Abstract
No other tissue in the body undergoes such a vast and extensive growth and remodeling in a relatively short period of time as the primate endometrium. Endometrial integrity is coordinated by ovarian hormones, namely, estrogens, progesterone, and the embryonic hormone chorionic gonadotropin (CG). These regulated events modulate the menstrual cycle and decidualization. The Notch family of transmembrane receptors regulate cellular proliferation, differentiation, and apoptosis, cellular processes required to maintain endometrial integrity. In two primate models, the human and the simulated pregnant baboon model, we demonstrated that Notch1 is increased during the window of uterine receptivity, concomitant with CG. Furthermore, CG combined with estrogens and progesterone up-regulate the level of Notch1, whereas progesterone increases the intracellular transcriptionally competent Notch1, which binds in a complex with progesterone receptor. Inhibition of Notch1 prevented decidualization, and alternatively, when decidualization is biochemically recapitulated in vitro, Notch1 is down-regulated. A focused microarray demonstrated that the Notch inhibitor, Numb, dramatically increased when Notch1 decreased during decidualization. We propose that in the endometrium, Notch has a dual role during the window of uterine receptivity. Initially, Notch1 mediates a survival signal in the uterine endometrium in response to CG from the implanting blastocyst and progesterone, so that menstrual sloughing is averted. Subsequently, Notch1 down-regulation may be critical for the transition of stromal fibroblast to decidual cells, which is essential for the establishment of a successful pregnancy.
During the normal menstrual cycle, biochemical and morphological changes in the endometrium occur as a consequence of ovarian steroid priming. During the menstrual cycle, a small window of time crafts an ideal milieu for blastocyst implantation. This tightly regulated period of time is known as the window of uterine receptivity and involves the coordination of multiple cellular and molecular events, triggered by the presence of the embryo within the uterus. During the early luteal phase, estrogen and progesterone receptors (PR) are found in endometrial stroma (1). Whereas progesterone antagonizes the proliferative effects of estradiol on the endometrial glands by down-regulating estrogen receptors, there is subsequent attenuation of the PR (2).
In addition to steroid hormone priming, successful implantation requires an elaborate dialogue between the embryonic secreted hormone chorionic gonadotropin (CG) and the hormonally primed endometrium, which rescues stromal fibroblasts from apoptosis and normal endometrial regression in the event of pregnancy (3). We have previously shown that CG induces alterations in endometrial morphology and endometrial gene expression (4, 5).
Failed implantation is a major limiting factor in women who have multiple miscarriages or after assisted reproductive therapies (6). Of the pregnancies that are lost, 50–75% represents a failure of the blastocyst to implant into the maternal endometrium (7). If implantation is successful, the endometrial stromal compartment forms the decidua, a morphologically and functionally distinct tissue, representing the maternal side of the feto-maternal interface.
Decidualization requires a modified uterine milieu that prevents apoptosis and promotes differentiation and trans-differentiation of the stromal fibroblast to a secretory cell called a decidual cell. Previously, our laboratory has demonstrated that the induction of α-smooth muscle actin (α-SMA) by CG may be essential to decrease the progesterone-regulated proliferation in stromal cells undergoing decidualization (8). After decidualization, stromal fibroblasts are prevented from undergoing apoptosis by the action of IGF-binding protein-1 (Igfbp1) and prolactin, both of which are markers of decidual cells (9, 10). In a well-established in vitro model, decidualization can be induced by cAMP, along with ovarian hormones. Coordinately, cAMP ligands have been shown to alter Notch signal activation in endothelial cells, pointing to a role of Notch in a cAMP-dependant decidualization process (11).
A large body of work has demonstrated that the highly conserved Notch signaling pathway mediates cell-to-cell signaling and ultimately influences cell proliferation, differentiation, survival, and apoptosis (12, 13) in various cell types (14–16). Although Notch receptors (12), ligands (17), and downstream effectors form a complex signaling pathway that plays multiple roles in a variety of normal tissues and malignancies, the physiological role of Notch in endometrial cell differentiation and embryo implantation have never been studied.
This work demonstrates a major physiological role for Notch1 in endometrial stromal cell differentiation both in vivo and in vitro. We suggest that Notch1 modulates processes underlying the differentiation of stromal fibroblasts to decidual cells, where Notch1 is induced by CG and regulated by ovarian progesterone. Furthermore, these molecules act in synergy to inhibit stromal cell apoptosis and permit decidualization to occur. A better understanding of the molecular mechanisms responsible for decidualization and implantation will improve the clinician's ability to detect, diagnose, and treat early pregnancy loss.
Materials and Methods
Simulated pregnancy model
The intrauterine endocrine milieu of pregnancy was simulated in normally cycling female baboons by infusing recombinant human CG (EMD-Serono, Rockland, MA) between d 6 and 10 after ovulation as previously described (4, 18). The 24-h infusion of 30 IU is equivalent to the amount of CG secreted by dispersed baboon trophoblast cells cultured in vitro in 24 h. Endometrial tissue was harvested on d 10 after ovulation for analysis. For PR antagonism studies, baboons were injected im with the PR antagonist onapristone (ZK 137.316; Schering AG, Berlin, Germany) at a dose of 1 mg/kg body weight per day between d 5 and 9 after ovulation in conjunction with human CG infusion, and endometrial tissues were collected on d 10 after ovulation (19). All animal studies were approved by the Animal Care Committee at the University of Illinois at Chicago.
Isolation and culture of human stromal cells
Human in vitro studies were performed using protocols for human uterine fibroblasts (HuF) cells. HuF cells were isolated from the decidua parietalis dissected from the placental membrane after normal vaginal delivery at the University of Illinois Hospital. Tissue was obtained after informed consent under a protocol approved by the Institutional Review Boards at both the University of Illinois at Chicago and Michigan State University. After trypsinization, dissociated cells were maintained and propagated in defined medium (phenol-red-free RPMI 1640) with or without 10% fetal bovine serum (FBS). From a single placenta, we isolate approximately 1.5 × 108 cells. These cells isolated from each individual patient represent a proliferating population of nondifferentiated stromal fibroblasts (20, 21). HuF cells isolated from a single placenta was used for multiple determinations but represented a single sample for statistical analysis. The analysis was based on the variance of data collected on our extensive in vitro decidualization studies using these cells. A sample size of six patients was used in total based on power analysis with α set at 0.05 and power equal to 0.7 based on group comparisons and one-way ANOVA. All studies were repeated three times and were performed in triplicate. From a single placenta, we isolate approximately 1.5 × 108 cells. These cells isolated from each individual patient represent a proliferating population of nondifferentiated stromal fibroblasts (20, 21) and were always used fresh between passages 3–5.
Hormonal treatment
Experiments were performed when cells reached 80–90% confluence. Phenol-red-free RPMI 1640 culture medium was used and changed every 2 d. Cells were treated with 10–100 IU/ml: recombinant human CG (EMD-Serono) in PBS, 36 nm estradiol-17β, and 1 μm medroxyprogesterone acetate (MPA) in ethanol with the appropriate controls. For decidualization studies, medium with the appropriate treatment paradigms containing 2% heat-treated charcoal-stripped FBS and was changed every 2 d. Cells were treated with 10 ng/ml IL-1β or 100 μm dibutyryl-cAMP (dbcAMP) in the presence of 36 nm estradiol-17β and 1 μm MPA for up to 12 d in triplicate with the appropriate controls (21) to induce in vitro decidualization. This combination of hormones has been used extensively in our laboratory on HuF cells (20–22). We have routinely used MPA instead of progesterone in all of our studies because it is more stable and less rapidly metabolized over time in culture.
Total cell lysate extraction
Cells were made quiescent by two washes with 1× PBS to remove traces of FBS and then incubated in serum-free medium for 24 h preceding treatment. After treatment, each 100-mm dish was rinsed with ice-cold PBS and the cells lysed on ice with 750 μl lysis buffer (0.5 m EGTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 25 mm Tris, 25 mm NaCl, 100 mm sodium pyrophosphate, and 10 mm NaF, with protease inhibitor cocktail). Cells were scraped and incubated in the lysis buffer cocktail on ice for 30 min, followed by centrifugation at 14,000 rpm for 15 min. The supernatant was aliquoted and frozen at −20 C until use. The protein concentration was estimated using the Bradford assay from Bio-Rad (Hercules, CA).
Immunoblotting
Twenty-five micrograms of protein from cell lysates were separated on 7% Tris-acetate gels (Invitrogen, Carlsbad, CA) using SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked for 1 h at room temperature in Tris-buffered saline and 0.1% Tween 20 containing 5% (wt/vol) nonfat milk and then incubated overnight with primary antibodies against Notch1 C-20 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), Notch1 Val1744 (1:1000; Cell Signaling Technology, Danvers, MA), Hes5 (1:500; Santa Cruz), Hey1 (1:500; Santa Cruz), PR (1:2000; courtesy of Dean P. Edwards, Baylor College of Medicine, Dallas, TX), or β-actin (1:5000; Sigma Chemical Co., St. Louis, MO) in Tris-buffered saline and 0.1% Tween 20 containing 5% (wt/vol) nonfat milk at 4 C. Incubation in primary antibody was followed by incubation in the respective secondary antibodies labeled with horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA) at a 1:20,000 dilution for 1 h at room temperature. Immunocomplexes were visualized by enhanced chemiluminescence (GE Amersham, Piscataway, NJ). All membranes were probed for β-actin, which served as a loading control.
Immunocytochemistry
Tissues
For immunostaining, endometrium was fixed overnight in 4% paraformaldehyde (vol/vol), followed by thorough washing in 70% ethanol, and tissues were processed, embedded in paraffin, and sectioned. Sections from paraffin-embedded tissue were cut at 5 μm and mounted on slides. Sections were deparaffinized in a graded alcohol series, and tissues were preincubated with 10% normal rabbit or goat serum in PBS (pH 7.5) and then incubated with polyclonal 1:500 Notch1 C-20, (1:500; Santa Cruz) and 1:1000 PR (courtesy of Dean P. Edwards, Baylor College of Medicine). Controls consisted of preimmune serum at the same dilution. On the following day, sections were washed in PBS and incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Immunoreactivity was detected by using the Vectastain Elite ABC kit and visualized as brown chromogen staining. Nuclear counterstaining was done with hematoxylin. Sections were blindly reviewed and scored quantitatively using positive pixel counts and the percent staining of each compartment, according to the intensity of diaminobenzidine chromogen deposition, normalized to nuclear staining. Pictures of five fields (0.09 mm2 each) per slide were taken using a Nuance multispectral imaging system (CRi) at ×400 magnification and multispectral acquisition software. The images were processed by Nuance image processing software version 1.6.8 to measure the spectral absorbance curve of each of the stains and then were unmixed. The percentage of positive staining was then quantified and expressed as the percentage of positive pixels to total pixels of the analyzed area. Comparison of two means was made with Student's t test. A P value <0.05 was considered statistically significant.
Cells
HuF cells were plated in two-well chamber slides and grown to 70–80% confluence. The medium was changed to serum-free RPMI overnight before treatment with inhibitors as specified, followed by hormonal treatment for the time period indicated. After each treatment time point, cells were washed three times with PBS and fixed in 4% paraformaldehyde for 20 min at 4 C and rinsed with PBS. Cells were permeabilized with 0.1% saponin in Tris-buffered saline at 37 C for 10 min and blocked in 5% normal goat serum in 0.1% saponin Tris-buffered saline for 30 min at room temperature. They were then incubated with anti-Notch1 C-20 (1:250) antibody overnight at 4 C in a humid chamber. Nonspecific rabbit IgG was used at the same concentration as the procedural control. After washing three times in PBS, they were subjected to fluorescein isothiocyanate-labeled antirabbit secondary antibody (1:200 dilution) at room temperature in the dark for 1 h, washed three times in PBS, and mounted using Vectashield hard-set mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories). Images were visualized with a Nikon Eclipse E400 series fluorescent microscope (Fryer Co., Huntley, IL) and captured using a digital Spot Advanced Camera and the Image-Pro Plus software package (Media Cybernetics, Bethesda, MD).
Immunoprecipitation
Cells were homogenized in lysis buffer [25 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Triton X-100, and protease inhibitor cocktail (Sigma)]. Lysates were kept on ice for 30 min and centrifuged for 15 min at 100,000 × g. The resulting supernatant fractions were precleared using a mixture of protein G-agarose beads (Pierce, Rockford, IL) and nonimmune mouse IgG-conjugated Sepharose beads (Jackson ImmunoResearch) for 1 h at room temperature. Precleared lysate (250 μg) was brought up to 1 ml incubated with 5 μg of the PR antibody with 10 μl of protein G-agarose beads at 4 C for 3 h. Immunocomplexes were recovered by centrifugation (3000 × gmax for 30 sec) and washed four times with 1 ml lysis buffer and once with 50 mm HEPES (pH 7.4). A 50-μl aliquot of each resultant supernatant was saved for Western analysis after each immunoprecipitation cycle with the Notch1 C-20 antibody (Santa Cruz).
Transfection of Notch1 small interfering RNA (siRNA) and dominant negative (DN)-mastermind
HuF cells were seeded in six-well dishes at 50,000 cells per well in RPMI. Lipofectamine 2000 was used to transfect the cells with the DN-mastermind, empty construct (MigR) plasmid, or the Notch1 siRNA and scrambled sequence expression vectors. The plasmids were dissolved in Lipofectamine 2000 (Invitrogen) diluted in serum and antibiotic-free OptiMEM, and the cells were incubated for 6–8 h. A GFP plasmid was used to measure and confirm transfection efficiency. After this incubation, the wells were washed and incubated in fresh phenol-red-free RPMI 1640 with serum and antibiotics. At approximately 80% confluence and 48 h after transfection, the cell culture medium for stromal cells was changed to serum-free medium and left overnight. The next day, 72 h after transfection, cells were treated with or without 50 IU CG for 24–48 h. Finally, the medium was removed, cells were washed with PBS, and total cell lysates were extracted.
RNA isolation and RT-PCR
Cells were rinsed twice with 1× PBS, and total RNA was extracted from cells using the RNeasy kit from QIAGEN (Valencia, CA). RNA preparations were then deoxyribonuclease treated and purified with the RNeasy kit (QIAGEN). RNA quality was analyzed using the Nanodrop 1000 (Thermo Fischer Scientific, Pittsburgh, PA) by assessing their absorbance ratio at 260:280 nm. One microgram of each of the RNA samples was analyzed by electrophoresis on a 1.5% agarose gel to confirm integrity. One microgram of RNA was used for the reverse transcription reaction using the iScript cDNA synthesis kit (Bio-Rad). The remaining RNA samples were stored in ribonuclease-free water until subsequent use.
Gene expression levels were measured by real-time RT-PCR SYBR Green analysis using the ABI Prism 7700 Sequence Detector System according to manufacturer's instructions (Applied Biosystems, Foster City, CA). Real-time primers were designed using Primer Express open-source software. For Notch1, Numb, Hey1, Hey1, LRF, and 18S, real-time RT-PCR was done by using RNA samples from three HuF cell lines, always in triplicates. Data analysis is based on the Δ/ΔCt method with normalization of the raw data to 18S gene expression. RT-PCR primer sequences were as follows: 18S forward 5′-TGATTAAGTCCCTGCCCTTTGT-3′ and reverse 5′-TCAAGTTGCACCGTCTTCTCAG-3′. Notch1 forward 5′-GTCAACGCCGTAGATGACC-3′ and reverse 5′-TTGTTAGCCCCGTTCTTCAG-3′. Numb forward 5′-TCTGTGCCCCAGATAGGAAC-3′ and reverse 5′-TCTTGGCATCTTGCAACTGT-3′.
Notch focused array
Day-12 control and decidual cDNA was mixed with ready-to-use PCR SYBR master mix and aliquoted with equal volumes to each of the 96 wells of the same plate ran in triplicates (SABiosciences Notch Signaling Pathway Array, Valencia, CA). Plates were run with a PCR cycling program on ABI Prism 7700 Sequence Detector System according to manufacturer's instructions (Applied Biosystems). Controls are also included on each array for genomic DNA contamination, RNA quality, and general PCR performance.
ELISA
IL-11 concentrations were measured in culture supernatants to access the extent of decidualization (see Hormonal treatment above). HuF cell culture supernatants were collected and frozen at each time point during the experimental time course. IL-11 levels were measured using an ELISA kit (Diagnostic Systems Laboratories, Inc., Webster, TX) according to the manufacturer's specifications (21).
Statistical analysis
One-way ANOVA was used to test the null hypothesis of group differences, followed by the Student's t test for pair-wise comparison. Each experiment was repeated three times in triplicate, and a P < 0.05 was considered significant.
Results
Notch1 is variably regulated through the menstrual cycle
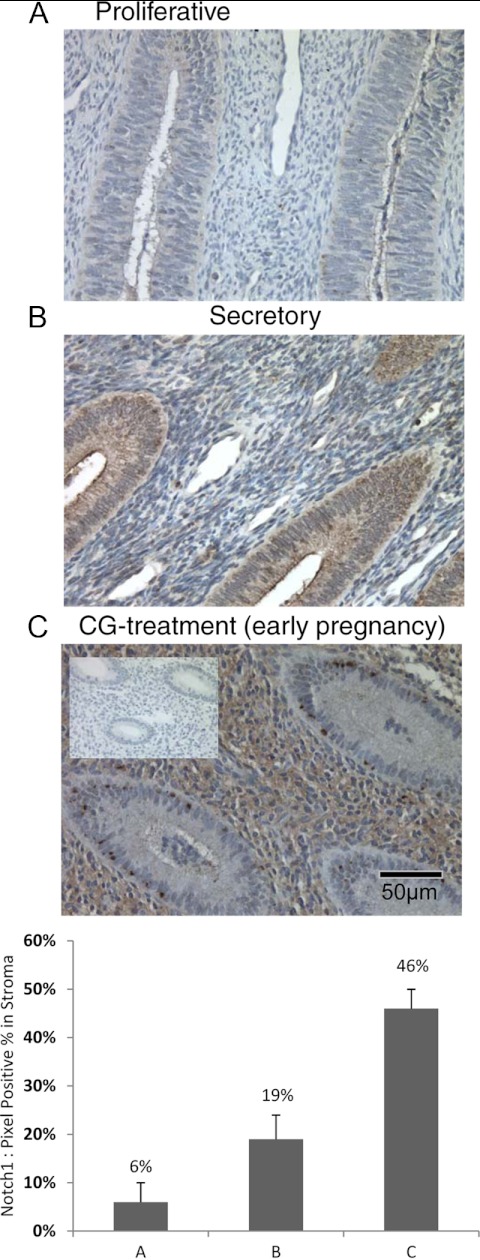
During the late proliferative phase (d 7–12 after menstruation), immunohistochemical staining of Notch1 is absent to minimal (6%) in the stromal and epithelial compartment of normally cycling baboon endometrium (Fig. 1A). As the endometrium progressed to a secretory phenotype (d 10–14 after ovulation), nominal stromal immunoreactivity began to appear (19%); however, the apical tips of the luminal epithelium and the glandular epithelium stained strongly for Notch1 (Fig. 1B). Infusion of human CG, to recapitulate the early events of pregnancy, at physiological levels significantly enhanced Notch1 immunoreactivity in the stromal compartment to 46% (18, 23). Glandular epithelial staining remained positive, but not as intensely (Fig. 1C). Nonspecific rabbit IgG was used as a negative control and showed no immunoreactivity.
Fig. 1.
Notch1 expression is regulated during the menstrual cycle in the baboon. Panel A, Notch1 protein expression parallels previously documented CG/LH receptor localization in the baboon endometrium. During the proliferative phase, staining is faint in both stromal and epithelial cells. Panel B, In contrast, during the secretory phase, the glandular epithelial cells stained positively and stromal staining was confined to the cells around spiral arteries. Panel C, In CG-treated animals, which mimics the early events of pregnancy, Notch1 protein expression is greatly enhanced in the stromal compartment, whereas epithelial cell staining is reduced. Magnification, ×40.
Chorionic gonadotropin increases Notch1
In vivo infusion of CG alters both the endometrial morphology and uterine gene expression patterns in the endometrium (4, 18), including Notch1. To corroborate in vivo data in a primate model, we investigated CG-dependant dose-response effects on Notch1 protein in human endometrial stromal fibroblasts (HuF) by Western blot analysis. In vitro cultures of HuF have been extensively used for studies on stromal cell function and decidualization because HuF cells respond to steroid hormones and have a functional LH/CG receptor (21, 24–28).
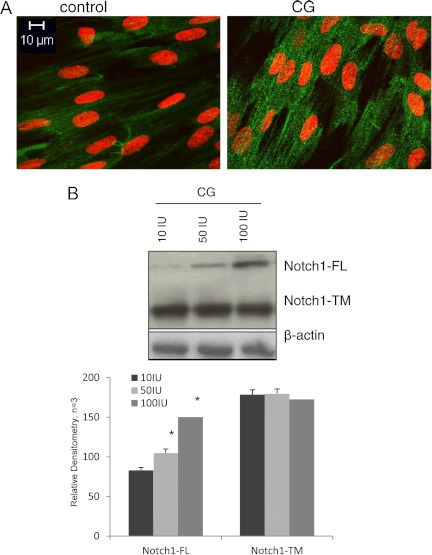
HuF cells were treated with 50 IU CG for 48 h, after 24 h of serum starvation. Cells were probed with anti-Notch1 immunofluorescent antibody, and confocal microscopy was performed, which demonstrated an increase in intracellular (IC) Notch1 fluorescence. 4′,6-Diamidino-2-phenylindole nuclear staining demonstrated that CG increased perinuclear Notch1, evident by the induction of green fluorescence surrounding the nucleus with CG treatment (Fig. 2A).
Fig. 2.
Notch1 protein is increased with CG in human uterine stromal fibroblasts. A, HuF treated with CG demonstrated increased Notch1 immunofluorescence throughout the cell cytoplasm. Notch1 staining is in green and nuclear staining in red. B, Western blot analysis for HuF treated with CG demonstrated that at 50 IU/ml, Notch1-FL increased. Notch1-TM did not show the same CG-induced protein induction with CG.
Furthermore, Western blot for Notch1 demonstrated that at 50 IU CG, Notch1 increased compared with controls. The presence of the Notch1 approximately 300-kDa [Notch1 full-length precursor (Notch1-FL)] and 120-kDa proteins [mature Notch1 transmembrane (Notch1-TM)] was confirmed in HuF cells by Western blot analysis (Fig. 2B).
CG and ovarian hormones synergize to increase Notch1
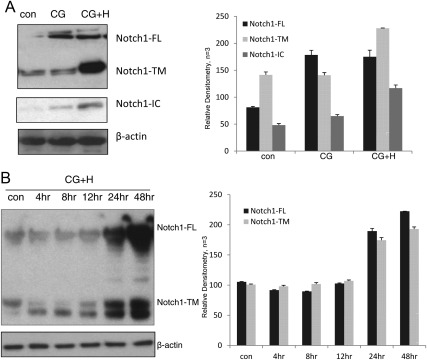
When we combined CG treatment with estradiol (E2) (36 nm) and MPA (1 μm), Notch1 protein levels were significantly increased, especially the functionally competent, Notch1-TM form. Additionally, 24 h estradiol and progesterone hormonal treatment (indicated as hormones, H) significantly increased protein levels. Importantly, an antibody specific to the Notch1 γ-secretase cleavage site (valine 1744) on the transcriptionally active IC fragment, demonstrated an increase in Notch1-IC after combined CG, estradiol, and progesterone treatment (Fig. 3A). This demonstrates that CG, combined with ovarian steroids, increased not only Notch1 protein expression but also its γ-secretase-mediated activation (Fig. 3B).
Fig. 3.
Steroid hormones and CG synergistically increase in Notch1 in human uterine fibroblasts. A, CG increased Notch1-FL; however, when hormones (H) (36 nm estradiol and 1 μm MPA) are added to HuF cells with CG, Western blot demonstrated that Notch1-TM and Notch1-IC increased in HuF cells after 24 h of culture. B, The CG+H synergism was demonstrated in a time-course analysis and was very evident at the 24-h time point of incubation. con, Control.
Progesterone up-regulates Notch1 activity
Progesterone is essential for the differentiation of the endometrium into a secretory phenotype, which is required for the establishment of pregnancy. Normally cycling female baboons were treated with the pure PR antagonist onapristone juxtaposed against the CG-stimulated pregnancy model, which provided a mechanism to decouple the specific roles of the individual hormones.
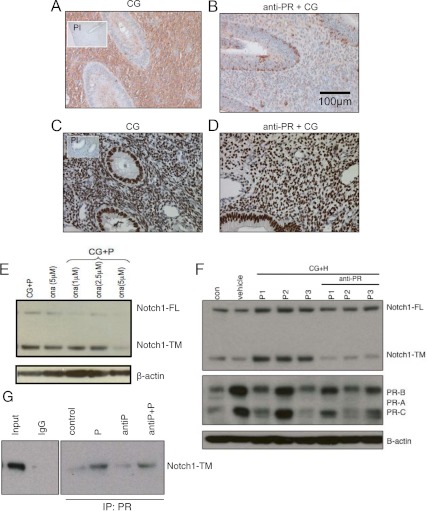
In contrast to the stromal induction of Notch1 seen during the window of uterine receptivity with CG, animals primed with PR antagonist did not demonstrate Notch1 immunoreactivity within the stroma, implying that Notch1 expression and signaling in the stroma requires progesterone in addition to CG (Fig. 4, A and B). Nevertheless, attenuated Notch1 immunoreactivity with anti-PR priming was not due to loss of PR because PR remained in the stroma and epithelium, as demonstrated by immunohistochemistry, with or without the PR antagonist (Fig. 4, C and D).
Fig. 4.
Progesterone is necessary for the CG-induced Notch1 induction in baboon endometrial stromal cells. A and B, Recapitulating the intrauterine endocrine milieu of early pregnancy with CG, increased Notch1 in the endometrial stroma. In baboons cotreated with onapristone, a pure PR antagonist (anti-PR), the stromal Notch1 is absent. Magnification, ×20. C and D, The inhibition of Notch1 in the presence of anti-PR is independent of PR status, as demonstrated by immunohistochemistry for PR in the endometrium, under both treatment paradigms. Magnification, ×20x. E, A dose-response experiment demonstrated that treatment of HuF with anti-PR (5 μm) abrogated the CG- plus hormone (H)-induced increase in Notch1. F, HuF cells from three separate isolations (P1–P3) demonstrated an increase in both the FL and TM forms of Notch1 with CG plus progesterone (P). However, the hormone-regulated increase in Notch1-TM was inhibited when cells were pretreated with anti-PR (5 μm) for 24 h before CG+H challenge. Furthermore, this was independent of the PR status of the cells. G, Immunoprecipitation (IP) with a PR antibody, followed by Western blot for Notch1, demonstrated that PR and Notch1 interact in a complex in the presence of progesterone, and this complex is not affected by anti-PR. PI, Preimmune.
In vitro studies demonstrated a synergistic induction of transcriptionally competent Notch1 by CG and ovarian hormones in HuF cells. Treatment of HuF cells with 1 μm MPA together with CG up-regulated Notch1 protein, especially Notch1-TM. Progesterone-mediated Notch1 induction was antagonized by 5 μm of the PR antagonist onapristone (Fig. 4E). Progesterone-mediated induction of Notch1-TM was validated in three different HuF cell isolates (P1, P2, and P3 and anti-P1, anti-P2, and anti-P3) from three individual patients. (Fig. 4F) with a statistically significant increase in Notch1-TM protein without a significant change in either Notch1-FL or PR protein expression as determined by densitometric scanning of the Western blots (n = 3). These data suggest that progesterone in the presence of CG increased the availability of cleaved Notch1 and that this increase is antagonized by the addition of onapristone, a specific PR antagonist independent of PR. Coimmunoprecipitation studies, in which the PR was pulled down from whole-cell lysate and immunoblotted with a Notch1-specific antibody, indicated that there is either a direct or indirect interaction between Notch1 and PR (Fig. 4G). PR coimmunoprecipitated with Notch1 both in the presence and absence of receptor antagonist (n = 4).
Attenuation of Notch1 leads to impaired decidualization
If Notch interferes with the differentiation of stromal cells into the decidual phenotype, we expect that markers of decidualization will be suppressed when Notch1 is inhibited. Notch1 was suppressed using either siRNA oligonucleotides for Notch1 or a dominant-negative construct for the Notch transcriptional coactivator mastermind (Mam) in a MigR vector. Transient transfection effectively abrogated Notch1 activation as determined by down-regulation of Notch1 protein and the helix-loop-helix Notch1 targets Hey1 and Hes5, confirming the Notch1 reduction had functional consequences (Fig. 5A).
Fig. 5.
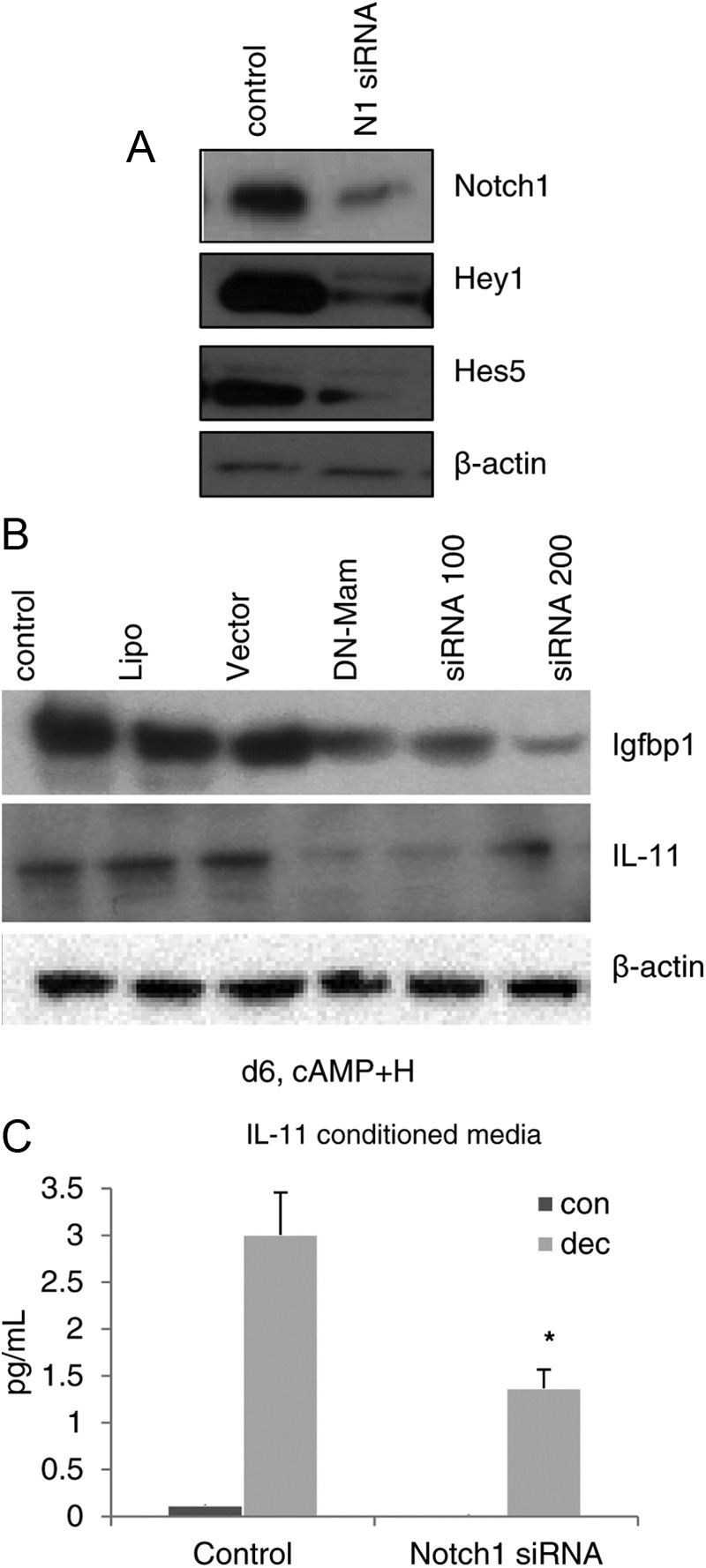
Silencing Notch1 inhibits in vitro decidualization. A, Inhibition of Notch1 in HuF cells using Notch1 siRNA decreased Notch1 and Notch1 targets Hey1 and Hes5, as evident on Western blot. B and C, Inhibition of Notch1, by transfection of HuF cells with DN-mastermind or siRNA oligos to Notch1 at different concentrations, inhibited the induction of decidual proteins Igfbp1 and IL-11 as assessed by Western blot (B) or ELISA for IL-11 (C). *, P < 0.05. con, Control; dec, decidualization stimulus; H, hormone; Lipo, Lipofectamine.
Inhibition of Notch1 clearly demonstrated that it is essential for in vitro decidualization of human stromal fibroblasts. In HuF cells, we recapitulated the decidual process by treating cells with 36 nm E2, 1 μm MPA, and 0.1 mm dbcAMP to induce decidualization (21). The decidual paradigm stimulated the production of Igfbp1 and IL-11, both well-established markers of decidualization. Expression of both decidual markers was significantly reduced (P < 0.05) after 6 d of the decidualization stimulus when Notch1 was inhibited (Fig. 5, B and C).
Decidualization down-regulates Notch1
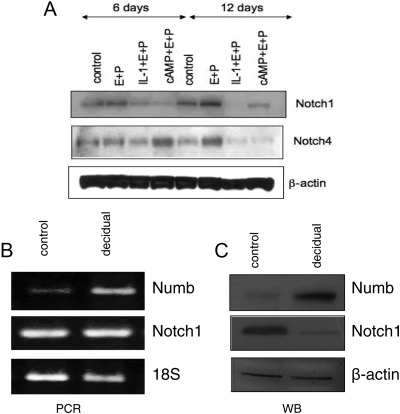
Notch1 is down-regulated when cells are decidualized in vitro, as demonstrated by Western blot. Concomitant with Notch1 down-regulation, the protein expression of the Notch1 target Notch4 (29) is decreased, as assessed by immunoblotting. To confirm that Notch1 reduction is associated with an in vitro decidualization, HuF cells were treated with an alternative decidualization stimulus (IL-1β with E2 and MPA). Again, Notch1 protein levels were significantly decreased compared with controls and/or hormonal treatment for 6 d as was the Notch1 target Notch4 (Fig. 6A). These changes are independent of Notch1 transcription because the mRNA levels for Notch1 do not change under these treatment conditions (data not shown).
Fig. 6.
Changes in Notch1 and Numb during decidualization. A, To confirm Notch1 down-regulation during in vitro decidualization, we used two different decidualization-inducing treatments [cAMP or IL-1β along with E2 and MPA (E+P)]. HuF cells were immunoblotted for Notch1 and Notch1 target Notch4 and compared with controls. Both Notch1 and Notch4 were down-regulated as a consequence of the decidualization stimulus. B, Numb transcript levels are increased after 12 d of in vitro decidualization, whereas Notch1 gene expression levels were unchanged. C, Protein expression for Numb corroborated transcript levels and increased during decidualization, whereas Notch1 is down-regulated. These data point to a possible mechanism for posttranscriptional inhibition of Notch1 during decidualization and cellular differentiation. WB, Western blot.
Identification of Notch pathways gene expression profiling in decidualization
Using a Notch pathway-focused microarray, we were able to compare Notch signaling genes that were significantly regulated during in vitro decidualization. After a 12-d in vitro culture with dbcAMP, E2, and MPA, we isolated RNA and reverse transcribed into cDNA from 12-d control vs. 12-d decidual HuF cells and conducted a focused pathway array.
Genes that were most significantly up- or down-regulated in the decidualized stromal cells vs. control included Frizzled homolog 2, Fzd2 (+397.75); WNT1 inducible signaling pathway protein 1, Wisp1 (+66.10); Numb (+18.76); Deltex homolog 1, Dtx1 (9.5); CASP8 and FADD-like apoptosis regulator, CFLAR (−83.29); and Hairy/enhancer-of-split related with YRPW motif-like, Hey1L (−18.69), among others (Table 1). All four members of the Notch receptor family (1–4) were down-regulated, but not significantly (fold change: −1.01, −1.22, 1.25, and −1.88, respectively) compared with control. Real-time quantitative PCR of HuF cells through decidualization (d 0–12) of Notch1 and -4 confirmed this finding (data not shown). Juxtaposed with the protein data that demonstrated Notch1 is down-regulated with decidualization, these data imply posttranscriptional regulation of Notch1 during decidualization.
Table 1.
Human decidual cell Notch-focused array: in vitro decidualization
| Symbol | Refseq | Description | Fold change |
|---|---|---|---|
| FZD2 | NM_001466 | Frizzled homolog 2 (Drosophila) | 397.75 |
| WISP1 | NM_003882 | WNT1-inducible signaling pathway protein 1 | 66.10 |
| NUMB | NM_003744 | Numb homolog (Drosophila) | 18.76 |
| MAP2K7 | NM_145185 | MAPK kinase 7 | 14.37 |
| STAT6 | NM_003153 | Signal transducer and activator of transcription 6, IL-4 induced | 13.89 |
| LMO2 | NM_005574 | LIM domain only 2 (rhombotin-like 1) | 12.91 |
| LOR | NM_000427 | Loricrin | 11.53 |
| DTX1 | NM_004416 | Deltex homolog 1 (Drosophila) | 9.50 |
| SHH | NM_000193 | Sonic hedgehog homolog (Drosophila) | 9.49 |
| DLL1 | NM_005618 | Delta-like 1 (Drosophila) | 8.77 |
| MYCL1 | NM_005376 | V-myc myelocytomatosis viral oncogene homolog 1, lung carcinoma derived (avian) | 5.48 |
| MMP7 | NM_002423 | Matrix metallopeptidase 7 (matrilysin, uterine) | 5.20 |
| IL2RA | NM_000417 | IL-2 receptor, α | 5.04 |
| FOS | NM_005252 | V-fos FBJ murine osteosarcoma viral oncogene homolog | 4.56 |
| FIGF | NM_004469 | C-fos-induced growth factor (vascular endothelial growth factor D) | 4.49 |
| KRT1 | NM_006121 | Keratin 1 (epidermolytic hyperkeratosis) | 4.30 |
| LRP5 | NM_002335 | Low-density lipoprotein receptor-related protein 5 | 4.23 |
| RFNG | NM_002917 | RFNG O-fucosylpeptide 3-β-N-acetylglucosaminyltransferase | 4.17 |
| SH2D1A | NM_002351 | SH2 domain protein 1A, Duncan's disease (lymphoproliferative syndrome) | 3.54 |
| KAT2B | NM_003884 | K(lysine) acetyltransferase 2B | 3.05 |
| HOXB4 | NM_024015 | Homeobox B4 | 3.04 |
| NFKB2 | NM_002502 | Nuclear factor of κ-light polypeptide gene enhancer in B cells 2 (p49/p100) | 3.04 |
| NEURL | NM_004210 | Neuralized homolog (Drosophila) | 3.02 |
| SMO | NM_005631 | Smoothened homolog (Drosophila) | 2.84 |
| FZD1 | NM_003505 | Frizzled homolog 1 (Drosophila) | 2.69 |
| GBP2 | NM_004120 | Guanylate-binding protein 2, interferon-inducible | 2.69 |
| PPARG | NM_015869 | Peroxisome proliferator-activated receptor γ | 2.25 |
| PAX5 | NM_016734 | Paired box 5 | 2.14 |
| HEY1 | NM_012258 | Hairy/enhancer-of-split related with YRPW motif 1 | 2.09 |
| ADAM10 | NM_001110 | ADAM metallopeptidase domain 10 | 2.01 |
| ADAM17 | NM_003183 | ADAM metallopeptidase domain 17 (tumor necrosis factor, α, converting enzyme) | 1.91 |
| CTNNB1 | NM_001904 | Catenin (cadherin-associated protein), β1, 88 kDa | 1.88 |
| AES | NM_001130 | Amino-terminal enhancer of split | 1.75 |
| HDAC1 | NM_004964 | Histone deacetylase 1 | 1.73 |
| EP300 | NM_001429 | E1A-binding protein p300 | 1.53 |
| PSEN2 | NM_000447 | Presenilin 2 (Alzheimer disease 4) | 1.51 |
| FZD6 | NM_003506 | Frizzled homolog 6 (Drosophila) | 1.47 |
| TEAD1 | NM_021961 | TEA domain family member 1 (SV40 transcriptional enhancer factor) | 1.40 |
| PSENEN | NM_172341 | Presenilin enhancer 2 homolog (Caenorhabditis elegans) | 1.35 |
| POFUT1 | NM_172236 | Protein O-fucosyltransferase 1 | 1.34 |
| TLE1 | NM_005077 | Transducin-like enhancer of split 1 [E(sp1) homolog, Drosophila] | 1.31 |
| PSEN1 | NM_000021 | Presenilin 1 (Alzheimer disease 3) | 1.29 |
| SUFU | NM_016169 | Suppressor of fused homolog (Drosophila) | 1.23 |
| AXIN1 | NM_003502 | Axin 1 | 1.17 |
| GSK3B | NM_002093 | Glycogen synthase kinase 3β | 1.14 |
| NCOR2 | NM_006312 | Nuclear receptor corepressor 2 | 1.13 |
| CCNE1 | NM_001238 | Cyclin E1 | 1.12 |
| CBL | NM_005188 | Cas-Br-M (murine) ecotropic retroviral transforming sequence | 1.11 |
| ERBB2 | NM_004448 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma-derived oncogene homolog (avian) | 1.11 |
| JAG1 | NM_000214 | Jagged 1 (Alagille syndrome) | 1.06 |
| PDPK1 | NM_002613 | 3-Phosphoinositide dependent protein kinase-1 | 1.06 |
| NFKB1 | NM_003998 | Nuclear factor of κ-light polypeptide gene enhancer in B cells 1 (p105) | 1.05 |
| NOTCH2NL | NM_203458 | Notch homolog 2 (Drosophila) N-terminal like | 1.01 |
| CDC16 | NM_003903 | Cell division cycle 16 homolog (Saccharomyces cerevisiae) | 1.01 |
| NOTCH1 | NM_017617 | Notch homolog 1, translocation-associated (Drosophila) | −1.01 |
| NOTCH2 | NM_024408 | Notch homolog 2 (Drosophila) | −1.22 |
| NOTCH3 | NM_000435 | Notch homolog 3 (Drosophila) | −1.25 |
| CDKN1A | NM_000389 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | −1.26 |
| HES1 | NM_005524 | Hairy and enhancer of split 1 (Drosophila) | −1.30 |
| PTCRA | NM_138296 | Pre T-cell antigen receptor α | −1.33 |
| JAG2 | NM_002226 | Jagged 2 | −1.35 |
| FZD7 | NM_003507 | Frizzled homolog 7 (Drosophila) | −1.65 |
| CD44 | NM_000610 | CD44 molecule (Indian blood group) | −1.73 |
| CHUK | NM_001278 | Conserved helix-loop-helix ubiquitous kinase | −1.88 |
| FZD3 | NM_017412 | Frizzled homolog 3 (Drosophila) | −1.88 |
| FZD4 | NM_012193 | Frizzled homolog 4 (Drosophila) | −1.88 |
| HR | NM_018411 | Hairless homolog (mouse) | −1.88 |
| IFNG | NM_000619 | Interferon, γ | −1.88 |
| IL17B | NM_014443 | Interleukin 17B | −1.88 |
| MFNG | NM_002405 | MFNG O-fucosylpeptide 3-β-N-acetylglucosaminyltransferase | −1.88 |
| NOTCH4 | NM_004557 | Notch homolog 4 (Drosophila) | −1.88 |
| NR4A2 | NM_006186 | Nuclear receptor subfamily 4, group A, member 2 | −1.88 |
| RUNX1 | NM_001754 | Runt-related transcription factor 1 (acute myeloid leukemia 1; aml1 oncogene) | −1.88 |
| SEL1 L | NM_005065 | Sel-1 suppressor of lin-12-like (C. elegans) | −1.88 |
| WNT11 | NM_004626 | Wingless-type MMTV integration site family, member 11 | −1.88 |
| ZIC2 | NM_007129 | Zic family member 2 (odd-paired homolog, Drosophila) | −1.88 |
| FOSL1 | NM_005438 | FOS-like antigen 1 | −2.30 |
| STIL | NM_003035 | SCL/TAL1 interrupting locus | −2.49 |
| CCND1 | NM_053056 | Cyclin D1 | −2.61 |
| SNW1 | NM_012245 | SNW domain containing 1 | −3.21 |
| GLI1 | NM_005269 | Glioma-associated oncogene homolog 1 (zinc finger protein) | −9.17 |
| LFNG | NM_001040167 | LFNG O-fucosylpeptide 3-β-N-acetylglucosaminyltransferase | −9.66 |
| HEYL | NM_014571 | Hairy/enhancer-of-split related with YRPW motif-like | −18.69 |
| CFLAR | NM_003879 | CASP8 and FADD-like apoptosis regulator | −83.29 |
Notch ligands Delta1 and Jagged1 were significantly up-regulated. Notch-specific N-glucosaminidyltransferase Lunatic Fringe (LFng) was dramatically decreased. This implies a shift from Δ-family ligand-mediated to Jagged family ligand-mediated Notch signaling, as Fringe-modified Notch has higher affinity for Δ-family ligands, whereas Fringe-unmodified Notch does not (30). In addition to activating Notch signaling, Notch1 ligands control the steady-state levels of Notch receptor at the cell surface and therefore regulate the availability of ligand-binding capability for Notch1 at the cell surface. High levels of Notch ligands can induce a ligand-mediated cis-inhibition, whereas low levels allow active Notch signaling (31). As such, multiple genes that are responsible for Notch cleavage at the cell surface were up-regulated; namely, ADAM10, ADAM17, MMP7, PSEN1, PSEN2, and PSENEN. Furthermore, E3 ubiquitin ligases that regulate the level of Notch receptor, which include DTX1 and NEURL, were up-regulated during decidualization, +9.5 and +3.02, respectively. The Notch endocytic inhibitor NUMB was 18.76-fold increased during decidualization, because Notch1 mRNA and protein levels are also decreased (see Table 1).
The Notch inhibitor Numb promotes Notch endocytosis and degradation and acts as a Notch antagonist (32), as does Deltex1 (33). In human endometrial stromal cells during decidualization, Numb also appears to act as a Notch antagonist. In our focused array, the transcript for Notch inhibitor NUMB was increased 18.76-fold during decidualization. Both RT-PCR and Western blot for Numb, after in vitro decidualization, corroborated the array data (Fig. 6, B and C). Numb is significantly increased during decidualization, when Notch1 protein (but not mRNA) is decreased. Even though Notch1 down-regulation during decidualization is a posttranscriptional phenomenon, Numb up-regulation is transcriptionally regulated.
Discussion
Notch1 signaling underlies multiple fundamental biological processes. As such, it is not surprising that expression levels are tightly controlled and multiple loss- and gain-of-function Notch pathologies underlie disease etiology. As such, a large body of information on Notch function has been focused on its role in disease and development. Here, we demonstrate that in the uterus, Notch has a physiological role in modulating endometrial integrity and is regulated by ovarian and placental hormones in a site-specific and temporally controlled manner.
Endometrial priming is essential for the successful establishment of pregnancy and is associated with a morphological and biochemical transformation of the endometrium. The baboon provides an ideal model to recapitulate and elucidate the events of early pregnancy because the hormonal cycles of baboon are easy to monitor, their menstrual cycle profiles are similar to those of women, and they are not endangered. Additionally, baboons breed easily in captivity and have a single placenta, like humans, with the additional benefit of fairly good access to tissues at the time of implantation, which demonstrated that Notch1 protein increased from the proliferative to the secretory phase of the uterine cycle. The staining pattern of Notch1 increased further in the presence of CG during the window of uterine receptivity. This regulated pattern implies that Notch1 may be critical to facilitate the proliferation and initial differentiation necessary to allow decidualization during the establishment of pregnancy, when the entire endometrium becomes the decidua of pregnancy, wherein cells are terminally differentiated (34, 35).
A dramatic effect of CG on the endometrial stromal fibroblasts is the induction of the cytoskeletal protein α-SMA (18). Like Notch1 induction, this response requires both CG and progesterone because antagonism of the PR inhibits the ability of CG to induce α-SMA (19). Because CG and PR synergize to regulate α-SMA expression (19), we evaluated the regulation of Notch1 by progesterone in vitro. Treatment of HuF cells with CG alone increased Notch1-FL, whereas CG and progesterone markedly increased the levels of Notch1-TM and active, cleaved Notch1-IC, suggesting that progesterone plays a central role in regulating Notch1 activity and that CG and progesterone synergize to activate Notch1, which in turn induces α-SMA, and inhibits stromal cell apoptosis. In endothelial cells, Notch activation results in a morphological and functional change consistent with mesenchymal transformation, which is similar to the stromal to decidual transdifferentiation that occurs during decidualization. Similar to the endometrial changes, endothelial cell alterations also involve cytoskeletal reorganization, characterized by the up-regulation of α-SMA because Notch directly regulates expression of α-SMA via canonical CSL (CBF1 in humans, RBPJk in mice, Suppressor of Hairless in Drosophila, LAG in C. elegans)-mediated transcriptional regulation (36, 37).
Notch signaling dictates cell fate and critically influences cell proliferation, differentiation, and apoptosis in the primate endometrium. In mammary epithelial cells, progesterone has been shown to override the inhibitory actions of estrogen on Notch activity (29), suggesting that progesterone plays an important role in the regulation of both the physiological and pathological functions of Notch1 in hormone-responsive tissues. In mammary progenitor cells, molecular profiling also demonstrated a paracrine role for progesterone because genes in the Notch signaling pathway were up-regulated in breast epithelium in response to progesterone (38). Similarly, in uterine stromal cells, we demonstrated a progesterone-regulated induction of Notch1. Concisely, CG increases Notch1-FL, and progesterone acts in synergy with CG to induce Notch1-IC in the primate endometrium during the endometrial window of uterine receptivity.
The uterus is a dynamic physiological system in which cellular proliferation, terminal differentiation, and apoptosis occur in a cell- and time-specific manner during the menstrual cycle and pregnancy. The role of Notch1 in regulating these pathways in decidual cells was confirmed in our previous studies in which we selectively ablated Notch 1 in the mouse uterus using a bigenic PRCre+/−/Notch1flox/flox mouse (39).
Our data suggest that Notch coordinates two distinct roles during the window of uterine receptivity. Initially, Notch1 mediates a survival signal in the endometrium in response to ovarian and embryonic hormones. As decidualization progresses, Notch1 is down-regulated to allow stromal cells to differentiate into the decidual phenotype. The pattern we observed is consistent with an initial burst of Notch1 activity during the early phase of decidualization, followed by down-regulation of Notch1 protein (presumably via Numb and Deltex1) and a shift from Δ- to Jagged-mediated Notch signaling. Notch signaling is notoriously complex and exquisitely dosage dependent. Our data suggest that Notch signal strength is tightly modulated by hormonal levels during decidualization. Levels must increase and subsequently decrease in a precisely timed manner for physiological decidualization to occur.
During decidualization, low expression of Notch1 is correlated with an up-regulation of Notch inhibitor Numb. The endocytic adaptor protein Numb acts as a Notch inhibitor by internalizing Notch1 into endosomal vesicles and directing it toward recycling or degradation (40). Notch1-IC has been shown to suppress the expression of Numb (32). Numb has been shown to act as a tumor suppressor (41), and Numb expression is inversely correlated to breast cancer prognosis; high levels of Numb correlate with good prognosis, lower levels with poor prognosis (42). Numb expression increased significantly during decidualization, which would suggest a physiological, the role for Numb as a tumor suppressor that prevents excessive, runaway activation of Notch1 that may lead to uncontrolled proliferation. Numb also coordinates the activation and biological competency of other cell cycle regulators, such as p53 (41). Numb recruits components of the ubiquitination machinery to the Notch receptor to facilitate Notch1 ubiquitination at the membrane, which in turn promotes Notch degradation, circumventing its nuclear translocation and downstream target gene activation. We have previously shown that decidualization in the baboon is accompanied by an increase in ubiquitin conjugates (43), which could promote Notch receptor turnover. Additionally, ligand cis-inhibition has been proposed to inhibit Notch during keratinocyte differentiation (44). Concomitantly, Notch inhibition maintains a population of stem cells for differentiation. A similar process could also occur in the uterus. It is also interesting to note that when Notch1 protein is decreased after decidualization, genes involved in Notch activation are up-regulated, implying a built-in feedback machinery on steady-state Notch levels or an important role for low-level, Jagged-mediated Notch signaling that may differ from that of high-level, Δ-mediated Notch signaling during early decidualization.
The possible functional role of a Notch1-PR complex deserves further investigation. Physical interaction of Notch1-IC with the estrogen receptor-α, resulting in activation of estrogen receptor-α-mediated transcription, has been described in breast cancer cells (45). Conversely, interaction of Notch1 with orphan nuclear receptor Nur77 in thymocytes inhibits Nur77 transcriptional activity (46). Thus, Notch1-IC can form complexes with other members or the nuclear receptor superfamily, with stimulatory or inhibitory effects. Whether Notch1 promotes PR-mediated transcription or acts as a feedback inhibitor of PR remains to be established.
In summary, our data in the baboon endometrium and human HuF cells demonstrate that Notch1 expression and activity during decidualization are dynamically regulated by the embryonic hormone CG and the ovarian hormones progesterone and estradiol. Furthermore, low expression of Notch1 can be correlated to shedding of the uterine lining and an inability of the uterus to accept an implanting embryo, making it tempting to speculate that levels of Notch1 in the luteal phase can be used as an early infertility marker in women undergoing multiple miscarriages. Decidualization is inhibited in the absence of Notch1 and progesterone (39, 47) and in gynecological conditions, such as endometriosis (48, 49). It is interesting to speculate that PR regulation of stromal Notch1 may suggest a possible role of Notch1 in diseases such as endometriosis, which is associated with progesterone resistance.
Acknowledgments
We thank Patty Mavrogianis and Kavita Sapru (University of Illinois) for technical assistance and Zuzana Strakova (University of Illinois) for helpful discussions.
This work was supported by NIH HD42280 (to A.T.F.) and TL1RR029879 (to Y.A.).
Disclosure Summary: The authors Y.A., L.M., and A.F. have nothing to declare.
Footnotes
- CG
- Chorionic gonadotropin
- dbcAMP
- dibutyryl-cAMP
- DN
- dominant negative
- E2
- estradiol
- FBS
- fetal bovine serum
- HuF
- human uterine fibroblasts
- IC
- intracellular
- Igfbp1
- IGF-binding protein-1
- MPA
- medroxyprogesterone acetate
- Notch1-FL
- Notch1 full-length precursor
- Notch1-TM
- Notch1 transmembrane
- siRNA
- small interfering RNA
- α-SMA
- α-smooth muscle actin.
References
- 1. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr 1988. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 67:334–340 [DOI] [PubMed] [Google Scholar]
- 2. Garcia E, Bouchard P, De Brux J, Berdah J, Frydman R, Schaison G, Milgrom E, Perrot-Applanat M. 1988. Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab 67:80–87 [DOI] [PubMed] [Google Scholar]
- 3. Lovely LP, Fazleabas AT, Fritz MA, McAdams DG, Lessey BA. 2005. Prevention of endometrial apoptosis: randomized prospective comparison of human chorionic gonadotropin versus progesterone treatment in the luteal phase. J Clin Endocrinol Metab 90:2351–2356 [DOI] [PubMed] [Google Scholar]
- 4. Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, Fazleabas AT. 2007. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology 148:618–626 [DOI] [PubMed] [Google Scholar]
- 5. Sherwin R, Catalano R, Sharkey A. 2006. Large-scale gene expression studies of the endometrium: what have we learnt? Reproduction 132:1–10 [DOI] [PubMed] [Google Scholar]
- 6. Spandorfer SD, Davis OK, Barmat LI, Chung PH, Rosenwaks Z. 2004. Relationship between maternal age and aneuploidy in in vitro fertilization pregnancy loss. Fertil Steril 81:1265–1269 [DOI] [PubMed] [Google Scholar]
- 7. Norwitz ER, Schust DJ, Fisher SJ. 2001. Implantation and the survival of early pregnancy. N Engl J Med 345:1400–1408 [DOI] [PubMed] [Google Scholar]
- 8. Kim JJ, Jaffe RC, Fazleabas AT. 1999. Insulin-like growth factor binding protein-1 expression in baboon endometrial stromal cells: regulation by filamentous actin and requirement for de novo protein synthesis. Endocrinology 140:997–1004 [DOI] [PubMed] [Google Scholar]
- 9. Jabbour HN, Critchley HO. 2001. Potential roles of decidual prolactin in early pregnancy. Reproduction 121:197–205 [DOI] [PubMed] [Google Scholar]
- 10. Tarantino S, Verhage HG, Fazleabas AT. 1992. Regulation of insulin-like growth factor-binding proteins in the baboon (Papio anubis) uterus during early pregnancy. Endocrinology 130:2354–2362 [DOI] [PubMed] [Google Scholar]
- 11. Yurugi-Kobayashi T, Itoh H, Schroeder T, Nakano A, Narazaki G, Kita F, Yanagi K, Hiraoka-Kanie M, Inoue E, Ara T, Nagasawa T, Just U, Nakao K, Nishikawa S, Yamashita JK. 2006. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler Thromb Vasc Biol 26:1977–1984 [DOI] [PubMed] [Google Scholar]
- 12. Miele L. 2006. Notch signaling. Clin Cancer Res 12:1074–1079 [DOI] [PubMed] [Google Scholar]
- 13. Miele L, Osborne B. 1999. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol 181:393–409 [DOI] [PubMed] [Google Scholar]
- 14. Jundt F, Anagnostopoulos I, Förster R, Mathas S, Stein H, Dörken B. 2002. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood 99:3398–3403 [DOI] [PubMed] [Google Scholar]
- 15. Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. 2002. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-κB and PPARγ. Cell Death Differ 9:842–855 [DOI] [PubMed] [Google Scholar]
- 16. Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, Maric D, Eberhart CG, Fine HA. 2005. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res 65:2353–2363 [DOI] [PubMed] [Google Scholar]
- 17. Mazella J, Liang S, Tseng L. 2008. Expression of Delta-like protein 4 in the human endometrium. Endocrinology 149:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. 1999. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA 96:2543–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banaszak S, Brudney A, Donnelly K, Chai D, Chwalisz K, Fazleabas AT. 2000. Modulation of the action of chorionic gonadotropin in the baboon (Papio anubis) uterus by a progesterone receptor antagonist (ZK 137.316). Biol Reprod 63:820–825 [DOI] [PubMed] [Google Scholar]
- 20. Kim JJ, Jaffe RC, Fazleabas AT. 1998. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod 59:160–168 [DOI] [PubMed] [Google Scholar]
- 21. Strakova Z, Srisuparp S, Fazleabas AT. 2000. Interleukin-1β induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology 141:4664–4670 [DOI] [PubMed] [Google Scholar]
- 22. Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT. 2005. In vivo infusion of interleukin-1β and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology 146:4097–4104 [DOI] [PubMed] [Google Scholar]
- 23. Kim JJ, Jaffe RC, Fazleabas AT. 1999. Blastocyst invasion and the stromal response in primates. Hum Reprod 14 Suppl 2:45–55 [DOI] [PubMed] [Google Scholar]
- 24. Cameo P, Szmidt M, Strakova Z, Mavrogianis P, Sharpe-Timms KL, Fazleabas AT. 2006. Decidualization regulates the expression of the endometrial chorionic gonadotropin receptor in the primate. Biol Reprod 75:681–689 [DOI] [PubMed] [Google Scholar]
- 25. Kim JJ, Fazleabas AT. 2004. Uterine receptivity and implantation: the regulation and action of insulin-like growth factor binding protein-1 (IGFBP-1), HOXA10 and forkhead transcription factor-1 (FOXO-1) in the baboon endometrium. Reprod Biol Endocrinol 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG. 2003. Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod 68:24–30 [DOI] [PubMed] [Google Scholar]
- 27. Strakova Z, Srisuparp S, Fazleabas AT. 2002. IL-1beta during in vitro decidualization in primate. J Reprod Immunol 55:35–47 [DOI] [PubMed] [Google Scholar]
- 28. Strakova Z, Szmidt M, Srisuparp S, Fazleabas AT. 2003. Inhibition of matrix metalloproteinases prevents the synthesis of insulin-like growth factor binding protein-1 during decidualization in the baboon. Endocrinology 144:5339–5346 [DOI] [PubMed] [Google Scholar]
- 29. Rizzo P, Miao H, D'Souza G, Osipo C, Song LL, Yun J, Zhao H, Mascarenhas J, Wyatt D, Antico G, Hao L, Yao K, Rajan P, Hicks C, Siziopikou K, Selvaggi S, Bashir A, Bhandari D, Marchese A, Lendahl U, Qin JZ, Tonetti DA, Albain K, Nickoloff BJ, Miele L. 2008. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res 68:5226–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kopan R, Ilagan MX. 2009. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fiuza UM, Klein T, Martinez Arias A, Hayward P. 2010. Mechanisms of ligand-mediated inhibition in Notch signaling activity in Drosophila. Dev Dyn 239:798–805 [DOI] [PubMed] [Google Scholar]
- 32. Chapman G, Liu L, Sahlgren C, Dahlqvist C, Lendahl U. 2006. High levels of Notch signaling down-regulate Numb and Numblike. J Cell Biol 175:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S. 2005. Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol 7:1191–1201 [DOI] [PubMed] [Google Scholar]
- 34. Afshar Y, Stanculescu A, Miele L, Fazleabas AT. 2007. The role of chorionic gonadotropin and Notch1 in implantation. J Assist Reprod Genet 24:296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cobellis L, Caprio F, Trabucco E, Mastrogiacomo A, Coppola G, Manente L, Colacurci N, De Falco M, De Luca A. 2008. The pattern of expression of Notch protein members in normal and pathological endometrium. J Anat 213:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noseda M, Fu Y, Niessen K, Wong F, Chang L, McLean G, Karsan A. 2006. Smooth Muscle α-actin is a direct target of Notch/CSL. Circ Res 98:1468–1470 [DOI] [PubMed] [Google Scholar]
- 37. Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A. 2004. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res 94:910–917 [DOI] [PubMed] [Google Scholar]
- 38. Lydon JP, Edwards DP. 2009. Finally! A model for progesterone receptor action in normal human breast. Endocrinology 150:2988–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Afshar Y, Jeong JW, Roqueiro D, DeMayo F, Lydon J, Radtke F, Radnor R, Miele L, Fazleabas A. 2011. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J 26:282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGill MA, Dho SE, Weinmaster G, McGlade CJ. 2009. Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem 284:26427–26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, Pece S, Di Fiore PP. 2008. NUMB controls p53 tumour suppressor activity. Nature 451:76–80 [DOI] [PubMed] [Google Scholar]
- 42. Stylianou S, Clarke RB, Brennan K. 2006. Aberrant activation of notch signaling in human breast cancer. Cancer Res 66:1517–1525 [DOI] [PubMed] [Google Scholar]
- 43. Bebington C, Bell SC, Doherty FJ, Fazleabas AT, Fleming SD. 1999. Localization of ubiquitin and ubiquitin cross-reactive protein in human and baboon endometrium and decidua during the menstrual cycle and early pregnancy. Biol Reprod 60:920–928 [DOI] [PubMed] [Google Scholar]
- 44. Lowell S, Benchoua A, Heavey B, Smith AG. 2006. Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol 4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hao L, Rizzo P, Osipo C, Pannuti A, Wyatt D, Cheung LW, Sonenshein G, Osborne BA, Miele L. 2010. Notch-1 activates estrogen receptor-α-dependent transcription via IKKα in breast cancer cells. Oncogene 29:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jehn BM, Bielke W, Pear WS, Osborne BA. 1999. Cutting edge: protective effects of notch-1 on TCR-induced apoptosis. J Immunol 162:635–638 [PubMed] [Google Scholar]
- 47. Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT. 2007. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod 13:323–332 [DOI] [PubMed] [Google Scholar]
- 48. Aghajanova L, Velarde MC, Giudice LC. 2010. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med 28:51–58 [DOI] [PubMed] [Google Scholar]
- 49. Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. 2012. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab 97:E35–E43 [DOI] [PMC free article] [PubMed] [Google Scholar]