Abstract
Type 2 diabetes mellitus (T2DM) is complex metabolic disease that arises as a consequence of interactions between genetic predisposition and environmental triggers. One recently described environmental trigger associated with development of T2DM is disturbance of circadian rhythms due to shift work, sleep loss, or nocturnal lifestyle. However, the underlying mechanisms behind this association are largely unknown. To address this, the authors examined the metabolic and physiological consequences of experimentally controlled circadian rhythm disruption in wild-type (WT) Sprague Dawley and diabetes-prone human islet amyloid polypeptide transgenic (HIP) rats: a validated model of T2DM. WT and HIP rats at 3 months of age were exposed to 10 weeks of either a normal light regimen (LD: 12:12-h light/dark) or experimental disruption in the light-dark cycle produced by either (1) 6-h advance of the light cycle every 3 days or (2) constant light protocol. Subsequently, blood glucose control, beta-cell function, beta-cell mass, turnover, and insulin sensitivity were examined. In WT rats, 10 weeks of experimental disruption of circadian rhythms failed to significantly alter fasting blood glucose levels, glucose-stimulated insulin secretion, beta-cell mass/turnover, or insulin sensitivity. In contrast, experimental disruption of circadian rhythms in diabetes-prone HIP rats led to accelerated development of diabetes. The mechanism subserving early-onset diabetes was due to accelerated loss of beta-cell function and loss of beta-cell mass attributed to increases in beta-cell apoptosis. Disruption of circadian rhythms may increase the risk of T2DM by accelerating the loss of beta-cell function and mass characteristic in T2DM.
Keywords: circadian disruption, constant light, beta-cell, type 2 diabetes, beta-cell mass, insulin secretion, insulin sensitivity
The circadian timing system allows the organism to adapt its internal metabolism to changes in the external environment created by daily fluctuations in the light/dark (LD) cycle. Thus, daily circadian oscillations in many physiological parameters such as cardiovascular function, thermoregulation, and glucose metabolism have long been characterized in mammalian physiology (Rutter et al., 2002). The “pacemaker” of the circadian system is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, composed of self-contained circadian oscillators governed by finely tuned transcriptional-translational feedback loops (Reppert and Weaver, 2002). Moreover, self-sustained circadian oscillators have been shown to be present in multiple peripheral tissues that are normally entrained by rhythmic cues from the SCN (Yamazaki et al., 2000). Misalignment of internal circadian oscillators with the external environment leads to deleterious health consequences and has long been associated with increased morbidity and mortality in humans (Reddy and O’Neill, 2010).
Recent epidemiological studies have linked disturbances in circadian rhythms to the development of type 2 diabetes (T2DM), a disease that afflicts nearly 10% of the American population (Bass and Takahashi, 2010; Reddy and O’Neill, 2010). T2DM is a complex metabolic disease, the pathophysiology of which involves an interaction between genetic predisposition and environmental triggers leading to induction of fasting and postprandial hyperglycemia. Insulin resistance and pancreatic beta-cell failure are both characteristic features of T2DM and play a major contributory role in the pathogenesis and progression of the disease (DeFronzo, 2009). Both beta-cell failure and insulin resistance undoubtedly contribute to pathogenesis of diabetes as both dysfunctions precede the development of overt hyperglycemia. Insulin resistance in T2DM primarily manifests at the level of the liver and the skeletal muscle, resulting in impaired insulin-stimulated glucose disposal and failure to adequately suppress hepatic glucose production (DeFronzo et al., 1982), which contributes to hyperglycemia. Additionally, induction of insulin resistance in patients with T2DM is associated with onset of a number of comorbidities associated with metabolic syndrome (e.g., hypertension and cardiovascular disease).
Beta-cell failure in T2DM is associated with a deficit in glucose-stimulated insulin secretion, which is already evident in individuals at risk for developing T2DM, with the impairment becoming more prominent in patients who eventually go on to develop T2DM. The decline in glucose-stimulated insulin secretion has been attributed to a defect in beta-cell function and the loss of beta-cell mass due to increased beta-cell apoptosis (Butler et al., 2003; Perley and Kipnis, 1967). Interestingly, most but not all genetic predispositions to T2DM appear to be linked to pancreatic beta-cell failure through the regulation of beta-cell function and/ or mass (Florez, 2008), whereas most environmental triggers such as obesity and physical inactivity have been shown to primarily influence insulin action (Yki-Jarvinen, 1995). While epidemiological evidence reveals a link between disturbances in circadian rhythms and T2DM, little is known about the mechanisms underlying this association (Silva et al., 2010).
In order to gain insight into the mechanisms by which circadian misalignment increases the risk of development of T2DM, we undertook studies in 3-month-old wild-type (WT) Sprague Dawley rats and diabetes-prone Sprague Dawley rats transgenic for human islet amyloid polypeptide (HIP rats). HIP rats develop diabetes by ~10 months of age, characterized by a progressive loss of pancreatic beta-cell mass and function due to accumulation of toxic human islet amyloid polypeptide oligomers and consequent induction of beta-cell endoplasmic reticulum (ER) stress (Haataja et al., 2008). The islet phenotype of diabetic HIP rats closely recapitulates the phenotype of islets seen in patients with T2DM also characterized by loss of beta-cell mass, beta-cell apoptosis, and ER stress (Huang et al., 2007). Thus, the prediabetic HIP rat provides an excellent model to explore the interaction between a genetic predisposition to beta-cell failure (characteristic of HIP rats) and novel environmental triggers such as circadian misalignment in the pathophysiology of T2DM (Butler et al., 2004). Therefore, in this study, we sought to address whether environmental disruption of the circadian system in vivo results in impaired glucoregulation and, if so, whether it is a consequence of beta-cell failure, decline in insulin action, or a combination of both.
MATERIALS AND METHODS
Animals
A total of 76 male Sprague Dawley rats (WT; n = 43) and rats overexpressing human islet amyloid polypeptide on the Sprague Dawley background (HIP rats; n = 33) were used in the current study. The generation of the HIP rats has been described in detail previously (Butler et al., 2004). All rats were bred and housed individually throughout the study at the University of California, Los Angeles animal housing facility and subjected to standard 12/12-h LD (lights on at 0600 h, lights off at 1800 h) prior to enrollment in the study. The University of California, Los Angeles Institutional Animal Care and Use Committee approved all surgical and experimental procedures.
Study Design
To establish the effects of disrupting circadian rhythms on diabetes development in vivo, we used 3-month-old diabetes-prone (HIP) and 3-month-old (WT) rats. At this stage, HIP rats exhibit fasting glucose levels and beta-cell mass comparable to WT rats (Butler et al., 2004). Two weeks prior to initiation of study protocols, WT and HIP rats were synchronized to standard living conditions with lights on at 0600 h and lights off at 1800 h in custom-made, environmentally controlled soundproof chambers. Subsequently, rats were randomly assigned into 3 experimental protocols for 10 weeks: (1) normal LD cycle (lights on at 0600 h, lights off at 1800 h) for 10 weeks, (2) 6-h advance of the LD cycle every 3 days designed to simulate chronic jet leg, and (3) 24-h constant light (LL) regimen (25-watt fluorescent tubes 12 in. above the cage at >100 lux light intensity) at a light intensity shown to create complete circadian arrhythmicity (Eastman and Rechtschaffen, 1983). A schematic representation of 3 light cycle protocols employed in the study is illustrated in Supplementary Figure S1. Fasting plasma glucose and body weight were measured every 2 weeks. Glucose measurements and subsequent hyperglycemic clamp studies were all conducted following a 14-h fast and consistently performed at 0800 h during the lights-on period in all groups. Studies in the LD group were performed during the regular (lights on at 0600 h, lights off at 1800 h) day cycle. Studies in the LL group were conducted during the (24-h lights-on) day cycle. Animals in the 6-h advance group shifted 6 h every 3 days and thus every 15 days returned to the “original” baseline LD cycle (lights on at 0600 h, lights off at 1800 h). Studies in 6-h advance group were conducted on the first day once the animals returned to the original LD cycle (lights on at 0600 h, lights off at 1800 h) at 0800 h during the lights-on period (Suppl. Fig. S1).
Implantation of Indwelling Catheters
Following 10 weeks of LD, LL, or 6-h advance, animals were anesthetized with isoflurane (2.5%) by inhalation until effect (Isoflurane Vapor 19.1; Summit Anesthesia, Portland, OR), and indwelling catheters were then inserted into the right internal jugular vein and left carotid artery for subsequent metabolic studies as previously described (Matveyenko et al., 2008). Rats were studied 5 days after surgical implantation of catheters and maintained preoperative body weight, food intake, and normal hematocrit (>40%) at the time of studies.
Validation of Circadian Disruption Protocols Induced by Changes in Light Cycles
To confirm disruptions of circadian rhythms, a subset of WT rats was used to determine patterns of locomotor activity in response to LD (n = 5), LL (n = 5), and 6-h light advance (n = 5) regimens. Gross motor activity was recorded using an optical beam infrared sensor system and Vital View software (Mini Mitter, Bend, OR) for a 10-day period in rats that were exposed to LD, LL, and 6-h advances for 4 weeks. Activity data were plotted and analyzed using ClockLab software (Actimetrics, Chicago, IL). To further validate the efficacy of circadian disruption protocols, WT (n = 9) and HIP (n = 8) rats were used to determine diurnal fluctuations in melatonin levels following LD, LL, and 6-h advance. Since no differences in melatonin secretion between WT and HIP rats were observed, the data were pooled to increase the power of analysis. Specifically, 1 week following implantation of indwelling catheters, 1 mL of blood was drawn from rats exposed to 10 weeks of LD (n = 6), 6-h advances (n = 6), and LL (n = 5) at 0500, 0800, 1400, and 2200 h. Melatonin levels in the LD group were assessed during the regular (lights on at 0600 h, lights off at 1800 h) cycle and in the LL group during the (24-h lights-on) cycle. Melatonin levels in the 6-h advance group were measured on the first day once the animals returned to the regular LD cycle (lights on at 0600 h, lights off at 1800 h). Blood was immediately centrifuged and stored for subsequent analysis for melatonin.
Measurements of Insulin Secretion and Insulin Sensitivity
To assess glucose and arginine-stimulated insulin secretion, a subset of LD (n = 13), 6-h advance (n = 11), and LL (n = 9) WT and HIP rats underwent a hyperglycemic clamp followed by an arginine bolus injection protocol as previously described (Matveyenko and Butler, 2006). In brief, following a 30-min equilibration period (−30 to 0 min), animals received an intravenous glucose bolus (375 mg/kg) followed by a variable 50% (wt/vol) glucose infusion to clamp arterial glucose at ~250 mg/dL (0–70 min). At t = 60 min, rats received a bolus injection of L-arginine solution (1 mmol/kg; Sigma, St. Louis, MO), and arterial blood samples (50 μL) were collected at 1 and 5 min and every 15 min thereafter during the clamp for immediate determination of plasma glucose and insulin concentrations. To assess insulin sensitivity, a subset of LD (n = 10), 6-h advance (n = 10), and LL (n = 11) WT and HIP rats underwent a hyperinsulinemic-euglycemic clamp as previously described (Matveyenko and Butler, 2006). Following an equilibration period, a constant infusion of regular human insulin (Novolin; Novo Nordisk, Princeton, NJ) at 4 mU·kg−1·min−1 was initiated and continued until the end of the clamp (0–120 min). Plasma glucose levels were determined every 10 min, and glucose (50% wt/vol) was infused (5–120 min) to clamp plasma glucose levels at ~100 mg/dL. Additional blood samples (~100 μL) were collected at baseline (−30 min) and during the clamp (10, 30, 50, 80, 120 min) for determination of plasma insulin and free fatty acid concentrations. Rates of exogenous glucose infusion were recorded to assess whole-body insulin sensitivity.
Immunohistochemistry and Immunofluorescence
Rats were euthanized by intravenous sodium pentobarbital 120 mg/kg. The pancreas was then rapidly removed from euthanized rats and fixed in 4% paraformaldehyde overnight at 4 °C. Paraffin-embedded pancreatic sections were stained first for hematoxylin/eosin and insulin (guinea-pig anti-insulin, 1:100; Zymed, Carlsbad, CA). The beta-cell mass was measured by first quantifying the pancreatic cross-sectional area positive for insulin and multiplying this by the pancreatic weight. In addition, sections were co-stained by immunofluorescence for insulin (guinea-pig anti-insulin, 1:100; Zymed) and terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL method; Roche Diagnostics, Mannheim, Germany) for quantification of beta-cell apoptosis, and insulin (guinea-pig anti-insulin, 1:100; Zymed) and Ki-67 (mouse anti-Ki-67, 1:50; Dako, Carpinteria, CA) for determination of beta-cell replication. All islets per pancreatic section (~100 islets) were examined in detail at ×200 magnification (×20 objective, ×10 ocular) for the total number of TUNEL and Ki-67 positive beta-cells. The frequency of TUNEL and Ki-67 expression in each animal was presented as a total number of stained beta-cells per total number of islets. Fluorescent slides were analyzed and imaged using a Leica DM600 microscope (Leica Microsystems, Wetzlar, Germany) and images acquired using OpenLab software (Improvision) and analyzed using ImagePro Plus software.
Analytical Procedures
Plasma glucose concentrations were measured by the glucose oxidase method (YSI Glucose Analyzer; YSI, Yellow Springs, OH). Plasma insulin was measured using competitive colorimetric enzyme-linked immunosorbent assay (Alpco Diagnostics, Salem, NH). Plasma melatonin levels were measured by competitive enzyme-linked immunosorbent assay (ELISA; IBL International, Hamburg, Germany).
Statistical Analysis
Statistical analysis was performed using ANOVA analysis with Fisher post-hoc were appropriate (Statistica, version 6; Statsoft, Tulsa, OK). Data in graphs and tables are presented as mean ± SEM. Findings were assumed statistically significant at p < 0.05.
RESULTS
Impact of LL and 6-h Advances on Diurnal Rhythms of Activity and Plasma Melatonin in Rats
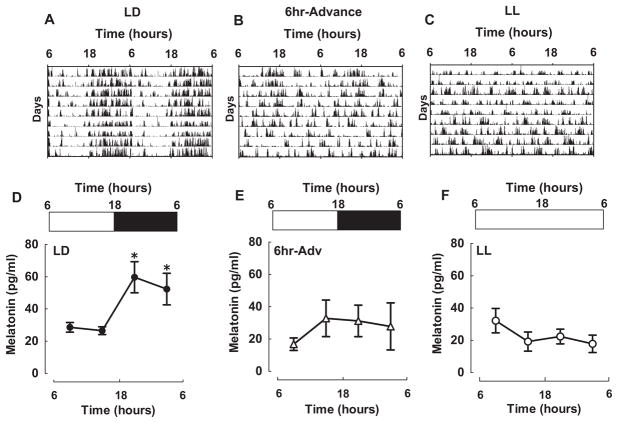
Three-month-old WT and diabetes-prone HIP rats were maintained on either control 12:12-h LD cycle (LD), 6-h advances of the light cycle once every 3 days (6-h ADV), or LL cycle (LL), and motor activity was recorded (Fig. 1). Consistent with previous studies, while rats in the LD cycle maintained robust diurnal activity rhythms, prolonged exposure to either LL or chronic light advances resulted in arrhythmicity of locomotor behavior (Fig. 1A–C). In addition, we examined diurnal melatonin secretion as a biomarker of circadian disruption (Mirick and Davis, 2008). In LD conditions, rats’ melatonin secretion demonstrated a typical robust nighttime rise with elevated plasma melatonin concentrations at 2200 and 0500 h (Fig. 1D; p < 0.001). Melatonin rhythms were disrupted in animals exposed to either 10 weeks of LL or 6-h ADV (p = 0.5; Fig. 1E,F). Individual records of melatonin secretion in LL and 6-h ADV were similar to the averaged data in that they lacked the daily peaks present in LD.
Figure 1.
Impact of LL and 6-h advances on diurnal rhythms of activity and plasma melatonin in rats. (A-C) Representative double-plotted actograms of rats exposed to either control light (A) regimen (12:12 h, LD; n = 5) or experimental changes in the LD cycle produced by (B) 6-h advance of the light cycle every 3 days for 10 weeks (6-h ADV; n = 5) or by 24-h constant light (LL; n = 5). (D-F) Diurnal levels of plasma melatonin in WT and HIP rats following 10-week exposure to LD (D), LL (E), or 6-h ADV (F). Data are expressed as mean ± SEM, *p < 0.05 statistical significance.
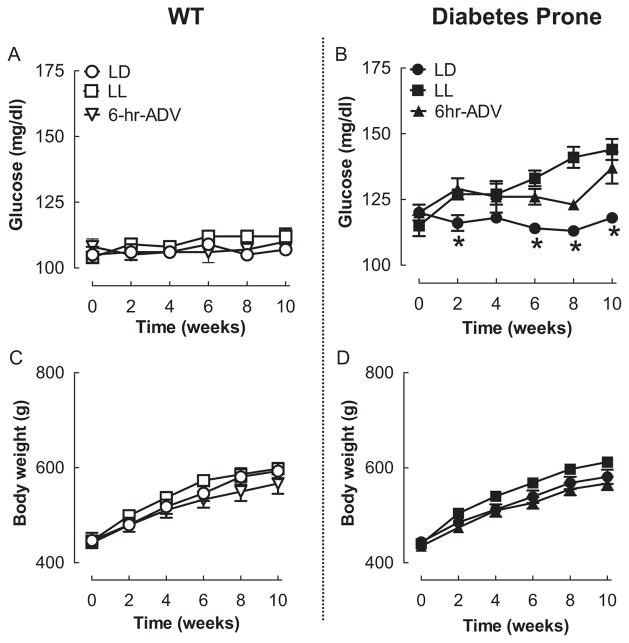
Effects of Circadian Rhythm Disruption on Weekly Plasma Glucose and Body Weight in WT and Diabetes-Prone HIP Rats
The fasting plasma glucose and body weight in WT and HIP rats were comparable among all 3 experimental light conditions at the beginning of the study (Fig. 2). In WT rats, 10 weeks of experimental disruption of circadian rhythms did not significantly alter fasting blood glucose concentrations as well as body weight, implying failure to modify regulation of glucose metabolism (Fig. 2A,C). HIP rats at the end of the 10-week study period (~5 months of age) already exhibited modestly elevated plasma glucose levels compared to WT (118 ± 2 vs. 107 ± 2, p < 0.01; Fig. 2). However, experimental disruption of circadian rhythms (LL and 6-h ADV) led to accelerated development and maintenance of hyperglycemia, which was more pronounced in the HIP LL group (Fig. 2B; p < 0.05 for LD vs. LL and 6-h ADV). Accelerated development of hyperglycemia in HIP rats exposed to LL or 6-h ADV was not associated with a significant increase in body weight versus LD (Fig. 2D). However, body weight in the HIP LL group tended to be elevated ~5% from 2 to 10 weeks (p = 0.08 for LL vs. LD).
Figure 2.
Effects of 10-week circadian rhythm disruption on fasting glucose and body weight. Influence of environmental circadian rhythm disruption on fasting glucose (A, B) and body weight (C, D) in WT (left panels) and diabetes-prone HIP (right panels) rats exposed to 10 weeks of LD (open and closed circles), LL (open and filled squares), or 6-h advance (open and filled triangles) regimens. Data are expressed as mean ± SEM. *p < 0.05 statistical significance for changes in plasma glucose in HIP LD light vs. HIP 6-h ADV and HIP LL.
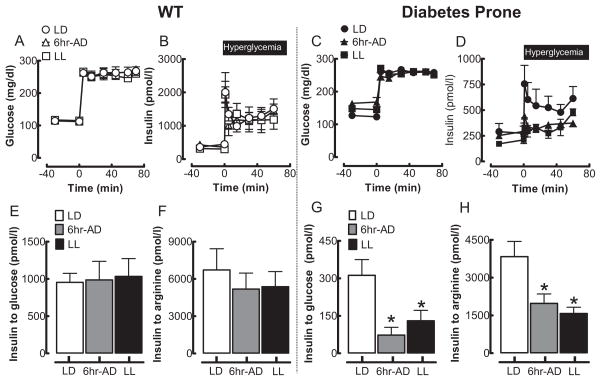
Effects of Circadian Rhythm Disruption on Pancreatic Beta-Cell Function in WT and Diabetes-Prone HIP Rats
Diminished insulin secretion in response to glucose and arginine is a characteristic abnormality in patients with T2DM (Perley and Kipnis, 1967; Ward et al., 1984). Reduction in glucose-stimulated insulin secretion typically represents defective beta-cell secretory function (glucose sensing, vesicle transport, etc.) and/or reduction in beta-cell mass. On the other hand, reduction in arginine-stimulated insulin secretion represents defective beta-cell maximal secretory capacity and thus serves as a surrogate of decline in beta-cell mass and/or insulin biosynthesis. Thus, to assess insulin secretion, we performed a hyperglycemic clamp in conjunction with an arginine bolus test in WT and diabetes-prone HIP rats (Fig. 3). By design, plasma glucose levels were matched at ~250 mg/dL among all experimental light conditions in HIP and WT rats from 0 to 70 min, and insulin secretion was subsequently assessed (Fig. 3A,C). There was no effect of disrupted circadian light rhythms in WT rats on either glucose- or arginine-stimulated insulin secretion (p = 0.71 and p = 0.96, respectively vs. LD; Fig. 3B–F). As previously reported (Matveyenko and Butler, 2006), prediabetic HIP rats at 5 months of age (similar to prediabetic humans) already exhibit a deficit in insulin secretion (Fig. 3C–H). However, in contrast to WT rats, in diabetes-prone HIP rats, disruption of circadian rhythms (LL and 6-h ADV) led to a significant further deterioration in glucose-stimulated (~65% compared to LD; p < 0.05) and arginine-stimulated (~50% compared to LD; p < 0.05) insulin secretion (Fig. 3D–H).
Figure 3.
Effects of 10-week circadian rhythm disruption on pancreatic beta-cell function in WT and HIP rats. Mean plasma glucose (A, C) and insulin (B, D) concentrations during the hyperglycemic clamp in WT (left panels) and diabetes-prone HIP rats (right panels) following 10-week exposure to LD (open and closed circles), LL (open and filled squares), or 6-h advance (open and filled triangles) regimen. Mean insulin response to glucose (E, G) and arginine (F, H) challenge during the hyperglycemic clamp in WT (left panel) and diabetes-prone HIP (right panels) rats following 10-week exposure to LD (open and closed circles), LL (open and filled squares), or 6-h advances (open and filled triangles) regimen. Data are expressed as mean ± SEM. *p < 0.05 statistical significance for insulin response to glucose and arginine vs. LD HIP.
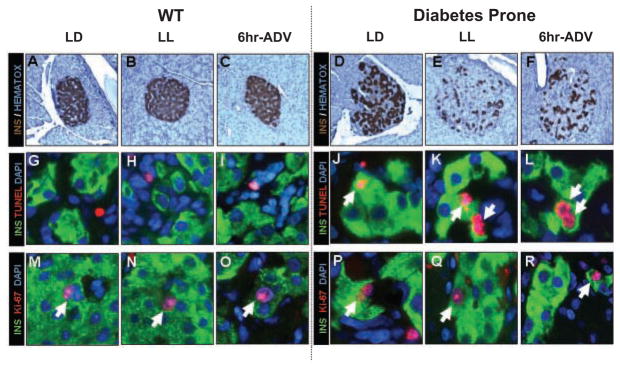
Effects of Circadian Rhythm Disruption on Pancreatic Beta-Cell Mass and Turnover in WT and Diabetes-Prone HIP Rats
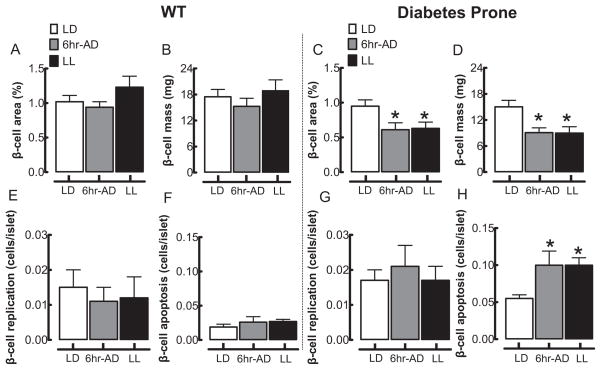
To elucidate potential mechanisms of impaired insulin secretion following circadian rhythm disruption in HIP rats, we next investigated beta-cell mass and turnover (Figs. 4, 5). Consistent with insulin secretion data (see above), in WT rats, beta-cell fractional area, beta-cell mass, and beta-cell turnover (replication and apoptosis) were not altered in LL and 6-h ADV groups compared to LD (Fig. 4A–O and Fig. 5A–F). As previously reported, HIP rats at 5 months of age (age of rats at the end of the 10-week study period) already exhibit a ~2-fold increased rate of beta-cell apoptosis compared to their WT counterparts (Fig. 5). However, chronic exposure of HIP rats to LL or 6-h ADV led to a further reduction in the beta-cell fractional area and beta-cell mass by ~40% compared to LD (p < 0.05; Fig. 4D-F and Fig. 5C,D). This decline in beta-cell mass following exposure to LL and 6-h ADV light regimens was associated with an increased rate of beta-cell apoptosis (p < 0.05 vs. LD; Fig. 4J–L and Fig. 5H) but no change in beta-cell replication (Fig. 4P-R and Fig. 5G).
Figure 4.
Effects of 10-week circadian rhythm disruption on pancreatic beta-cell mass and turnover. (A-F) Representative examples of pancreatic islets stained by immunohistochemistry for insulin (brown) and nuclear stain hematoxylin (blue) imaged at 20x in WT (left panels) and diabetes-prone HIP rats (right panels) following 10-week exposure to LD (A, D), LL (B, E), and 6-h advance (C, F) regimens. (G–L) Representative examples of pancreatic islets stained by immunofluorescence for insulin (green), marker of cell apoptosis TUNEL (red), and nuclear stain DAPI (blue) and (M–R) representative examples of pancreatic islets stained by immunofluorescence for insulin (green), marker of cell replication Ki-67 (red), and nuclear stain DAPI (blue) imaged at 60x in WT (left panels) and diabetes-prone HIP rats (right panels) following 10-week exposure to LD (M, P), LL (N, Q), and 6-h advances (O, R).
Figure 5.
Effects of 10-week circadian rhythm disruption on pancreatic beta-cell mass, beta-cell apoptosis, and replication. Mean beta-cell fractional area (A, C) and beta-cell mass (B, D) in WT (left panels) and diabetes-prone HIP rats (right panels) following 10-week exposure to LD (open bars), LL (black bars), and 6-h advances (gray bars). Mean beta-cell replication (E, G) and beta-cell apoptosis (F, H) in WT (left panels) and diabetes-prone HIP rats (right panels) following 10-week exposure to LD (open bars), LL (black bars), and 6-h advances (gray bars). Data are expressed as mean ± SEM. *p < 0.05 statistical significance for beta-cell area, beta-cell mass, and beta-cell apoptosis vs. LD HIP rats.
Effects of Circadian Rhythm Disruption on Insulin Sensitivity in WT and Diabetes-Prone HIP Rats
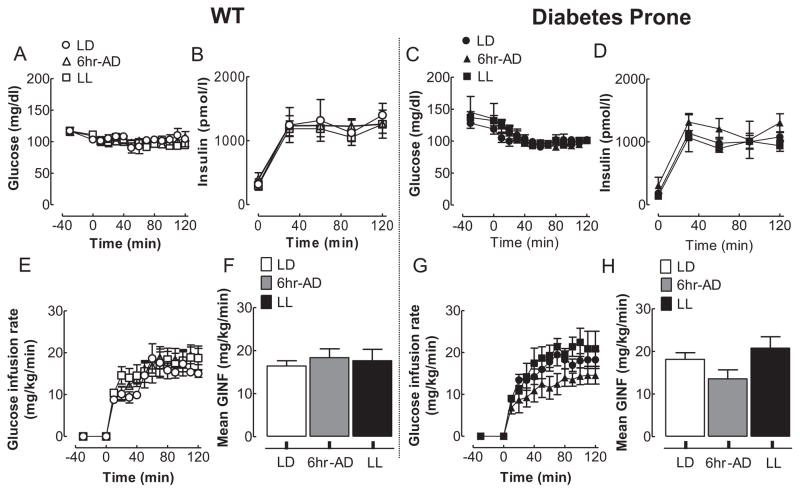
Since acceleration in the development of hyperglycemia in HIP rats may also be a consequence of deterioration in insulin action, we next examined insulin sensitivity by a hyperinsulinemic-euglycemic clamp method. By design, plasma glucose and insulin concentrations were matched during the hyperinsulinemic-euglycemic clamps among all experimental light conditions in HIP and WT rats (Fig. 6A–D). Whole-body insulin sensitivity, assessed by the mean glucose infusion rates during the hyperinsulinemic clamp, was comparable and not significantly different among all light regimens in both WT and HIP rats (Fig. 6E–H), implying that accelerated development of diabetes in HIP rats was primarily due to beta-cell failure.
Figure 6.
Effects of 10-week circadian rhythm disruption on insulin sensitivity in WT and diabetes-prone HIP rats. Mean glucose (A, C) and plasma insulin (B, D) concentrations during the hyperinsulinemic-euglycemic clamp (0–120 min) in WT (left panels) and diabetes-prone HIP rats (right panels) following 10-week exposure to LD (open and closed circles), LL (open and filled squares), or 6-h advances (open and filled triangles). Corresponding (E, G) and cumulative (F, H) glucose infusion rates during the hyperinsulinemic-clamp in WT (left panels) and diabetes-prone HIP rats (right panels) following 10-week exposure to LD (open and closed circles), LL (open and filled squares), or 6-h advances (open and filled triangles). Data are expressed as mean ± SE.
DISCUSSION
Recent studies have described disturbances in circadian rhythms as a predisposing environmental trigger for the development of T2DM (Bass and Takahashi, 2010; Reddy and O’Neill, 2010). In those vulnerable to T2DM, hyperglycemia occurs as a consequence of impaired insulin secretion in response to insulin resistance (Bergman et al., 1981) with characteristic islet pathology, including a deficit in beta-cell mass (Butler et al., 2003; Kloppel et al., 1985) and increased beta-cell apoptosis (Huang et al., 2007; Laybutt et al., 2007). In the present study, we sought to gain insight into pathophysiological mechanisms underlying the predisposition to T2DM following exposure to circadian misalignment. We report that circadian disruption brought about by frequent shifts in the light schedule or by LL led to accelerated development of diabetes in diabetes-prone HIP rats, deterioration in glucose-stimulated insulin secretion, and a decline in beta-cell mass attributed to an increase in beta-cell apoptosis. The current study was designed to examine effects of circadian disruption in an in vivo animal model, with the advantage of best approaching actions in humans with T2DM but with the limitation of precluding direct assessment whether resultant impaired insulin secretion is a direct consequence of beta-cell loss, beta-cell dysfunction, or a combination of both factors.
The potential role of beta-cell failure in mediating the deleterious effects of circadian rhythm disruption has been previously hypothesized (Bass and Takahashi, 2010). Spiegel and colleagues (1999) reported that sleep deprivation caused impaired glucose tolerance with decreased beta-cell glucose responsiveness, implying that circadian sleep disturbance may adversely affect insulin secretion. In support of this, a more recent study demonstrated that acute (10 days) circadian misalignment, designed to recapitulate shift work, led to impaired glucose tolerance, fasting hyperglycemia, and diminished beta-cell function (Scheer et al., 2009). These effects were independent of changes in feeding and sleep cycles (Scheer et al., 2009). Interestingly, Scheer and colleagues (2009) show that in a subset of individuals (~20%), 10 days of circadian misalignment led to the level of glucose intolerance classified as “diabetic” according to American Diabetes Association diagnostic criteria. It is intriguing to hypothesize that, as with other known environmental triggers such as obesity (Chan et al., 1994), circadian misalignment may promote the development of T2DM in the subset of the population with a genetic predisposition (Florez, 2008). Our data support this postulate by revealing that experimental disruption of circadian rhythms accelerated the development of diabetes only in rats characterized by a predisposition to beta-cell failure, where circadian misalignment had no discernable effect on beta-cell function or mass in WT animals.
In addition to environmental disruption of circadian rhythms, genetic mutations in core intracellular circadian clock components such as CLOCK and BMAL1 can also promote development of diabetes and beta-cell failure (Marcheva et al., 2010; Turek et al., 2005). Pancreatic islets express robust circadian rhythms in gene expression that are dampened in CLOCK and BMAL1 mutant mice (Marcheva et al., 2010). Furthermore, mutations of CLOCK and BMAL1 in mice (both global and beta-cell specific) result in the development of diabetes and glucose intolerance through the induction of beta-cell failure (Marcheva et al., 2010). Since more than 20% of global gene expression in some peripheral tissues (e.g., liver) is reported to be under circadian control (Reddy et al., 2006), it is not surprising that mutations in clock genes in peripheral tissue such as the pancreas can have a detrimental effect on genes regulating pancreatic beta-cell function and mass (Marcheva et al., 2010). Interestingly, abrupt shifts in environmental light cycles akin to the ones employed in our study have been shown to disrupt the temporal relationship between circadian oscillators within the SCN and peripheral tissues (Filipski et al., 2004; Yamazaki et al., 2000). Therefore, induction of advances and delays in the central clock brought about by alterations in light schedules during shift work can potentially disrupt circadian clock coordination in peripheral organs such as the beta-cell, thus potentially contributing to beta-cell failure.
Another mechanism by which alterations in light schedules utilized in our study can potentially disrupt circadian clock coordination in peripheral organs is through impact on feeding behavior. Both locomotor activity and feeding patterns are disrupted following exposure to LL (Granados-Fuentes et al., 2004), an observation partially confirmed by our studies (Fig. 1). Changes in feeding behavior have the potential to uncouple circadian oscillators in the peripheral tissues such as the liver and the pancreas from the SCN, via regulation of peripheral clock gene expression (Damiola et al., 2000). Therefore, potential aberration of feeding behavior associated with LL and 6-h ADV might have influenced our results as it pertains to effects on pancreatic beta-cell function and survival. Additional studies will be needed to evaluate the role of disrupted feeding patterns as a consequence of aberrant light schedules on beta-cell clock gene expression and consequent effects on beta-cell function and survival.
Recent genome-wide association studies have identified a link between variance in the melatonin receptor 1B gene (MTNR1B) and the onset of hyperglycemia and impaired insulin secretion (Bouatia-Naji et al., 2000; Lyssenko et al., 2009; Prokopenko et al., 2009; Ronn et al., 2009). These studies imply that actions of melatonin (a key circadian hormone pacemaker) on beta-cells may also be a potential link between circadian rhythm disturbances and increased susceptibility for T2DM. Circulating melatonin levels are diminished in conditions associated with circadian dysregulation and glucose intolerance such as shift work, nocturnal lifestyle, and aging (Karasek, 2004; Mirick and Davis, 2008). Interestingly, a role for melatonin in the regulation of glucose homeostasis and beta-cell function was first proposed more than 50 years ago (Milcou et al., 1957). Pinealectomy was shown to lead to the development of hyperglycemia associated with a decline in beta-cell function (Diaz and Blazquez, 1986; Mellado et al., 1989). Furthermore, melatonin has been shown to promote cell survival, particularly in response to Alzheimer beta-amyloid-induced cell death (Jang et al., 2005). This is particularly interesting since the mechanism of beta-cell failure in T2DM shares many parallels with neurodegenerative diseases such as Alzheimer disease (Haataja et al., 2008). Collectively, these data imply a possible role for melatonin in the regulation of beta-cell mass and/or function and raise the possibility that disruption of beta-cell melatonin signaling may contribute to development of T2DM associated with circadian rhythm disorders.
It is also important to emphasize that in addition to beta-cell failure, insulin resistance (often due to obesity) also plays a major contributory role to the pathogenesis of T2DM. Although in the current study, disruption of circadian rhythms did not adversely affect insulin sensitivity, the primary metabolic defect in HIP rats relates to beta-cell failure, and thus additional studies are needed to fully characterize the role of circadian rhythm disruption on the pathogenesis of insulin resistance. Indeed, disruption of circadian rhythms in humans as a consequence of shift work and sleep loss has been associated with insulin resistance. Additionally, sleep loss in humans is associated with an increase in food intake and preference for high-energy foods, which likely contributes to increased risk for obesity, insulin resistance, and, subsequently, T2DM (Spiegel et al., 2005; Van Cauter et al., 2008).
To conclude, we report that 10 weeks of circadian misalignment induced by either exposure to LL or 6-h advance of the light cycle every 3 days leads to the accelerated development of diabetes due to deterioration in glucose-stimulated insulin secretion and beta-cell loss in diabetes-prone HIP rats. Our studies provide potential mechanisms by which disturbances in circadian rhythms can increase the risk of T2DM development in genetically predisposed individuals and emphasize that conditions of circadian misalignment that have become commonplace in our “24/7” society may have severe health consequences.
Supplementary Material
Acknowledgments
This work was supported by the Larry Hillblom Foundation and by grants from the National Institutes of Health (DK089003 and DK063491 to A.V.M.). We thank Ryan Galasso, Larry Hillblom Islet Research Center at University of California, Los Angeles, for excellent technical support. We are indebted to William Larkin, University of California, Los Angeles, for help with building environmentally controlled circadian chambers. We also thank Dr. Peter Butler, Larry Hillblom Islet Research Center at the University of California, Los Angeles, for insightful comments and critical discussions.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary online material for this article is available on the Journal of Biological Rhythms website at http://jbr.sagepub.com/supplemental.
References
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: Measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP rat): A new model for type 2 diabetes. Diabetes. 2004;53:1509–1516. doi: 10.2337/diabetes.53.6.1509. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA. Banting Lecture: From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: A common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982;23:313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Diaz B, Blazquez E. Effect of pinealectomy on plasma glucose, insulin and glucagon levels in the rat. Horm Metab Res. 1986;18:225–229. doi: 10.1055/s-2007-1012279. [DOI] [PubMed] [Google Scholar]
- Eastman C, Rechtschaffen A. Circadian temperature and wake rhythms of rats exposed to prolonged continuous illumination. Physiol Behav. 1983;31:417–427. doi: 10.1016/0031-9384(83)90061-6. [DOI] [PubMed] [Google Scholar]
- Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- Florez JC. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia. 2008;51:1100–1110. doi: 10.1007/s00125-008-1025-9. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. 2004;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- Jang MH, Jung SB, Lee MH, Kim CJ, Oh YT, Kang I, Kim J, Kim EH. Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neurosci Lett. 2005;380:26–31. doi: 10.1016/j.neulet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–1729. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveyenko AV, Butler PC. Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55:2106–2114. doi: 10.2337/db05-1672. [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Veldhuis JD, Butler PC. Measurement of pulsatile insulin secretion in the rat: Direct sampling from the hepatic portal vein. Am J Physiol Endocrinol Metab. 2008;295:E569–E574. doi: 10.1152/ajpendo.90335.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado C, Rodriguez V, de Diego JG, Alvarez E, Blazquez E. Effect of pinealectomy and of diabetes on liver insulin and glucagon receptor concentrations in the rat. J Pineal Res. 1989;6:295–306. doi: 10.1111/j.1600-079x.1989.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Milcou SM, Vrejoin G, Marcean R, Nanu L. Effect of a hypoglycemic pineal hormone on the endocrine pancreas in alloxanized animals: Norphological study [in French] Ann Endocrinol (Paris) 1957;18:621–627. [PubMed] [Google Scholar]
- Mirick DK, Davis S. Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol Biomarkers Prev. 2008;17:3306–3313. doi: 10.1158/1055-9965.EPI-08-0605. [DOI] [PubMed] [Google Scholar]
- Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: Studies in normal and diabetic subjects. J Clin Invest. 1967;46:1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, et al. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Reddy AB, O’Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20:36–44. doi: 10.1016/j.tcb.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Ronn T, Wen J, Yang Z, Lu B, Du Y, Groop L, Hu R, Ling C. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia. 2009;52:830–833. doi: 10.1007/s00125-009-1297-8. [DOI] [PubMed] [Google Scholar]
- Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CM, Sato S, Margolis RN. No time to lose: Workshop on circadian rhythms and metabolic disease. Genes Dev. 2010;24:1456–1464. doi: 10.1101/gad.1948310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: A novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74:1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Role of insulin resistance in the pathogenesis of NIDDM. Diabetologia. 1995;38:1378–1388. doi: 10.1007/BF00400597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.