Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is caused by heterozygous mutations in either PKD1 or PKD2, genes that encode polycystin (PC) -1 and -2, respectively 1. We show here that tumor necrosis factor-alpha (TNF-α), an inflammatory cytokine present in human ADPKD cystic fluid, disrupts the localization of PC2 to the plasma membrane and primary cilia through a scaffold protein, FIP2, which is induced by TNF-α. Treatment of mouse embryonic kidney organ cultures with TNF-α resulted in formation of cysts, and this effect was exacerbated in the Pkd2+/− kidneys. TNF-α also stimulated cyst formation in vivo in Pkd2+/− mice. In contrast, treatment of Pkd2+/− mice with the TNF-α inhibitor, etanercept, prevented cyst formation. These data reveal a pathway connecting TNF-α signaling, polycystins and cystogenesis, the activation of which may reduce functional PC2 below a critical threshold, precipitating the ADPKD cellular phenotype.
It was proposed that cystogenesis in ADPKD results from the clonal expansion of tubule epithelial cells acquiring “second hit” mutations inactivating the functional copy of the PKD gene 2. However, in mice with a hypomorphic Pkd1 allele, reduction in Pkd1 expression was sufficient to initiate cystogenesis 3. The high frequency at which cysts develop in human ADPKD suggests that non-genetic factors may contribute to cyst formation 4. One of the possible physiological factors is the pro-inflammatory cytokine, TNF-α 5. TNF-α mRNA and protein are markedly increased after hypertensive stress 6 and renal injury 7, conditions that are associated with ADPKD 4. Urinary tract infections are also frequent complications in ADPKD 8. TNF-α was found to increase progressively with age in cystic kidneys of cpk mice 9 and was also present in human ADPKD cyst fluids 10.
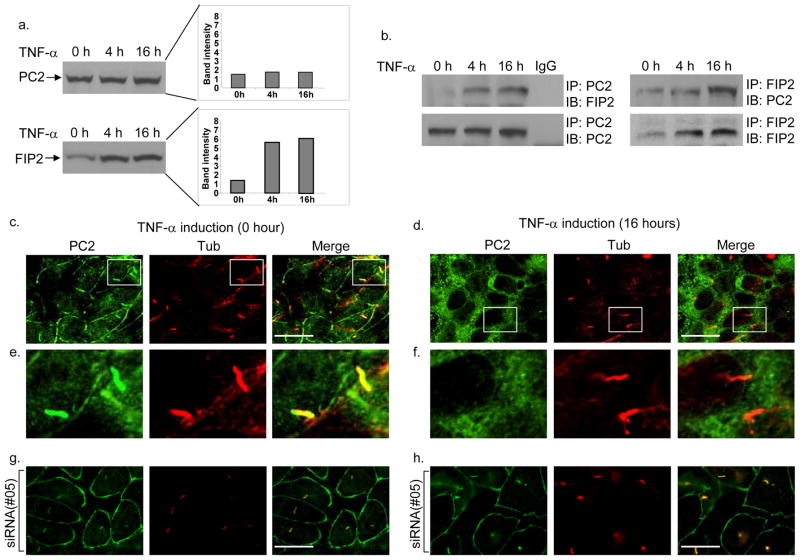
To investigate the connection between TNF-α and ADPKD, we have focused on FIP2 11, a TNF-α-induced protein previously found in a 2-hybrid screen as a potential binding partner with PC2 12. Immunoblot analysis confirmed that TNF-α elevated the expression of FIP2, but not PC2, by 3–4 fold (Fig. 1a), and PC2 and FIP2 co-immunoprecipitated from inner medullary collecting duct (IMCD) cell lysates when antibody against either protein was used (Fig. 1b).
Figure 1.
Effects of TNF-α on FIP2 and PC2 in IMCD cells. a, Western blot analysis shows increased FIP2 protein levels but not PC2 protein levels upon TNF-α stimulation for 4 hr or 16 hr. b, Co-immunoprecipitation of endogenous FIP2 and PC2 with anti-PC2 or FIP2 antibody. TNF-α induction for 4 hr or 16 hr resulted in increased amounts of FIP2 pulled down with PC2, and PC2 pulled down with FIP2. IgG: control antibody. c and d, Double immunofluorescence staining of endogenous PC2 (green) and acetylated tubulin (tub, red) before (c) or after (d) treatment with TNF-α for 16 hr, observed with confocal microscopy. Scale bars, 10 μm. e and f, enlarged cilia images in the boxed regions in c and d, respectively. g and h, Transfection of siRNA#05 against FIP2 prevented TNF-α-induced mislocalization of PC2 in IMCD cells.
Since FIP2 is involved in vesicular trafficking 13,14, we tested the possibility that TNF-α could affect the normal localization of PC2, a calcium channel 1, through induction of FIP2. Immunofluorescence staining using an antibody to PC2 (96525 15) revealed that while endogenous PC2 localized to the plasma membrane and in >95% of the primary cilia observed (Fig. 1c,e and Supplemental movie 1 online) in untreated IMCD cells, cells treated with TNF-α showed a striking loss of PC2 staining from these locations (100% observed). Instead, PC2 was enriched within perinuclear regions (Fig. 1d,f and Supplemental movie 2 online) overlapping partially with a Golgi marker (Supplemental Fig. 1a online). This effect of TNF-α was further confirmed using antibody YCC2 16 (Supplemental Fig. 1b,c online), though this antibody only recognized PC2 on the cilia but not other membrane structures 17. The loss of membrane localization of PC2 was not due to cell death, as the TNF-α-treated cells showed normal morphology and were negative for TUNEL staining (Supplemental Fig. 2 online). To test if the effect of TNF-α on PC2 localization was FIP2-dependent, siRNA was used to knock down FIP2 expression (Supplemental Fig. 3a,b online) and was found to restore normal PC2 localization in TNF-α treated cells (Fig. 1g,h, and Supplemental Fig. 3c,d online).
PC2 functions in a complex with PC1 18,19, and as such immunoprecipitation assays were performed to test the effect of TNF-α on PC1-PC2 complex formation. TNF-α treatment disrupted the PC1- PC2 interaction, even though the expression levels of PC1 and PC2 remained the same as in untreated cells (Supplemental Fig. 4a,b online). Ciliary localization of PC1, however, was not affected in TNF-α-treated IMCD cells (Supplemental Fig. 4c–f online).
The above cellular data raised the possibility that TNF-α could promote cyst formation by disrupting the normal localization and a key interaction of PC2. To test this hypothesis we modified a mouse embryonic kidney organ culture assay 20 by using E15.5 kidneys and a low concentration of cAMP, such that there was no cyst formation (0/32 kidneys observed) in the absence of TNF-α (see Methods). Treatment for five days with TNF-α resulted in numerous cyst-like structures in wild-type embryonic kidneys (Fig. 2a; 20/20 cultured kidneys treated with 6.25–12.5 ng/ml TNF-α). Significantly, in Pkd2+/− embryonic kidneys cyst formation occurred at TNF-α concentration as low as 1.5 ng/ml. (Fig. 2b). The cysts that formed in TNF-α-treated kidneys were positive for DBA, a collecting duct marker, or LTA, a proximal tubule marker (Supplemental Fig. 5 online), suggesting that the TNF-α-induced cysts originated from these nephron segments.
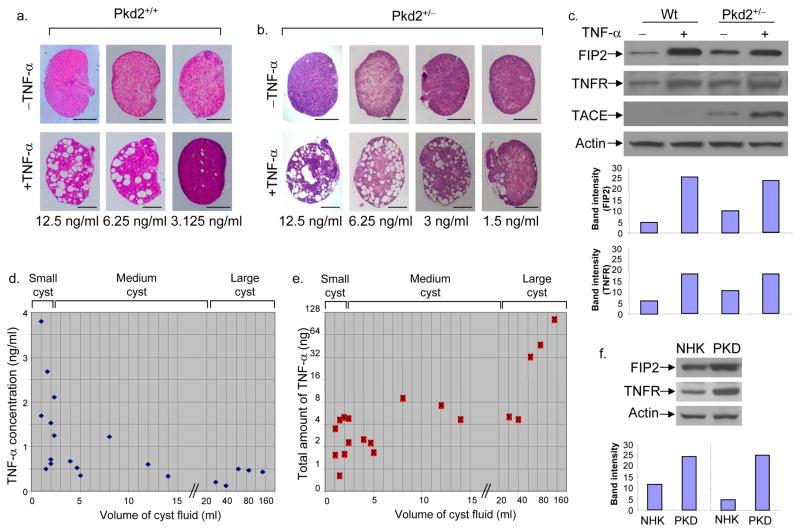
Figure 2.
TNF-α triggers cyst formation in cultured embryonic kidneys and is present in human ADPKD cyst fluid. a, Treatment of wild-type cultured kidneys from E15.5 embryos with various concentrations of TNF-α for 5 days triggered cyst formation. Each vertical pair represents left and right kidneys from the same embryo. Scale bars, 500 μm. b, Treatment with different concentrations of TNF-α for 5 days triggered cyst formation in Pkd2+/− cultured kidneys from E15.5 embryos. Scale bars, 500 μm. c, Immunoblot analysis of FIP2, TNFR-I and TACE protein levels in wild-type and Pkd2+/− embryonic kidneys with or without TNF-α (6.25 ng/ml) stimulation for 5 days. The band intensity of FIP2 (top) and TNFR (bottom) are shown as histograms. d, e, Quantification of TNF-α levels in ADPKD human cysts by ELISA. The data represent 20 cysts from 10 ADPKD patients. The horizontal axis indicates cyst volumes; the vertical axis indicates TNF-α concentration (d); and the total TNF-α amount in each cyst (e). f, Immunoblot analysis of FIP2 and TNFR-I protein levels, comparing cultured normal human kidney (NHK) cells and ADPKD cyst lining (PKD). The band intensity of FIP2 (left) and TNFR (right) are shown as histograms.
Immunoblot analysis confirmed that TNF-α increased the expression of FIP2 by 4–5 fold in wild-type embryonic kidneys (Fig. 2c). Interestingly, FIP2 levels in non-treated Pkd2+/− kidneys were markedly higher (>2 fold) than in wild-type kidneys, suggesting that PC2 negatively affects the expression level of FIP2. FIP2 level in Pkd2+/− kidneys was further stimulated 2–3 fold by TNF-α treatment (Fig. 2c). Moreover, a similar pattern of expression for TNF-α receptor, TNFR-I, was also detected across the kidney samples examined (Fig. 2c), suggesting that exposure to TNF-α or Pkd2 heterozygosity could render the kidney more sensitive to TNF-α through an increase in TNFR-I level. TNF-α converting enzyme (TACE), while undetectable in wild-type kidneys, was clearly observed in Pkd2+/− embryonic kidneys and its level was further elevated ~3 fold after TNF-α treatment (Fig. 2c).
The effective concentrations of TNF-α for cyst induction observed with kidney organ cultures were in the ng/ml range, much higher than the previously reported concentration of TNF-α in human cyst fluids (in the range of 10–73 pg/ml) 10. To investigate this discrepancy, we quantified the concentration of TNF-α in human ADPKD cyst fluids collected from 10 ADPKD kidneys on ice and immediately frozen in liquid nitrogen. Our results demonstrate a significant accumulation of TNF-α with concentrations in ng/ml range in small cysts (Fig. 2d), and an inverse relationship between cyst volume and TNF-α concentration. However, the total amount of TNF-α was higher in larger cysts (Fig. 2e), indicating that TNF-α accumulated as cysts grew. The highest level of TNF-α (3.8 ng/ml) was observed in the most freshly collected cyst fluid that had not been frozen (Fig. 2d). The difference between our measurements and those described previously may be due to differences in the size of the cysts or the method in fluid collection/storage. Interestingly, TACE was also found in cyst fluids at a concentration of 9.0 ± 0.9 ng/ml (n=15), in contrast to being undetectable in normal human urine samples. Soluble TNFR-I and II were also present in cyst fluids at 2.2 ± 0.3 ng/ml and 2.1 ± 0.4 ng/ml (n=15), respectively, which were slightly elevated compared to normal urine (~1.5 ng/ml). Previous work showed that this moderate level of soluble TNFRs augmented rather than inhibited TNF-α activity through a stabilization/buffering effect 21,22. The presence of TACE and soluble TNFRs in ADPKD cyst fluids could both contribute to the accumulation of TNF-α, which may be directly produced by renal cells 7. Immune cells, which infiltrate the kidney in response to renal lesions and infections, are another possible source for TNF-α 7.
To test further if the level of FIP2 was elevated in human cystic kidneys, the amount of FIP2 protein was compared between primary cultures of normal human kidney cells (NHK) and ADPKD human cyst lining cells (PKD). As shown in Fig. 2f, the level of FIP2 in the PKD cells was ~2 fold higher compared to that in NHK cells, similar to the observed FIP2 level difference between Pkd2+/− and Pkd2+/+ mouse embryonic kidneys (Fig. 2c). The levels of TNFR-I also exhibited an increase (~4 fold) in cyst lining cells over NHK cells.
To test the effect of TNF-α on cyst formation in vivo, 4 week old Pkd2+/− mice received intra-peritoneal injections of TNF-α (0.5 μg per gram mouse body weight, 1 injection/wk, 4 weeks). No cysts were observed in the control group (n=10), by contrast 4 of the 10 TNF-α-treated mice developed unilateral cysts (Table 1). This experiment was also repeated using 8.5 week old mice (0.5 μg per gram mouse body weight, 1 injection/wk, 10 weeks). While the control group developed cysts at the expected frequency (21.3%) 23, 42.8% (6 out of 14 mice) of the experimental group developed cysts (Table 1), including two mice that developed multiple bi-lateral small cysts (Supplemental Fig. 6 online). This result shows that TNF-α stimulates cyst development in Pkd2+/− mice.
Table 1. TNF-αstimulated cyst formation in 8 or 18 weeks Pkd2+/− kidneys.
TNF-α stimulated cyst formation in 8.5 or 18.5 weeks Pkd2+/− kidneys. Group 1: 4 weeks old Pkd2+/− mice were injected intra-peritoneally with TNF-α (0.5 μg of TNF-α per gram mouse body weight, 1 injection/wk, 4 weeks). Whereas no cysts were observed in the control group (n=10), 4 of the 10 TNF-α-treated mice developed unilateral cysts. Group 2: 8 week old Pkd2+/− mice were injected intra-peritoneally with same amount TNF-α as in group 1 per week for 10 weeks. While the control group developed cysts at an expected frequency (21.3%), 42.8% of the experimental group developed cysts, including two mice (out of 14) that developed multiple small cysts
| 8.5 weeks Pkd2+/− mice | 18.5 weeks Pkd2+/− mice | ||||
|---|---|---|---|---|---|
| TNF-α | Treated | Untreated | Treated | Untreated | |
| Total (mouse) | 10 | 10 | 14 | 61 | |
| Number of mice with kidney cysts | 1 large cyst | 3 | 0 | 2 | 8 |
| 2–3 cysts | 1 | 0 | 2 | 4 | |
| Multi. Uni-lateral cysts | 0 | 0 | 0 | 1 | |
| Multi. Bi-lateral cysts | 0 | 0 | 2 | 0 | |
| Sub-total | 4 | 0 | 6 | 13 | |
| Total % with cysts | 40% | 0% | 42.8% | 21.8% | |
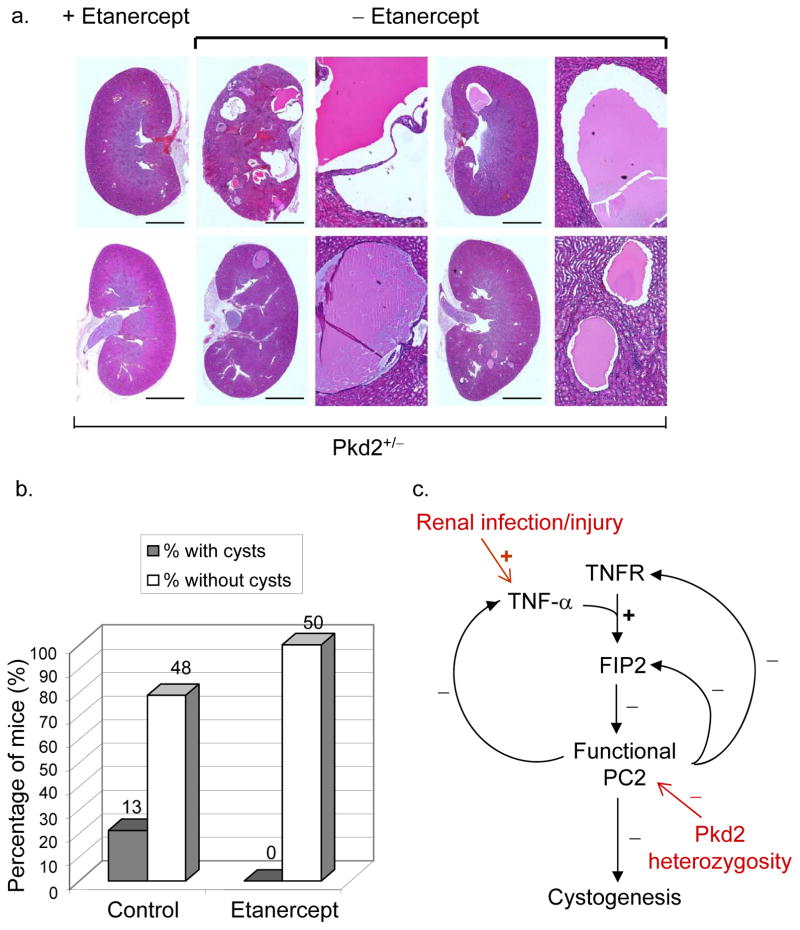
To test if inhibition of TNF-α could alleviate cyst formation, 8.5-week old Pkd2+/− mice were treated with etanercept, a TNF-α inhibitor (subcutaneously administered at 125 μg/mouse/week). Etanercept is a Food and Drug Administration-approved drug that neutralizes circulating TNF-α with well-characterized pharmacodynamics 24. After 10 weeks of treatment, the control group developed cysts at the expected frequency, but none of etanercept-treated mice developed kidney cysts (n=50) (Fig. 3a,b). Fisher’s exact test yielded a p-value of 0.0005, indicating that neutralization of TNF-α significantly prevented cyst formation in Pkd2+/− mice.
Figure 3.
TNF-α inhibitor, etanercept, prevents cyst formation in Pkd2+/− mice. a, Kidney histology sections from Pkd2+/− mice injected intraperitoneally, from week 8.5 to week 18.5, with etanercept at 125 μg/mouse/week (right column) or phosphate buffered saline (PBS) (middle and right columns). For control examples (-etanercept), the panel to the right of each kidney section displays an enlarged image of a cyst in that section. Scale bars, 4 mm. b, Quantitative result of etanercept treatment experiments. The number above each bar indicates the number of mice represented by the bar. p=0.0005 (Fisher’s exact test). c. A functional network connecting TNF-α signaling, polycystin complex and cystogenesis. Three double-negative feedback loops can be observed: between PC2 function and the physiological response that leads to TNF-α production; between PC2 level and FIP2 expression; between PC2 and TNFR expression. +: positive effect; −: negative effect. In red: genetic and non-genetic factors that could influence the pathway leading to cystogenesis.
Taken together, these findings suggest that TNF-α is a potent factor that promotes renal cyst development, especially in the ADPKD genetic background, and are consistent with the previous finding that a TACE inhibitor reduced cyst formation in the bpk mouse model of autosomal recessive PKD 25. The functional network that connects TNF-α, polycystin and cyst development contains three double-negative feedback loops (Fig. 3c), which are important motifs in biological networks that could generate bi-stable responses to small, transient signals 26. We hypothesize that this network structure could contribute significantly to the transition from normal tubule development to cystic disease onset in response to perturbation of PC2 by TNF-α in the ADPKD heterozygous genetic background. A recent study found that TNF-α can also activate mTOR pathway through IKKβ 27, and inhibition of mTOR has been shown to revert cystogenesis in PKD kidneys 28. Thus, the effect of etanercept observed in this work may not be limited to the mechanism suggested in this study. Moreover, such mechanisms do not argue against key contributions from genetic changes, such as loss of heterozygosity 2. Nonetheless, unraveling non-genetic factors that contribute to the onset of cystogenesis may be a useful approach toward therapeutic intervention of ADPKD.
Methods
Cell culture
IMCD (ATCC catalog no. CRL-2123) cells were cultured in Dulbecco’s modified Eagle’s medium/F12 medium supplemented with 10% (v/v) fetal bovine serum (Invitrogen). For TNF-α treatment experiments, it was important that cells were cultured for 7–9 days at 37 °C to allow sufficient time for cilia growth before addition of TNF-α. The concentration of TNF-α (Genzyme Diagnostic, Cambridge, MA) was 200 ng/ml for all cell culture experiments. For the FIP2 knock-down experiment, cells were cultured for 7 days prior to siRNA transfection. After 48 hours, cells were either harvested for Western blot analysis or treated with TNF-α for 16 hours, followed by fixation for immunostaining.
Embryonic kidney organ culture
Embryonic kidneys were dissected in PBS (with calcium and magnesium) plus penicillin-streptomycin-glutamine (GIBCO, Grand Island, NY) from embryos of C57BL/6 Pkd2+/− mice or their wild type counterparts at E15.5. The dissected kidneys were cultured at 37°C in DMEM/F12 containing 2 mM L-glutamine, 10 mM Hepes, 5 μg/ml insulin, 5μg/ml transferrin, 2.8 mM selenium, 25 ng/ml prostaglandin E1, 32 pg/ml T3 and 250 U/ml penicillin-streptomycin. The kidneys were cultured with or without TNF-α (Genzyme Diagnostic, Cambridge, MA) at different concentrations for 48 hours and then 50 μM 8-Bromo-cyclic AMP (Sigma, St. Louis, MO) was added for 5 days. The cultured kidneys were then fixed with 4% paraformaldehyde (PFA) in PBS for 6 hours, washed with PBS twice for 5 minutes each and transferred to 70% EtOH for short-term storage at room temperature or for more extended storage at 4°C. The fixed kidney samples were subsequently processed for Hematoxylin and Eosin staining following common histology protocol.
TUNEL assay
Cells treated with or without TNF-α were fixed with 4% PFA. TUNEL assay was performed using a fluorescent apoptosis-detection system (R&D systems, Minneapolis, MN). Fixed cells treated with TACS-nuclease were used as the positive control.
Immunoprecipitation and Western blot analysis
Immunoprecipitation and Western blotting were performed on whole-cell lysates as previously described 29. The antibodies used for Western blotting included rabbit polyclonal anti-FIP2 11, rabbit anti-PC2 polyclonal antibody 96525 15, rabbit anti-PC1 polyclonal antibody 96521 15, rabbit anti-TNFR-I polyclonal antibody, and rabbit anti-TACE polyclonal antibody (NeoMarkers, Fremont, CA). Secondary antibodies used include: goat-anti-rabbit IgG-fluorescein isothiocyanate (FITC Molecular Probes, Eugene, OR), goat-anti-mouse IgG–Texas Red, (1:500 dilution; Molecular Probes). For western blotting, goat-anti-rabbit Ig-horseradish peroxidase (HRP or goat-anti-mouse IgG–HRP, 1:10,000 dilution; Amersham Pharmacia Biotech) were used as secondary antibodies.
RNAi to inhibite FIP2 expression
The oligo sequences used for FIP2 RNAi (Dharmacon, Chicago, IL) were listed below. #05 Sense sequence: 5′-GCUAUGAAAGGGCGAUUUGUU; #05 Antisense sequence: 5′-PCAAAUCGCCCUUUCAUAGCUU. #6 Sense sequence: 5′-UGAGCUGCCUGACUGAGAAUU; #6 Antisense sequence: 5′-PUUCUCAGUCAGGCAGCUCAUU. #07 Sense sequence: 5′-GAAAUGCAGUGCCGACACGUU; #07 Antisense sequence: 5′-PCGUGUCGGCACUGCAUUUCUU. #08 Sense sequence: 5′-CCAUGAAGCUAAAUAAUCAUU; #08 Antisense sequence: 5′-PUGAUUAUUUAGCUUCAUGGUU. Both sense and anti-sense oligos were used to form short RNA duplexes. The transfection was performed using the DharmaFECT siRNA transfection reagent (Dharmacon, Chicago, IL).
Immunofluorescence microscopy
Immunofluorescence was carried out as previously described 29. Primary antibodies were used at the following dilutions: FIP2 (1:100), PC1 (1:500), PC2 (1:500), and flag (1:500). Secondary antibodies used included goat-anti-rabbit IgG-fluorescein isothiocyanate (Molecular Probes, Eugene, OR) and goat-anti-mouse IgG-Texas Red (1:500 dilution; Molecular Probes). Images were captured on an inverted microscope (Axiovert 200M, Carl Zeiss, inc.) equipped with a spinning disc confocal head (Yogogawa), Argon-Krypton laser system (Prairie Technologies, Inc.), and ORCA-ER CCD camera (Hamamatsu). Images were acquired using the Metamorph software (Molecular Devices) and 3D image reconstruction was performed using the Volocity (Improvision, Inc.) software.
Collection of human ADPKD cyst fluids
Cyst fluids were collected from 10 ADPKD kidneys (9 end stage and 1 early stage removed due to severe pain) by aspiration with a syringe and a needle. The kidneys were maintained at 4 °C throughout the cyst fluid collection. The collected cyst fluids were cleared by centrifugation and then snap-frozen in liquid nitrogen and stored at −80 °C until analyzed. Cysts were described as small, medium, and large based on the cyst fluid volumes at 1–2.5 ml, 2.5–20 ml, and >20 ml, respectively.
ELISA
The concentrations and total amounts of TNF-α in individual cyst fluids were measured using the DuoSet ELISA Development kit for human TNF-α/TNFSF1A (R&D Systems, Minneapolis, MN). The concentrations of TACE and soluble TNFRs in ADPKD cyst fluids and normal human urine samples were measured by using the DuoSet ELISA Development kits for human TACE/ADAM17 (R&D Systems, Minneapolis, MN), for human sTNF-RI/TNFRSF1A (R&D Systems, Minneapolis, MN) and for human sTNF RII/TNFRSF1B (R&D Systems, Minneapolis, MN), respectively, and following the manufacturer’s instructions.
TNF-α and etanercept treatments of mice
Pkd2 mutant mice (Pkd2-183) were kindly provided by Stephan Somlo (Yale University). For TNF-α(Genzyme Diagnostic, Cambridge, MA) treatments, mice were intraperitoneally injected weekly, from week 4 (day 28) to week 8.5 (day 60), or from week 8.5 (day 60) to week 18.5 (day 130), with 0.5 μg of TNF-α per gram mouse body weight per week or phosphate buffered saline (PBS). For TNF-α inhibitor etanercept (EMGEN, Thousand Oaks, CA) treatment experiments, mice were intraperitoneally injected weekly, from week 8.5 (day 60) to week 18.5 (day 130), with 125 μg of etanercept per mouse per week or phosphate buffered saline (PBS).
Supplementary Material
Acknowledgments
We thank Stefan Somlo (Yale Medical School) for providing the Pkd2+/− mice and YCC2 antibody; Jing Zhou (Harvard Medical School) for the 96525 and 96521 antibodies; and Trisha Nichols, Brian Slaughter, Norman Pavelka, Praveen Suraneni, Gail Reif, and Jean-Philippe Rey (Stowers Institute for Medical Research) for technical assistance, and Jared Grantham and Robb Krumlauf for helpful discussion. This work was supported by funds from the Stowers Institute for Medical Research to R.L. and a PKD Center grant from the National Institutes of Health (P50 DK05301-07) to J.P.C. and D.P.W. and a PKD foundation grant to X.L.
References
- 1.Wilson PD. Polycystic kidney disease. The New England journal of medicine. 2004;350:151–64. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 2.Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87:979–87. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- 3.Lantinga-van Leeuwen IS, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–77. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 4.Martinez JR, Grantham JJ. Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis Mon. 1995;41:693–765. doi: 10.1016/s0011-5029(05)80007-0. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14:185–91. doi: 10.1016/s1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 6.Todorov V, Muller M, Schweda F, Kurtz A. Tumor necrosis factor-alpha inhibits renin gene expression. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1046–51. doi: 10.1152/ajpregu.00142.2002. [DOI] [PubMed] [Google Scholar]
- 7.Vielhauer V, Mayadas TN. Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Seminars in nephrology. 2007;27:286–308. doi: 10.1016/j.semnephrol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Peters DJ, Breuning MH. Autosomal dominant polycystic kidney disease: modification of disease progression. Lancet. 2001;358:1439–44. doi: 10.1016/S0140-6736(01)06531-X. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, et al. Increased endothelin and endothelin receptor mRNA expression in polycystic kidneys of cpk mice. J Am Soc Nephrol. 1993;4:1064–72. doi: 10.1681/ASN.V441064. [DOI] [PubMed] [Google Scholar]
- 10.Gardner KD, Jr, Burnside JS, Elzinga LW, Locksley RM. Cytokines in fluids from polycystic kidneys. Kidney Int. 1991;39:718–24. doi: 10.1038/ki.1991.87. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Kang J, Horwitz MS. Interaction of an adenovirus E3 14.7-kilodalton protein with a novel tumor necrosis factor alpha-inducible cellular protein containing leucine zipper domains. Molecular and cellular biology. 1998;18:1601–10. doi: 10.1128/mcb.18.3.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Luo Y, SMN, MSH, Zhou J. TNF-alpha regulates polycystin-2 trafficking and its interaction with polycystin-1 and fibrocystin. J Am Soc Nephrol. 2004;15:12A–13A. [Google Scholar]
- 13.Hattula K, Peranen J. FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr Biol. 2000;10:1603–6. doi: 10.1016/s0960-9822(00)00864-2. [DOI] [PubMed] [Google Scholar]
- 14.Sahlender DA, et al. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol. 2005;169:285–95. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003 doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, et al. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274:28557–65. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- 17.Geng L, et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. Journal of cell science. 2006;119:1383–95. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–98. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 19.Vandorpe DH, et al. The cytoplasmic C-terminal fragment of polycystin-1 regulates a Ca2+-permeable cation channel. J Biol Chem. 2001;276:4093–101. doi: 10.1074/jbc.M006252200. [DOI] [PubMed] [Google Scholar]
- 20.Magenheimer BS, et al. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(−) Co-transporter-dependent cystic dilation. J Am Soc Nephrol. 2006;17:3424–37. doi: 10.1681/ASN.2006030295. [DOI] [PubMed] [Google Scholar]
- 21.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. The Journal of experimental medicine. 1992;175:323–9. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Groote D, Grau GE, Dehart I, Franchimont P. Stabilisation of functional tumor necrosis factor-alpha by its soluble TNF receptors. European cytokine network. 1993;4:359–62. [PubMed] [Google Scholar]
- 23.Wu G, et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–88. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 24.Mann DL, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 25.Dell KM, et al. A novel inhibitor of tumor necrosis factor-alpha converting enzyme ameliorates polycystic kidney disease. Kidney Int. 2001;60:1240–8. doi: 10.1046/j.1523-1755.2001.00963.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferrell JE., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–8. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee DF, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 28.Shillingford JM, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–71. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, et al. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat Cell Biol. 2005;7:1102–12. doi: 10.1038/ncb1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.