Abstract
Context
Chamber studies in adult humans indicate reduced responses to acute ozone with increasing age. Age-related changes in TNFα have been observed. TNFα induced inflammation is predominantly mediated through TNFR1.
Objective
To examine the impact of aging on inflammatory responses to acute ozone exposure in mice and determine the role of TNFR1 in age-related differences.
Materials and methods
Wildtype and TNFR1 deficient (TNFR1−/−) mice aged 7 or 39 weeks were exposed to ozone (2 ppm for 3 h). Four hours after exposure, bronchoalveolar lavage (BAL) was performed and BAL cells, cytokines, chemokines, and protein were examined.
Results
Ozone-induced increases in BAL neutrophils and in neutrophil chemotactic factors were lower in 39- versus 7-week-old wildtype, but not (TNFR1−/−) mice. There was no effect of TNFR1 genotype in 7-week-old mice, but in 39-week-old mice, BAL neutrophils and BAL concentrations of MCP-1, KC, MIP-2, IL-6 and IP-10 were significantly greater following ozone exposure in TNFR1−/− versus wildtype mice. BAL concentrations of the soluble form of the TNFR1 receptor (sTNFR1) were substantially increased in 39-week-old versus 7-week-old mice, regardless of exposure.
Discussion and conclusion
The data suggest that increased levels of sTNFR1 in the lungs of the 39-week-old mice may neutralize TNFα and protect these older mice against ozone-induced inflammation.
Keywords: Bronchoalveolar lavage, neutrophil, sTNFR1, TNFα
Introduction
Ozone (O3) is a common air pollutant that causes significant morbidity and mortality. O3 exposure causes cough, dyspnea, reductions in lung function, increased susceptibility to lung infections, hospitalizations, increased asthma attacks (Hazucha et al., 1996; Alexis et al., 2000; Fauroux et al., 2000; Tolbert et al., 2000; Gent et al., 2003; Triche et al., 2006; Chiu et al., 2009), and even excess deaths (Bell et al., 2005; Levy et al., 2005).
O3 exposure causes lung epithelial injury leading to inflammation. This inflammatory response includes the generation of numerous cytokines and chemokines, resulting in an influx of neutrophils (Hazucha et al., 1996; Johnston et al., 1999; Alexis et al., 2000; Johnston et al., 2000a; Shore et al., 2000; Shore et al., 2003; Johnston et al., 2006; Lu et al., 2006). Exposure chamber studies in human subjects indicate a reduction in the response to acute O3 with increasing age in adult human subjects (McDonnell et al., 1993; Hazucha et al., 2003), but the mechanistic basis for this reduction has not been established. Both ourselves (Shore et al., 2002) and others (Vancza et al., 2009) have reported reductions in the inflammatory response to O3 in young adult versus neonatal mice, but there have been no comparisons of pulmonary responsiveness to O3 in mice older than 15 weeks. To determine whether we could model adult human age-related declines in the response to O3 in mice, we examined the inflammatory response to acute O3 exposure in 7- and 39-week-old mice. Although it is difficult to exactly map ages across mice and men, the 7-week-old mice can be considered similar to late adolescent/young adult humans, while the 39-week-old mice can be considered similar to middle aged humans.
Studies in both mice and humans have demonstrated a role for TNFα in responses to O3. For example, genetic linkage studies in mice have implicated a role for a locus containing TNFα in the neutrophil influx that occurs after exposure to O3 (Kleeberger, 2003). In humans, decrements in pulmonary function induced by O3 exposure are augmented in subjects homozygous for the −308G polymorphism in the promoter of the TNFα gene (Yang et al., 2005). Studies using anti-TNF antibodies or TNFα receptor deficient mice, also indicate that TNFα is required for O3-induced airway hyperresponsiveness and inflammation, but not lung injury in mice, although the requirement for TNFα appears to depend on the strength and duration of the O3 exposure (Cho et al., 2001; Shore et al., 2001; Cho et al., 2007; Matsubara et al., 2009). The role of TNFα in the induction of cytokines and chemokines that occurs after acute O3 has not been established, although TNFα does appear to be required for induction of IL-6 after prolonged exposure to O3 (Cho et al., 2007).
TNFα can bind to either of two membrane bound receptors, TNFR1 and TNFR2. Most studies indicate that inflammation induced by TNFα is largely mediated through TNFR1 activation (Vandenabeele et al., 1995; Peschon et al., 1998; MacEwan, 2002; Naude et al., 2011), although TNFR2 mediated inflammation has been reported (Lucas et al., 1997; Akassoglou et al., 2003; Ramesh et al., 2003; Vielhauer et al., 2005). Soluble forms of these receptors (sTNFR1 and sTNFR2) created by cleavage from cell membranes are also present in blood and other body fluids, and have been considered by some to act as endogenous inhibitors of TNFα (Ulich et al., 1993; Hale et al., 1995; Yagi et al., 2010). Age-related elevations in serum TNFα, and in serum sTNFR1 and sTNFR2 have been reported (Dobbs et al., 1999; Brüünsgaard et al., 2003; Scalzo et al., 2009; de Gonzalo-Calvo et al., 2010), but it is unclear what the relative impact of these changes is, and whether they are relevant to the lungs. The impact of age-related changes in TNFα signaling on pulmonary responses to O3 is essentially unknown.
To determine whether differences in TNFα signaling contributed to age-related differences in the inflammatory response to O3, studies were performed both in wildtype (WT) mice and in mice genetically deficient in TNFR1. We chose to focus on TNFR1 because our interest was in the inflammatory response to O3, and because most studies indicate that TNFα-induced inflammation is mediated through TNFR1 (Vandenabeele et al., 1995; Peschon et al., 1998; MacEwan, 2002; Naude et al., 2011).
In mice, increased pulmonary oxidative stress occurs during aging with an onset consistent with the older (39-week-old) mice in this study (Calvi et al., 2011). Moreover, the anti-oxidants metallothionein (MT) and heme oxygenase-1 (HO-1) are strongly induced by acute O3 exposure (Johnston et al., 2000b; Williams et al., 2007; Vasu et al., 2010), and have been shown to play important roles in attenuating subsequent inflammatory responses to O3 (Hisada et al., 2000; Inoue et al., 2008). Because induction of MT-1, MT-2, and HO-1 by other stimuli has been shown to require TNFα (Oguro et al., 2002; Chiu et al., 2003; Quintana et al., 2007), we also measured the mRNA expression of MT-1, MT-2, and HO-1 to determine 1) whether age-related differences in inflammation were due to greater expression of these anti-oxidants; and 2) whether the induction of these enzymes by O3 was TNF dependent.
We also examined a possible role of amphiregulin (Areg) in age- and TNFR1-related differences in the inflammatory response to O3. Areg is one of a family of growth factors that act via the epidermal growth factor receptor (EGFR). Importantly, Areg is induced by O3 exposure (Vasu et al., 2010) and others have reported roles for Areg both in promoting epithelial proliferation (Lee et al., 2011) and in limiting the pulmonary inflammation induced in a bleomycin model of pulmonary fibrosis (Fukumoto et al., 2010). Areg can also be induced by TNFα (Sisto et al., 2010).
Methods
Animals
Breeding pairs of TNFR1 deficient (TNFR1−/−) mice were purchased from The Jackson Labs (Bar Harbor, ME). The mice have been backcrossed through at least 6 generations to C57BL/6 mice, which were therefore used as WT controls. Mice were bred in the animal facilities of the Harvard School of Public Health and were studied at either 7 or 39 weeks of age. Mice of both genders were used. It is noteworthy that alveolar development is complete by about 5 weeks of age in the mouse, although lung growth continues with somatic growth thereafter (Mund et al., 2008). The procedures were approved by the Harvard Medical Area animal use committee. Mice were on a 12 h light/dark cycle (6 AM on/6 PM off) and were exposed mid morning.
Ozone exposure
Mice were exposed to O3 (2 ppm for 3 h) and studied 4 h after the cessation of exposure. The 4-h time point was chosen because our primary interest was in the acute inflammatory response induced by O3: many of the cytokines and chemokines induced by O3 peak at approximately this time point and then decline to near air exposed values by 24 h (Johnston et al., 2007). During exposure, mice were placed in individual wire mesh cages within a stainless steel and plexiglass exposure chamber. Both TNFR1−/− and age-matched WT mice were exposed at the same time within the same chamber. Control mice were exposed in an identical but distinct chamber to room air. Details of the O3 exposure and monitoring system have been previously described (Johnston et al., 2005b).
Bronchoalveolar lavage
Four hours after O3 exposure, mice were euthanized with an overdose of sodium pentobarbital. A canula was inserted into the trachea, and the lungs were lavaged with 1 ml of PBS containing 0.6 mM EDTA. The lavage was repeated, and the two samples were pooled and placed on ice until centrifuged at 2000 rpm at 4°C for 10 min. The pellet was resuspended in 1 ml of Hank’s Balanced Salt solution (Sigma-Aldrich, St. Louis, MO) and total BAL cells counted using a hemacytometer. Aliquots of cells were centrifuged onto glass slides, air-dried, and stained with with Hema 3⊠ (Biochemical Sciences, Inc., Swedesboro, NJ). Cell differentials were determined by counting 200–300 cells under 400× magnification. The BAL supernatant was frozen at −80°C until analyzed. BAL IL-6, KC, MIP-2, IP-10, MCP-1, and sTNFR1 were measured by Quantikine ELISAs or DuoSet⊠ ELISA development systems (R&D Systems Inc., Minneapolis, MN) according to the manufacturer’s instructions. BAL protein was measured using the Bradford protein assay procedure (Bio-Rad; Hercules, CA).
We instilled the same amount of fluid (1 ml × 2) for the BAL procedure in the 7- and 39-week-old mice. However, the 39-week-old mice were substantially larger, and likely had larger lungs and an increased amount of lung lining fluid. Therefore, there would have been greater dilution of lung lining fluid by the BAL fluid in the younger mice, leading to underestimation, relative to the 39-week-old mice, of actual BAL concentrations of cytokines and chemokines. Indeed, even in the air exposed mice, BAL concentrations of all cytokines and chemokines measured were greater in the 39-week-old than in the 7-weekold mice. Consequently, in order to compare the effect of O3 on BAL cytokines and chemokines across age groups, we used the process described by Vancza et al. (2009) and expressed the values in O3-exposed mice as a ratio of the air exposed values in mice of the same age and genotype. For BAL cells, we could not do this because BAL neutrophils were virtually absent in air exposed mice. Consequently, we expressed BAL cell types as total cell numbers.
RNA extraction and real time PCR
The lungs were excised and the left lung was immersed in RNAlater (Qiagen, Valencia, CA). Lung tissue was homogenized with lysis buffer containing 1% 2-mercaptoethanol using TissueLyser LT (Qiagen) at 50 Hz for 5 min. The resulting lysate was cleared by centrifugation, and total RNA precipitated with an equal volume of 70% ethanol. Total RNA was then purified using RNeasy columns (Qiagen) with a DNase II digestion step to remove genomic DNA. RNA concentration and purity was determined using a small volume spectrophotometer (Nanodrop, Thermo Scientific, Waltham, MA). 1 µg of total RNA was converted into cDNA using a commercial kit containing a combination of oligo-dt and random hexamer primers with a further cDNA sample treatment with RNase (SuperScript III for qRT-PCR, Invitrogen, Carlsbad, CA). MT-1, MT-2, HO-1, Areg, and claudin-4 (Cldn-4) mRNA were quantified using real time PCR (7300 Real-Time PCR Systems, Applied Biosystems, Carlsbad, CA) with primers described in Table 1 and SYBR-green detection. The delta Ct (ΔCt) was obtained by subtracting Ct values for 18S ribosomal RNA from the gene of interest. Changes in mRNA were expressed relative to values from the 7-week-old WT O3 group, to obtain ΔΔCt values. Expression was calculated by RQ = 2−ΔΔCt (Livak et al., 2001).
Table 1.
Primers used for PCR.
| Gene | Primers |
|---|---|
| MT-1 | Forward: CACGACTTCAACGTCCTGAG |
| Reverse: TGCACTTGCAGTTCTTGCAG | |
| MT-2 | Forward: CGATGGATCCTGCTCCTG |
| Reverse: ACTTGTCGGAAGCCTCTTTG | |
| HO-1 | Forward: AAGCCGAGAATGCTGAGTTC |
| Reverse: TCCAGGGCCGTGTAGATATG | |
| Claudin-4 | Forward: AACATCGTCACGGCACAGAC |
| Reverse: CGAGCATCGAGTCGTACATC | |
| Amphiregulin | Forward: TTCATGGCGAATGCAGATAC |
| Reverse: TGTCATCCTCGCTGTGAGTC | |
| 18S ribosomal RNA | Forward: GTAACCCGTTGAACCCCATT |
| Reverse: CCATCCAATCGGTAGTAGCG |
Statistics
The significance of exposure, age, and genotype related changes in BAL cells, cytokines, chemokines, and in gene expression was assessed by factorial ANOVA using STATISTICA software (StatSoft®; Tulsa, OK). Fisher’s least significant difference (LSD) test was used as a follow-up to determine the significance of differences between individual groups. The results are expressed as mean and SE. A p value less than 0.05 was considered significant.
Results
Body weight
The 39-week-old mice weighed significantly more than the 7-week-old mice (p < 0.001). However, there was no significant effect of genotype on body weight. The 7- and 39-week-old WT mice weighed 19.2 ± 0.8 and 42.0 ± 2.1 g, respectively, and the 7- and 39-week-old TNFR1−/− mice weighed 22.5 ± 1.1 and 45.2 ± 2.6 g, respectively.
Bronchoalveolar lavage cells
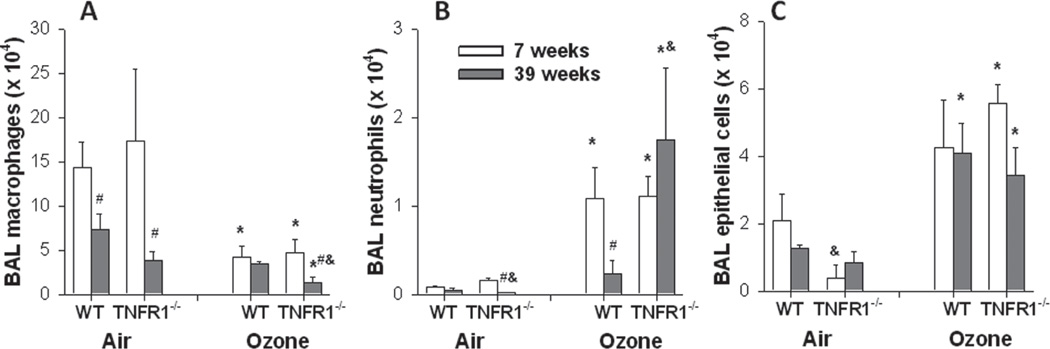
Compared to air, O3 exposure caused a significant increase in the numbers of neutrophils (p < 0.001) and epithelial cells (p < 0.001) in the BAL and a reduction in the numbers of macrophages (p < 0.001) (Figure 1). No effect of O3 on BAL lymphocytes was observed in any experimental group (data not shown). For BAL neutrophils, there were significant effects of age on the response to O3 that varied by genotype. The number of BAL neutrophils was significantly lower in 39-week-old versus 7-week-old WT mice exposed to O3, whereas this age-related effect was abolished in O3-exposed TNFR1−/− mice (Figure 1). Similar results were obtained when the neutrophils were expressed as a percentage of total cells (data not shown).
Figure 1.
Effect of age on the number of macrophages (A), neutrophils (B), and epithelial cells (C) in bronchoalveolar lavage (BAL) of WT and TNFR1−/− mice exposed to air or ozone (O3) (2 ppm for 3 h). Mice were studied 4 h after O3 exposure. Results are mean ± SE of data from 3 to 6 mice in each group. *p < 0.05 versus genotype and age-matched mice exposed to air; #p < 0.05 versus genotype and exposure matched 7-week-old mice; &p < 0.05 versus exposure and age-matched WT mice.
BAL cytokines and chemokines
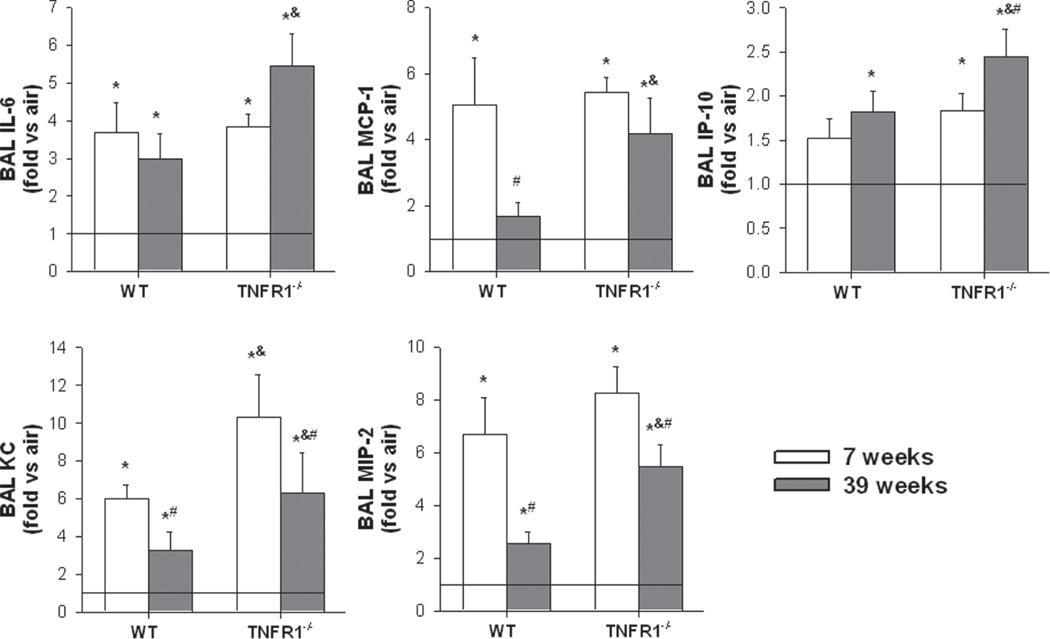
In order to examine the mechanistic basis for the reduced O3-induced recruitment of neutrophils that occurred in the 39-week-old wildtype mice, we measured BAL concentrations of a number of cytokines and chemokines that have been implicated in neutrophil recruitment following acute O3 exposure (Figure 2). As described above, BAL cytokines and chemokines from O3-exposed mice were normalized to their respective age- and genotype-matched air exposed controls (represented by the horizontal lines in Figure 2 at a “fold versus air” of 1). Factorial ANOVA indicated that compared to air, O3 caused an increase in BAL IL-6, MCP-1, IP-10, KC, and MIP-2 (p < 0.001 in each case; Figure 2). In wildtype mice, a significant effect of age on the response to O3 was observed for MCP-1, KC, and MIP-2, which were all lower in 39 versus 7-week-old mice, whereas for IL-6 and IP-10, no age-related difference was observed for O3-exposed WT mice. However, in TNFR1 deficient mice, only BAL KC and MIP-2 were significantly lower in O3-exposed 39 versus 7-week-old mice (Figure 2). In 7-week-old mice O3-exposed mice, TNFR1 deficiency had no effect on BAL cytokines and chemokines except for BAL KC, which was higher in the TNFR1−/− than the WT mice. In contrast, in the 39-week-old O3-exposed mice, all cytokines and chemokines were higher in the TNFR1−/− versus WT mice.
Figure 2.
Effect of age on bronchoalveolar lavage (BAL) concentrations of IL-6 (A), MCP-1 (B), IP-10 (C), KC (D), and MIP-2 (E), in WT and TNFR1−/− mice exposed to O3. Results are mean ± SE of data from 3 to 6 mice in each group. The data are expressed as fold change relative to genotypeand age-matched air controls, which are represented by the horizontal line. *p < 0.05 versus genotype and age-matched mice exposed to air; #p < 0.05 versus genotype and exposure matched 7-week-old mice; &p < 0.05 versus exposure and age-matched WT mice.
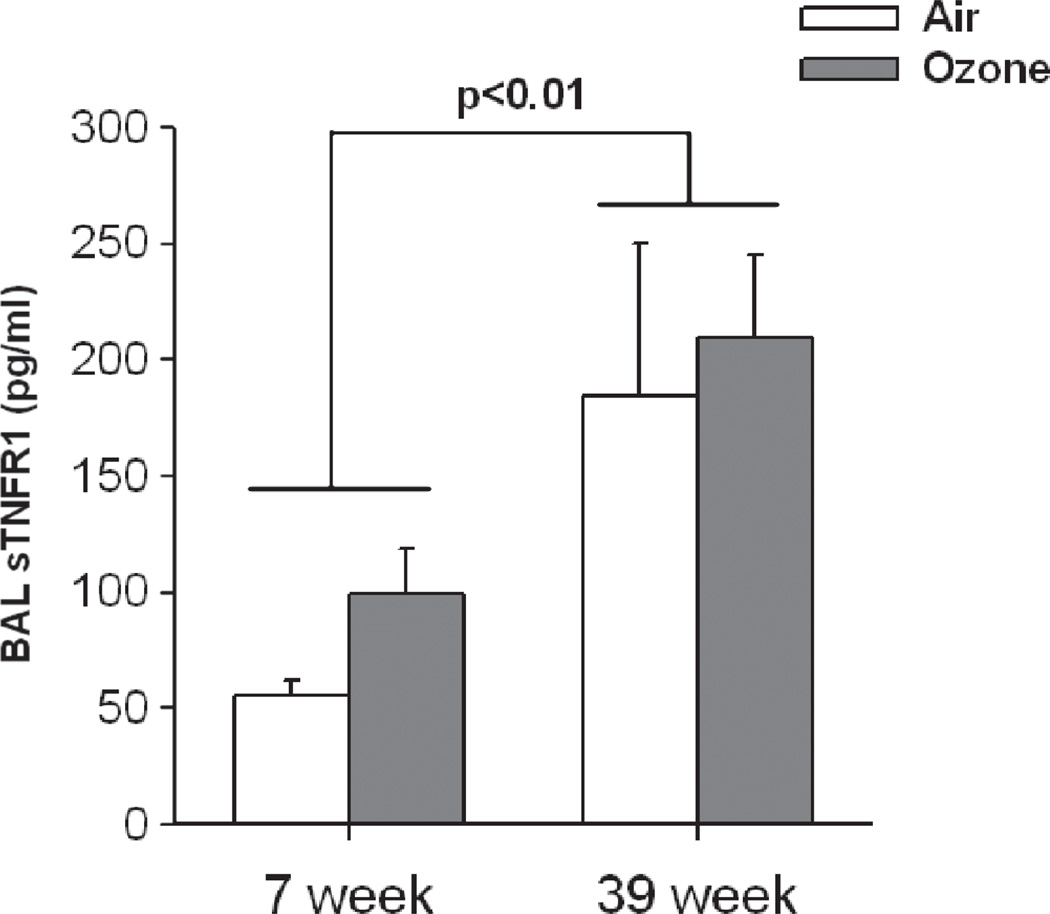
We also measured age-related changes in BAL concentrations of the soluble form of the TNFR1 receptor (sTNFR1) (Figure 3). Compared to air, O3 exposure did not cause any significant change in BAL sTNFR1. However, there was a significant effect of age (p < 0.01). BAL sTNFR1 was substantially increased in 39-week-old versus 7-week-old mice, regardless of exposure.
Figure 3.
Effect of age on bronchoalveolar lavage (BAL) concentrations of soluble form of TNFR1 (sTNFR1) in WT mice exposed to air or O3. Results are mean ± SE of data from 3 to 6 mice in each group. There was a significant increase in sTNFR1 in the older mice.
Lung injury
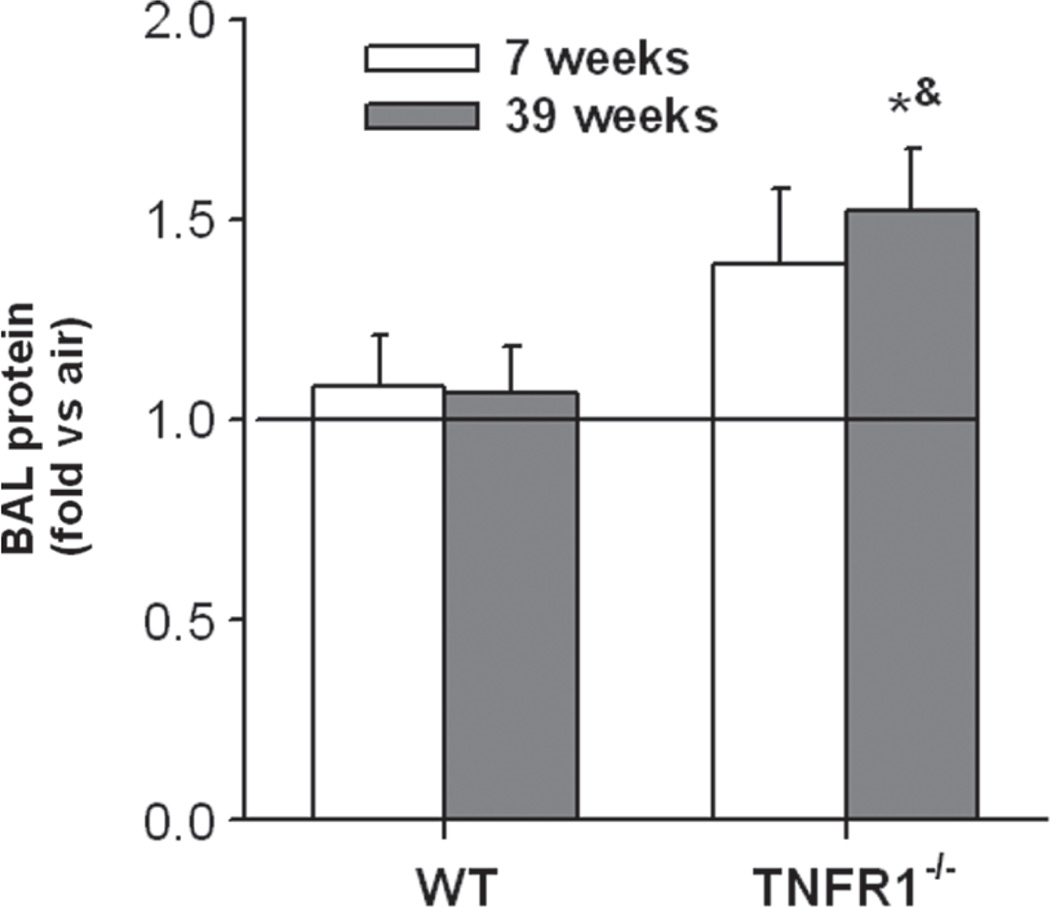
O3 exposure is known to cause injury to lung epithelial cells leading to increased lung permeability and an increase in BAL protein (Bhalla, 1999). As described above, BAL protein from O3-exposed mice was normalized to their respective age- and genotype-matched air exposed controls (represented by the horizontal lines in Figure 4 at a “fold versus air” of 1). O3 exposure caused a significant increase in BAL protein in TNFR1−/− but not WT mice, but the effect only reached statistical significance in the 39-week-old TNFR1−/− mice (Figure 4). Consistent with lack of effect of O3 in the wildtype mice, we have previously reported that O3 induced increases in BAL protein are minimal 4 h post- exposure (the time point used in this study), but increase substantially by 24 h after exposure (Lang et al., 2008).
Figure 4.
Effect of age on bronchoalveolar lavage protein concentration in WT and TNFR1−/− mice exposed to O3. Results are mean ± SE of data from 3 to 6 mice in each group, and are expressed as fold change relative to the genotype- and age-matched air controls which are represented by the horizontal line. *p < 0.05 versus genotype and age-matched mice exposed to air; &p < 0.05 versus exposure and age-matched WT mice.
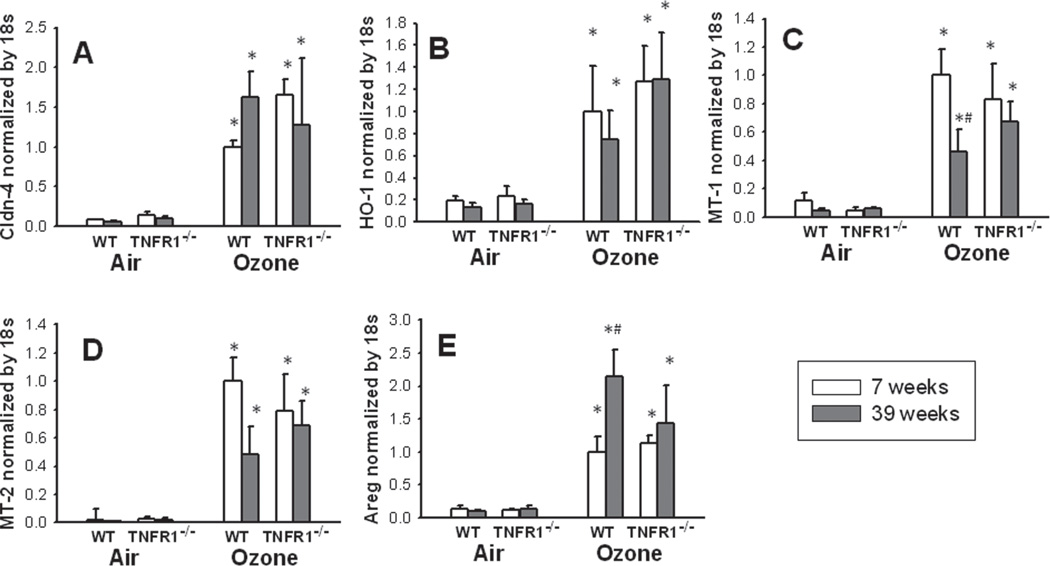
Because of the impact of TNFR1 deficiency on lung epithelial barrier integrity (Figure 4), and because the tight junction protein claudin-4 has been shown to play an important role in alveolar epithelial barrier function (Wray et al., 2009; Mitchell et al., 2011) and to be impacted by TNF (Mazzon et al., 2007; Wang et al., 2009), we also measured claudin-4 mRNA expression in lung tissue from these mice (Figure 5A). Compared to air, O3 exposure resulted in a marked increase in claudin-4 gene expression in mice of both ages and both genotypes (Figure 5A) However, there was no significant impact of either age or genotype on claudin-4 expression.
Figure 5.
Effect of age and TNFR1 deficiency on pulmonary mRNA expression of claudin-4 (Cldn-4) (A), heme oxygenase-1 (HO-1) (B), metallothionein-1 (MT-1) (C), metallothionein-2 (MT-2) (D), and amphiregulin (Areg) (E). Results are mean ± SE of data from 3 to 6 mice in each group. Gene expression was normalized for 18S and expressed relative to the 7-week-old wildtype (WT) O3 exposed group. *p < 0.05 versus genotype and age-matched mice exposed to air; #p < 0.05 versus 7-week-old genotype and exposure matched mice.
Anti-oxidant gene expression
The mRNA expression of HO-1, MT-1, and MT-2 was markedly increased by O3 exposure in mice of both genotypes and both ages (Figure 5B, C, D). MT-1 expression was significantly reduced in O3-exposed 39-week-old versus 7-week-old WT mice, but there was no effect of TNFR1 deficiency on MT-1 expression in O3-exposed mice. A similar trend was observed for MT-2, but did not reach statistical significance (p < 0.085). There was no significant effect of either age or TNFR1 genotype on O3-induced HO-1 expression.
Areg expression
We also examined a possible role of Areg in age- and TNFR1-related differences in the inflammatory response to O3. Compared to air, O3 exposure caused a marked increase in Areg expression in mice of both ages and both genotypes (Figure 5E). Areg was induced to a greater extent in 39-week-old than in 7-week-old O3-exposed wildtype mice, but there was no effect of TNFR1 genotype.
Discussion
Our data indicate that in mice, age-related differences in the inflammatory response to acute O3 exposure vary with TNFR1 expression. In WT mice, the influx of BAL neutrophils was lower (Figure 1B), and BAL concentrations of MCP-1, KC, and MIP-2 were also reduced in the 39-weekold versus 7-week-old mice (Figure 2). In contrast, the decreased inflammatory responses in the older mice reverted to robust responses in TNFR1−/− mice (Figures 1B and 2). MT and HO-1 expression did not appear to account for the age- or TNFR1-related differences in the inflammatory response to O3 (Figure 5). However, we did observe that BAL sTNFR1 was significantly increased in the 39 week versus 7-week-old mice (Figure 3). Taken together, the data suggest that this increase in sTNFR1 may neutralize TNF in the lung and protect the 39-weekold mice against O3-induced inflammation.
One important technical issue requires consideration. As discussed in the Methods section, in order to correct for age-related differences in dilution of lung lining fluid by a fixed amount of instilled BAL fluid, we expressed the BAL concentrations of IL-6, MCP-1, IP-10, KC, MIP-2, and protein in the O3-exposed mice as a ratio of the respective age- and genotype-matched air values (Figures 2 and 4), a procedure previously employed by ourselves and others in correcting for age-related differences in BAL moieties (Shore et al., 2002; Vancza et al., 2009). Hence, in Figures 2 and 4, the air exposed values have all been set to a value of “1”, represented by the horizontal lines. However, in the case of sTNFR1, we were also interested in how age might affect even the resting (air exposed) values, since sTNFR1 in the lung lining fluid may be acting to neutralize TNFα. The magnitude of the age-related difference in BAL sTNFR1 was quite marked—almost 4-fold greater in the 39 versus 7-week-old air exposed mice (Figure 3). However, an increase was expected on the basis of issues of dilution alone: in the young mice, with smaller lungs, there would have been greater dilution of lung lining fluid by the BAL fluid than in the old mice in which we instilled the same volume of fluid. To estimate how much of the apparent increase in BAL sTNFR1 in the 39- versus 7-week-old mice was simply the result of differences in dilution and how much was real, we computed the ratio of the air exposed values of the 39- versus the 7-week-old mice for all the other moieties measured in BAL fluid. The average of these ratio’s was 1.5-fold. Thus, the concentration of sTNFR1 in BAL fluid was elevated in the 39-week-old mice substantially more (4-fold) than would be expected on the basis of issues of dilution alone (1.5-fold). A similar age-related increase in sTNFR1 has been reported in human serum (Scalzo et al., 2009).
Acute O3 exposure caused an increase in neutrophils and epithelial cells and a reduction in macrophages in BAL fluid. The increase in neutrophils is the classic manifestation of cellular inflammation with O3 exposure, while the increase in epithelial cells likely reflects epithelial injury. The reduction in macrophages is thought to represent cell activation leading to increased adherence to the pulmonary epithelium, making them more difficult to remove with lavage. Importantly, we observed a reduction in neutrophil recruitment following O3 exposure in 39 versus 7-week-old wildtype mice after O3 exposure (Figure 1B). To address the mechanistic basis for this reduction in BAL neutrophils, we measured BAL concentrations of IL-6, KC, MIP-2, IP-10, and MCP-1 (Figure 2). Each of these cytokines and chemokines has been implicated in the influx of neutrophils that occurs following acute O3 exposure in mice (Zhao et al., 1998; Michalec et al., 2002; Johnston et al., 2005a; Johnston et al., 2005b; Lang et al., 2008). BAL KC, MIP-2, and MCP-1 were each reduced in 39 versus 7-week-old wildtype mice exposed to O3 (Figure 2), suggesting that reductions in these chemokines may be contributing to the reduction in neutrophil recruitment that occurred in the 39-week-old mice. Elder et al. (Elder et al., 2000) also reported a reduction in O3-induced neutrophil recruitment to the lung in aged (22-month-old) versus young (10-week-old) rats, and Stiles et al. (1988) reported fewer centriacinar lesions in the lungs of O3-exposed aged (15-month-old) versus young adult (8-week-old) rats. Hamade et al. (Hamade et al., 2010) reported that the bradycardia induced by O3 exposure was reduced in older (1 year) versus younger (5 months) mice. However, to our knowledge, this is the first report of the impact of increasing age on O3-induced pulmonary inflammation in adult mice.
Reduced pulmonary responses to O3 have also been observed in older human subjects. In a study utilizing 240 human nonsmokers aged 18–60 years, Hazucha et al. observed that the decline in FEV1 that was induced by acute O3 exposure in a chamber study was smaller in older (>35 years) than in younger (≤ 35 years) subjects (Hazucha et al., 2003). In another chamber study, McDonnell et al. (1993) also observed smaller O3-induced reductions in FEV1 in older adult human subjects, but the oldest individuals in their study were only 32 years of age. In contrast, Korrick et al. (1998) observed no age-related differences in decline in FEV1 in a study of over 500 individuals exposed to ambient O3 while hiking Mount Washington. While such observations appear to be at variance with the greater susceptibility of older individuals to O3 induced respiratory related emergency room visits and hospital admissions (Delfino et al., 1997; Moolgavkar et al., 1997; Yang et al., 2003), it is important to recognize that these studies (Delfino et al., 1997; Moolgavkar et al., 1997; Yang et al., 2003), included not just individuals healthy enough to participate in chamber studies or extended hikes, but also individuals who may have had underlying respiratory complications.
We used a concentration of 2 ppm O3 in this study. This concentration is higher than the ambient O3 concentrations that would be observed even in the most polluted cities in the world. However, in mice, such concentrations are routinely used by many investigators in studying the acute effects of O3 exposure on respiratory outcomes (Slade et al., 1997; Johnston et al., 2000b; Cho et al., 2001; Kierstein et al., 2008; Matsubara et al., 2009; Williams et al., 2009), because rodents are much less susceptible to O3 than humans (Hatch et al., 1994; Slade et al., 1997). Hatch et al. (1994) estimated, based on the concentration of ozone reaction product accumulated in BAL cells and fluids from stable isotopically labeled O3, that rodents require much higher concentrations of O3 to achieve the same O3 dose as humans. Indeed, because of such species differences, Slade et al. (1997) have estimated that the O3 exposure we used here is roughly equivalent in dose to that employed by Hazucha et al. (2003) and McDonnell et al. (1993) in their examination of age-related differences in the acute response to O3 in humans, even though the concentration differed by about 5-fold.
We cannot rule out the possibility that the reduced inflammation observed in the 39-week-old mice (Figure 1B and 2) is the result of a lower inhaled dose of O3. The inhaled dose is the product of exposure time, exposure concentration, and minute ventilation during the exposure (Weister, 1987). Dauger et al. reported no differences in minute ventilation normalized for body weight between room air exposed 30-, 100-, and 210-day-old mice (Dauger et al., 2003). However, in rodents, minute ventilation declines during O3 exposure, which may account for at least part of their reduced susceptibility to O3 described above. Arito et al. (1997) measured O3-induced reductions in ventilation in 4–6 versus 20–22-month-old rats, and found that the magnitude of O3-induced reductions in ventilation was greater in the younger animals. Since reducing ventilation also reduces the inhaled dose of O3, the inhaled dose of the older rats would have been higher than that of the younger rats, which would be predicted to result in greater inflammation. We have also reported age-related differences in this effect of O3 in mice, although the age span we studied (2–12 weeks of age) was quite different from that used by Arito et al. (Shore et al., 2000). In contrast to their results, we reported that very young (2-week-old) mice have little reduction (hence a higher net dose of O3) compared to 12-week-old mice, in which minute ventilation declined by more than 60% during a 3 h 2 ppm exposure such as employed here (Shore et al., 2000). The age-related effect appeared to plateau between 4 and 8 weeks of age, but we did not measure effects of O3 on ventilation in mice older than 12 weeks.
Others have reported that laboratory rodents housed as these mice were, with ad libitum feeding and lack of exercise, typically become overweight as they age (Martin et al., 2010). We considered the possibility that age-related increases in adiposity might be contributing to the age-dependent responses to O3 that we observed (Figures 1 and 2). However, we have previously reported increased responses to acute O3 exposure in obese mice (Shore et al., 2003; Johnston et al., 2006; Lu et al., 2006), whereas reduced responses to O3 were observed in the older mice in this study (Figures 1 and 2). Moreover, although no direct markers of adiposity were assessed, body weight was not different in the wildtype and TNFR1 deficient 39-week-old mice, even though their responses to O3 were substantially different (Figures 1 and 2). Thus, the data suggest that age, rather than adiposity, accounts for the reduced responses to O3 observed in 39- versus 7-week-old mice.
Whereas O3-induced neutrophil influx into the lungs was reduced in 39- versus 7-week-old wildtype mice, this was not the case in TNFR1−/− deficient mice (Figure 1B). Indeed, although there was little impact of TNFR1 deficiency in the 7-week-old mice, in the 39-week-old mice TNFR1 deficiency enhanced neutrophil influx (Figure 1B) and increased many of the BAL cytokines and chemokines examined. We were surprised by these observations, since in mice, both TNF inhibition and TNFR1 or TNFR2 deficiency have been reported to inhibit the neutrophilic inflammation induced by exposure to 0.3 ppm O3 for 48–72 h in this strain of mice (Kleeberger et al., 1997; Cho et al., 2001), although the nature of that exposure was quite different from what was employed in this study. We have previously reported that neither TNFR1 nor TNFR2 deficiency affects the neutrophil influx observed 24 h after exposure to 2 ppm O3 for 3 h (Shore et al., 2001), the same type of exposure employed here. Those data were obtained in young adult mice and hence are consistent with the lack effect of TNFR1 deficiency on neutrophil recruitment observed in the 7-week-old mice reported here. However, the augmented inflammatory response observed with TNFR1 deficiency in the 39-week-old mice was unexpected, since it was opposite in direction to what would be expected if the pro-inflammatory actions of TNFα were involved in mediating these inflammatory responses to O3.
The anti-oxidants, MT and HO-1, are each induced by acute O3 exposure (Johnston et al., 2000b). Moreover, induction of these enzymes by O3 is functionally important: O3-induced inflammation is augmented in mice genetically deficient in MT-1 (Inoue et al., 2008), or in rats in which HO-1 is chemically inhibited (Hisada et al., 2000). TNFα is capable of inducing MT-1, MT-2, and HO-1 (Kaji et al., 1993; Sato et al., 1994). Indeed, induction of MT-1 and MT-2 after traumatic brain injury is reduced in mice deficient in TNFR1 (Quintana et al., 2007). Similarly, TNFR1−/− deficient mice had reduced liver expression of HO-1 and associated increased injury in a model of acetaminophen induced hepatotoxicity (Chiu et al., 2003). TNF is also required for induction of HO-1 in liver following LPS (Oguro et al., 2002). Based on these observations, we reasoned that greater expression of these anti-oxidants in the 39-week-old mice might be contributing to their protection against O3-induced inflammation (Figures 1 and 2) and that TNFR1 deficiency might reduce expression of these anti-oxidants, resulting in loss of this protection. Our data do not support this hypothesis. In O3-exposed mice, both MT-1 and MT-2 were reduced in the 39-week-old versus 7-week-old mice while HO-1 was not impacted, and TNFR1 deficiency did not affect expression of any of these enzymes (Figure 5).
We observed greater induction of Areg mRNA in 39 versus 7-week-old wildtype mice exposed to O3, whereas no age-related difference was observed in TNFR1−/− mice (Figure 5E). Areg is one of a family of ligands that act on the EGFR receptor, and Areg has been reported to increase airway epithelial proliferation (Lee et al., 2011), which could be important in protection against the epithelium damaging effects of O3. Indeed, exogenous administration of Areg protects mice against the pulmonary inflammation induced by bleomycin exposure (Fukumoto et al., 2010). We do not know whether there were differences in Areg protein as well as mRNA expression in the 39-versus 7-week-old mice. However, it is conceivable that the augmented expression of Areg in 39-week-old mice (Figure 5E) may be contributing to the relative protection of the 39-week-old mice against O3-induced inflammation (Figures 1 and 2), and that loss of this protective agerelated increase in Areg expression in TNFR1−/− mice may be contributing to their augmented responses to O3.
We observed an age-related increase in sTNFR1 in the lung lining fluid (Figure 3). sTNFR1 is the extracellular domain of the p55 TNF receptor (TNFR1) that is released from the cell surface by proteolytic cleavage, via the enzyme TACE (ADAM17), that is also responsible for shedding of TNFα from the cell surface. We do not know the mechanistic basis for this age-related increase in sTNFR1, but it may be related to oxidative stress, since others have reported elevations in oxidative stress in lungs of mice with aging (Calvi et al., 2011), and since oxidative stress promotes activation of TACE (Brill et al., 2009). Importantly, sTNFR1 can exert anti-inflammatory actions by neutralizing TNFα (Yagi et al., 2010). Administration of sTNFR1 with TNFα blocks the neutrophil influx into the peritoneal cavity induced by i.p. injection of TNFα alone (Hale et al., 1995). Similarly, co-injection of endotoxin and sTNFR1 intratracheally attenuates the pulmonary neutrophilia induced by endotoxin alone (Ulich et al., 1993). Such effects of endotoxin are in part mediated by TNFα. Thus, the observation that BAL sTNFR1 was elevated in 39- versus 7-week-old mice (Figure 3), may explain why neutrophil influx and induction of neutrophil chemotactic factors induced by O3 were limited in the 39-week-old mice (Figures 1B and 2). This hypothesis is supported by the observation that when TNFR1 was deleted, neutrophil influx and BAL chemokine levels were restored in the 39-week-old mice. The data are consistent with the hypothesis that deletion of TNFR1 led to increased TNFα and increased activation of the remaining TNF receptor, TNFR2. Although the TNFR1 dominates over TNFR2 for many inflammatory effects of TNFα (Vandenabeele et al., 1995; Peschon et al., 1998; MacEwan, 2002; Naude et al., 2011). TNFR2 can still activate NF-κB (MacEwan, 2002; Gupta et al., 2005), a transcription factor important for expression of many of the chemokines induced by O3 Indeed, TNFR2 but not TNFR1 is required for several types of inflammatory conditions. For example, TNFR2 is required for renal injury and inflammation in a mouse model of acute renal failure induced by cisplatin, whereas TNFR1 is not (Ramesh et al., 2003). TNFR2 but not TNFR1 is required for the induction of glomerular injury and inflammation in a mouse model of glomerulonephritis (Vielhauer et al., 2005). TNFα acting on TNFR2 receptors in brain microvessels is also required for upregulation of ICAM1 and leukocyte recruitment in a model of cerebral malaria (Lucas et al., 1997). Similarly, we have previously reported that TNFR2 is required for O3-induced airway hyperresponsiveness in mice, whereas TNFR1 is not (Shore et al., 2001). Thus, it is possible that increased inflammatory responses to O3 observed in TNFR1 deficient mice are being mediated by TNFR2.
In summary, the pulmonary inflammation induced by acute exposure to 2 ppm O3 for 3 hours was reduced in 39- versus 7-week-old mice. The age-related reduction in BAL neutrophils, cytokines, and chemokines was largely abolished in TNFR1−/− mice. The data suggest that increased levels of sTNFR1 present in the lungs of the older mice may neutralize TNF and protect them against O3-induced inflammation.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Environmental Health Sciences grants ES-013307 and ES-000002.
Footnotes
Declaration of interest
The authors have no other declarations of interest to report.
References
- Akassoglou K, Douni E, Bauer J, Lassmann H, Kollias G, Probert L. Exclusive tumor necrosis factor (TNF) signaling by the p75TNF receptor triggers inflammatory ischemia in the CNS of transgenic mice. Proc Natl Acad Sci USA. 2003;100:709–714. doi: 10.1073/pnas.0236046100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis N, Urch B, Tarlo S, Corey P, Pengelly D, O’Byrne P, Silverman F. Cyclooxygenase metabolites play a different role in ozone-induced pulmonary function decline in asthmatics compared to normals. Inhal Toxicol. 2000;12:1205–1224. doi: 10.1080/08958370050198548. [DOI] [PubMed] [Google Scholar]
- Arito H, Takahashi M, Iwasaki T, Uchiyama I. Age-related changes in ventilatory and heart rate responses to acute ozone exposure in the conscious rat. Ind Health. 1997;35:78–86. doi: 10.2486/indhealth.35.78. [DOI] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- Brill A, Chauhan AK, Canault M, Walsh MT, Bergmeier W, Wagner DD. Oxidative stress activates ADAM17/TACE and induces its target receptor shedding in platelets in a p38-dependent fashion. Cardiovasc Res. 2009;84:137–144. doi: 10.1093/cvr/cvp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüünsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Calvi CL, Podowski M, D’Alessio FR, Metzger SL, Misono K, Poonyagariyagorn H, Lopez-Mercado A, Ku T, Lauer T, Cheadle C, Talbot CC, Jr, Jie C, McGrath-Morrow S, King LS, Walston J, Neptune ER. Critical transition in tissue homeostasis accompanies murine lung senescence. PLoS ONE. 2011;6:e20712. doi: 10.1371/journal.pone.0020712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Gardner CR, Dambach DM, Brittingham JA, Durham SK, Laskin JD, Laskin DL. Role of p55 tumor necrosis factor receptor 1 in acetaminophen-induced antioxidant defense. Am J Physiol Gastrointest Liver Physiol. 2003;285:G959–G966. doi: 10.1152/ajpgi.00219.2003. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Cheng MH, Yang CY. Air pollution and hospital admissions for pneumonia in a subtropical city: Taipei, Taiwan. Inhal Toxicol. 2009;21:32–37. doi: 10.1080/08958370802441198. [DOI] [PubMed] [Google Scholar]
- Cho HY, Morgan DL, Bauer AK, Kleeberger SR. Signal transduction pathways of tumor necrosis factor–mediated lung injury induced by ozone in mice. Am J Respir Crit Care Med. 2007;175:829–839. doi: 10.1164/rccm.200509-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-alpha receptors. Am J Physiol Lung Cell Mol Physiol. 2001;280:L537–L546. doi: 10.1152/ajplung.2001.280.3.L537. [DOI] [PubMed] [Google Scholar]
- Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest. 2003;123:530–538. doi: 10.1378/chest.123.2.530. [DOI] [PubMed] [Google Scholar]
- de Gonzalo-Calvo D, Neitzert K, Fernández M, Vega-Naredo I, Caballero B, García-Macía M, Suárez FM, Rodríguez-Colunga MJ, Solano JJ, Coto-Montes A. Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radic Biol Med. 2010;49:733–737. doi: 10.1016/j.freeradbiomed.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Murphy-Moulton AM, Burnett RT, Brook JR, Becklake MR. Effects of air pollution on emergency room visits for respiratory illnesses in Montreal, Quebec. Am J Respir Crit Care Med. 1997;155:568–576. doi: 10.1164/ajrccm.155.2.9032196. [DOI] [PubMed] [Google Scholar]
- Dobbs RJ, Charlett A, Purkiss AG, Dobbs SM, Weller C, Peterson DW. Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism. Acta Neurol Scand. 1999;100:34–41. doi: 10.1111/j.1600-0404.1999.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Elder AC, Gelein R, Finkelstein JN, Cox C, Oberdörster G. Pulmonary inflammatory response to inhaled ultrafine particles is modified by age, ozone exposure, and bacterial toxin. Inhal Toxicol. 2000;12(Suppl 4):227–246. doi: 10.1080/089583700750019585. [DOI] [PubMed] [Google Scholar]
- Fauroux B, Sampil M, Quénel P, Lemoullec Y. Ozone: a trigger for hospital pediatric asthma emergency room visits. Pediatr Pulmonol. 2000;30:41–46. doi: 10.1002/1099-0496(200007)30:1<41::aid-ppul7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Fukumoto J, Harada C, Kawaguchi T, Suetsugu S, Maeyama T, Inoshima I, Hamada N, Kuwano K, Nakanishi Y. Amphiregulin attenuates bleomycin-induced pneumopathy in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L131–L138. doi: 10.1152/ajplung.90576.2008. [DOI] [PubMed] [Google Scholar]
- Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, Leaderer BP. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- Gupta S, Gollapudi S. Molecular mechanisms of TNF-alpha-induced apoptosis in aging human T cell subsets. Int J Biochem Cell Biol. 2005;37:1034–1042. doi: 10.1016/j.biocel.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Hale KK, Smith CG, Baker SL, Vanderslice RW, Squires CH, Gleason TM, Tucker KK, Kohno T, Russell DA. Multifunctional regulation of the biological effects of TNF-alpha by the soluble type I and type II TNF receptors. Cytokine. 1995;7:26–38. doi: 10.1006/cyto.1995.1004. [DOI] [PubMed] [Google Scholar]
- Hamade AK, Misra V, Rabold R, Tankersley CG. Age-related changes in cardiac and respiratory adaptation to acute ozone and carbon black exposures: interstrain variation in mice. Inhal Toxicol. 2010;22(Suppl 2):84–94. doi: 10.3109/08958378.2010.503974. [DOI] [PubMed] [Google Scholar]
- Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, Costa DL, McKee J. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. 1994;150:676–683. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- Hazucha MJ, Folinsbee LJ, Bromberg PA. Distribution and reproducibility of spirometric response to ozone by gender and age. J Appl Physiol. 2003;95:1917–1925. doi: 10.1152/japplphysiol.00490.2003. [DOI] [PubMed] [Google Scholar]
- Hazucha MJ, Madden M, Pape G, Becker S, Devlin R, Koren HS, Kehrl H, Bromberg PA. Effects of cyclo-oxygenase inhibition on ozone-induced respiratory inflammation and lung function changes. Eur J Appl Physiol Occup Physiol. 1996;73:17–27. doi: 10.1007/BF00262805. [DOI] [PubMed] [Google Scholar]
- Hisada T, Salmon M, Nasuhara Y, Chung KF. Involvement of haemoxygenase-1 in ozone-induced airway inflammation and hyperresponsiveness. Eur J Pharmacol. 2000;399:229–234. doi: 10.1016/s0014-2999(00)00369-1. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Kaewamatawong T, Shimada A, Suzuki J, Yanagisawa R, Tasaka S, Ishizaka A, Satoh M. Role of metallothionein in lung inflammation induced by ozone exposure in mice. Free Radic Biol Med. 2008;45:1714–1722. doi: 10.1016/j.freeradbiomed.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Oberdörster G, Gelein R, Finkelstein JN. Newborn mice differ from adult mice in chemokine and cytokine expression to ozone, but not to endotoxin. Inhal Toxicol. 2000a;12:205–224. doi: 10.1080/08958370050165067. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Reed CK, Avissar NE, Gelein R, Finkelstein JN. Antioxidant and inflammatory response after acute nitrogen dioxide and ozone exposures in C57Bl/6 mice. Inhal Toxicol. 2000b;12:187–203. doi: 10.1080/089583700196239. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Stripp BR, Reynolds SD, Avissar NE, Reed CK, Finkelstein JN. Inflammatory and antioxidant gene expression in C57BL/6J mice after lethal and sublethal ozone exposures. Exp Lung Res. 1999;25:81–97. doi: 10.1080/019021499270448. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am J Respir Cell Mol Biol. 2007;37:477–484. doi: 10.1165/rcmb.2006-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol. 2005a;288:L61–L67. doi: 10.1152/ajplung.00101.2004. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol. 2005b;288:L390–L397. doi: 10.1152/ajplung.00007.2004. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R126–R133. doi: 10.1152/ajpregu.00306.2005. [DOI] [PubMed] [Google Scholar]
- Kaji T, Yamamoto C, Tsubaki S, Ohkawara S, Sakamoto M, Sato M, Kozuka H. Metallothionein induction by cadmium, cytokines, thrombin and endothelin-1 in cultured vascular endothelial cells. Life Sci. 1993;53:1185–1191. doi: 10.1016/0024-3205(93)90536-c. [DOI] [PubMed] [Google Scholar]
- Kierstein S, Krytska K, Sharma S, Amrani Y, Salmon M, Panettieri RA, Jr, Zangrilli J, Haczku A. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergen-sensitized mice. Allergy. 2008;63:438–446. doi: 10.1111/j.1398-9995.2007.01587.x. [DOI] [PubMed] [Google Scholar]
- Kleeberger SR. Genetic aspects of susceptibility to air pollution. Eur Respir J Suppl. 2003;40:52s–56s. doi: 10.1183/09031936.03.00403003. [DOI] [PubMed] [Google Scholar]
- Kleeberger SR, Levitt RC, Zhang LY, Longphre M, Harkema J, Jedlicka A, Eleff SM, DiSilvestre D, Holroyd KJ. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat Genet. 1997;17:475–478. doi: 10.1038/ng1297-475. [DOI] [PubMed] [Google Scholar]
- Korrick SA, Neas LM, Dockery DW, Gold DR, Allen GA, Hill LB, Kimball KD, Rosner BA, Speizer FE. Effects of ozone and other pollutants on the pulmonary function of adult hikers. Environ Health Perspect. 1998;106:93–99. doi: 10.1289/ehp.9810693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JE, Williams ES, Mizgerd JP, Shore SA. Effect of obesity on pulmonary inflammation induced by acute ozone exposure: role of interleukin-6. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1013–L1020. doi: 10.1152/ajplung.00122.2007. [DOI] [PubMed] [Google Scholar]
- Lee J, Ryu SH, Kang SM, Chung WC, Gold KA, Kim ES, Hittelman WN, Ki Hong W, Koo JS. Prevention of bronchial hyperplasia by EGFR pathway inhibitors in an organotypic culture model. Cancer Prev Res (Phila) 2011;4:1306–1315. doi: 10.1158/1940-6207.CAPR-10-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JI, Chemerynski SM, Sarnat JA. Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology. 2005;16:458–468. doi: 10.1097/01.ede.0000165820.08301.b3. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- Lucas R, Juillard P, Decoster E, Redard M, Burger D, Donati Y, Giroud C, Monso-Hinard C, De Kesel T, Buurman WA, Moore MW, Dayer JM, Fiers W, Bluethmann H, Grau GE. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol. 1997;27:1719–1725. doi: 10.1002/eji.1830270719. [DOI] [PubMed] [Google Scholar]
- MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci USA. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S, Takeda K, Jin N, Okamoto M, Matsuda H, Shiraishi Y, Park JW, McConville G, Joetham A, O’Brien RL, Dakhama A, Born WK, Gelfand EW. Vgamma1+ T cells and tumor necrosis factor-alpha in ozone-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2009;40:454–463. doi: 10.1165/rcmb.2008-0346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon E, Cuzzocrea S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respir Res. 2007;8:75. doi: 10.1186/1465-9921-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell WF, Muller KE, Bromberg PA, Shy CM. Predictors of individual differences in acute response to ozone exposure. Am Rev Respir Dis. 1993;147:818–825. doi: 10.1164/ajrccm/147.4.818. [DOI] [PubMed] [Google Scholar]
- Michalec L, Choudhury BK, Postlethwait E, Wild JS, Alam R, Lett-Brown M, Sur S. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol. 2002;168:846–852. doi: 10.4049/jimmunol.168.2.846. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2011;301:L40–L49. doi: 10.1152/ajplung.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolgavkar SH, Luebeck EG, Anderson EL. Air pollution and hospital admissions for respiratory causes in Minneapolis-St. Paul and Birmingham. Epidemiology. 1997;8:364–370. doi: 10.1097/00001648-199707000-00003. [DOI] [PubMed] [Google Scholar]
- Mund SI, Stampanoni M, Schittny JC. Developmental alveolarization of the mouse lung. Dev Dyn. 2008;237:2108–2116. doi: 10.1002/dvdy.21633. [DOI] [PubMed] [Google Scholar]
- Naudé PJ, den Boer JA, Luiten PG, Eisel UL. Tumor necrosis factor receptor cross-talk. FEBS J. 2011;278:888–898. doi: 10.1111/j.1742-4658.2011.08017.x. [DOI] [PubMed] [Google Scholar]
- Oguro T, Takahashi Y, Ashino T, Takaki A, Shioda S, Horai R, Asano M, Sekikawa K, Iwakura Y, Yoshida T. Involvement of tumor necrosis factor alpha, rather than interleukin-1alpha/beta or nitric oxides in the heme oxygenase-1 gene expression by lipopolysaccharide in the mouse liver. FEBS Lett. 2002;516:63–66. doi: 10.1016/s0014-5793(02)02502-4. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- Quintana A, Giralt M, Molinero A, Campbell IL, Penkowa M, Hidalgo J. Analysis of the cerebral transcriptome in mice subjected to traumatic brain injury: importance of IL-6. Neuroimmunomodulation. 2007;14:139–143. doi: 10.1159/000110637. [DOI] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003;285:F610–F618. doi: 10.1152/ajprenal.00101.2003. [DOI] [PubMed] [Google Scholar]
- Sato M, Sasaki M, Hojo H. Differential induction of metallothionein synthesis by interleukin-6 and tumor necrosis factor-alpha in rat tissues. Int J Immunopharmacol. 1994;16:187–195. doi: 10.1016/0192-0561(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Scalzo P, Kümmer A, Cardoso F, Teixeira AL. Increased serum levels of soluble tumor necrosis factor-alpha receptor-1 in patients with Parkinson’s disease. J Neuroimmunol. 2009;216:122–125. doi: 10.1016/j.jneuroim.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Shore SA, Abraham JH, Schwartzman IN, Murthy GG, Laporte JD. Ventilatory responses to ozone are reduced in immature rats. J Appl Physiol. 2000;88:2023–2030. doi: 10.1152/jappl.2000.88.6.2023. [DOI] [PubMed] [Google Scholar]
- Shore SA, Johnston RA, Schwartzman IN, Chism D, Krishna Murthy GG. Ozone-induced airway hyperresponsiveness is reduced in immature mice. J Appl Physiol. 2002;92:1019–1028. doi: 10.1152/japplphysiol.00381.2001. [DOI] [PubMed] [Google Scholar]
- Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 2001;164:602–607. doi: 10.1164/ajrccm.164.4.2001016. [DOI] [PubMed] [Google Scholar]
- Sisto M, Lisi S, Lofrumento DD, Ingravallo G, Mitolo V, D’Amore M. Expression of pro-inflammatory TACE-TNF-β-amphiregulin axis in Sjogren’s syndrome salivary glands. Histochem Cell Biol. 2010;134:345–353. doi: 10.1007/s00418-010-0735-5. [DOI] [PubMed] [Google Scholar]
- Slade R, Watkinson WP, Hatch GE. Mouse strain differences in ozone dosimetry and body temperature changes. Am J Physiol. 1997;272:L73–L77. doi: 10.1152/ajplung.1997.272.1.L73. [DOI] [PubMed] [Google Scholar]
- Stiles J, Tyler WS. Age-related morphometric differences in responses of rat lungs to ozone. Toxicol Appl Pharmacol. 1988;92:274–285. doi: 10.1016/0041-008x(88)90387-0. [DOI] [PubMed] [Google Scholar]
- Tolbert PE, Mulholland JA, MacIntosh DL, Xu F, Daniels D, Devine OJ, Carlin BP, Klein M, Dorley J, Butler AJ, Nordenberg DF, Frumkin H, Ryan PB, White MC. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;151:798–810. doi: 10.1093/oxfordjournals.aje.a010280. [DOI] [PubMed] [Google Scholar]
- Triche EW, Gent JF, Holford TR, Belanger K, Bracken MB, Beckett WS, Naeher L, McSharry JE, Leaderer BP. Low-level ozone exposure and respiratory symptoms in infants. Environ Health Perspect. 2006;114:911–916. doi: 10.1289/ehp.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich TR, Yin S, Remick DG, Russell D, Eisenberg SP, Kohno T. Intratracheal administration of endotoxin and cytokines. IV. The soluble tumor necrosis factor receptor type I inhibits acute inflammation. Am J Pathol. 1993;142:1335–1338. [PMC free article] [PubMed] [Google Scholar]
- Vancza EM, Galdanes K, Gunnison A, Hatch G, Gordon T. Age, strain, and gender as factors for increased sensitivity of the mouse lung to inhaled ozone. Toxicol Sci. 2009;107:535–543. doi: 10.1093/toxsci/kfn253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- Vasu VT, Oommen S, Lim Y, Valacchi G, Hobson B, Eirserich JP, Leonard SW, Traber MG, Cross CE, Gohil K. Modulation of ozone-sensitive genes in alpha-tocopherol transfer protein null mice. Inhal Toxicol. 2010;22:1–16. doi: 10.3109/08958370902838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhauer V, Stavrakis G, Mayadas TN. Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest. 2005;115:1199–1209. doi: 10.1172/JCI23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lopez-Fraga M, Rynko A, Lo DD. TNFR and LTbetaR agonists induce follicle-associated epithelium and M cell specific genes in rat and human intestinal epithelial cells. Cytokine. 2009;47:69–76. doi: 10.1016/j.cyto.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weister MJ, Williams TB, King E, Menache MG, Muller FJ. Ozone uptake in awake Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 1987;89:429–437. doi: 10.1016/0041-008x(87)90162-1. [DOI] [PubMed] [Google Scholar]
- Williams AS, Eynott PR, Leung SY, Nath P, Jupp R, De Sanctis GT, Resnick R, Adcock IM, Chung KF. Role of cathepsin S in ozone-induced airway hyperresponsiveness and inflammation. Pulm Pharmacol Ther. 2009;22:27–32. doi: 10.1016/j.pupt.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Williams AS, Issa R, Leung SY, Nath P, Ferguson GD, Bennett BL, Adcock IM, Chung KF. Attenuation of ozone-induced airway inflammation and hyper-responsiveness by c-Jun NH2 terminal kinase inhibitor SP600125. J Pharmacol Exp Ther. 2007;322:351–359. doi: 10.1124/jpet.107.121624. [DOI] [PubMed] [Google Scholar]
- Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–L227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther. 2010;18:1857–1864. doi: 10.1038/mt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IA, Holz O, Jörres RA, Magnussen H, Barton SJ, Rodríguez S, Cakebread JA, Holloway JW, Holgate ST. Association of tumor necrosis factor-alpha polymorphisms and ozone-induced change in lung function. Am J Respir Crit Care Med. 2005;171:171–176. doi: 10.1164/rccm.200402-194OC. [DOI] [PubMed] [Google Scholar]
- Yang Q, Chen Y, Shi Y, Burnett RT, McGrail KM, Krewski D. Association between ozone and respiratory admissions among children and the elderly in Vancouver, Canada. Inhal Toxicol. 2003;15:1297–1308. doi: 10.1080/08958370390241768. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol. 1998;274:L39–L46. doi: 10.1152/ajplung.1998.274.1.L39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.