijnzeel AW, Haanstra JR, Wiegant VM, Adan RA. 2005. Differential regulation of agouti-related protein and neuropeptide Y in hypothalamic neurons following a stressful event. J Mol Endocrinol 35:159–164 [DOI] [PubMed] [Google Scholar]
- 57. Dellovade TL, Merchenthaler I. 2004. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology 145:736–742 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.