Abstract
Background
The male gametophyte developmental programme can be divided into five phases which differ in relation to the environment and pollen hydration state: (1) pollen develops inside the anther immersed in locular fluid, which conveys substances from the mother plant – the microsporogenesis phase; (2) locular fluid disappears by reabsorption and/or evaporation before the anther opens and the maturing pollen grains undergo dehydration – the dehydration phase; (3) the anther opens and pollen may be dispersed immediately, or be held by, for example, pollenkitt (as occurs in almost all entomophilous species) for later dispersion – the presentation phase; (4) pollen is dispersed by different agents, remaining exposed to the environment for different periods – the dispersal phase; and (5) pollen lands on a stigma and, in the case of a compatible stigma and suitable conditions, undergoes rehydration and starts germination – the pollen–stigma interaction phase.
Scope
This review highlights the issue of pollen water status and indicates the various mechanisms used by pollen grains during their five developmental phases to adjust to changes in water content and maintain internal stability.
Conclusions
Pollen water status is co-ordinated through structural, physiological and molecular mechanisms. The structural components participating in regulation of the pollen water level, during both dehydration and rehydration, include the exine (the outer wall of the pollen grain) and the vacuole. Recent data suggest the involvement of water channels in pollen water transport and the existence of several molecular mechanisms for pollen osmoregulation and to protect cellular components (proteins and membranes) under water stress. It is suggested that pollen grains will use these mechanisms, which have a developmental role, to cope with environmental stress conditions.
Keywords: Pollen, water status, dehydration, rehydration, angiosperm pollen, pollination
INTRODUCTION
Organisms, and their cells, require mechanisms to maintain internal stability in the face of developmental and environmental changes. The American physiologist Walter Cannon (1871–1945) termed this ability ‘homeostasis’ (homeo means ‘the same’ and stasis means ‘standing or staying’). Homeostasis may thus be defined as the ability of an organism or cell to maintain internal equilibrium by adjusting its physiological processes. In this review, we pinpoint five phases during male gametophyte development and functioning that require maintenance of specific water levels, via the use of various structural, physiological and molecular mechanisms. These five phases differ in the optimum water/hydration levels needed to suit specific biological functions, as detailed further on. The significance of the existence of potential ‘water homeostasis control points’ will also be addressed in relation to maintaining pollen quality and function upon exposure to environmental stresses.
Changes in pollen volume and water content during the developmental programme
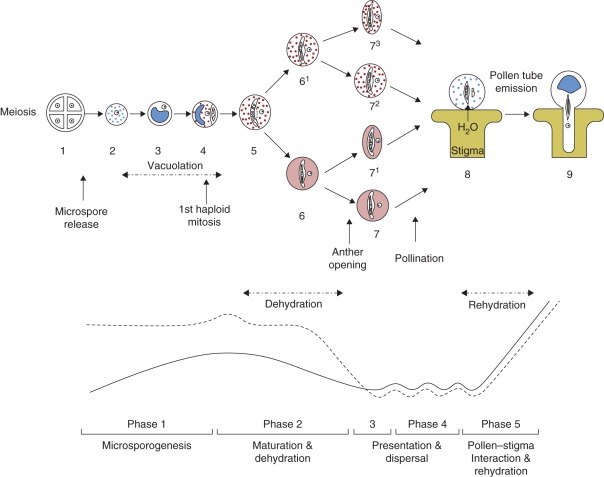
The male gametophyte developmental programme can be divided into five phases which differ in relation to the environment and pollen hydration state. (1) Pollen develops inside the anther immersed in locular fluid, which conveys substances from the mother plant – the microsporogenesis phase. (2) The locular fluid disappears by reabsorption and/or evaporation before the anther opens (Pacini et al., 2006, and references therein) and the maturing pollen grains undergo dehydration – the dehydration phase. (3) The anther opens: pollen may be dispersed immediately, or be held for later dispersal by, for example, pollenkitt, as occurs in almost all entomophilous species (Pacini et al., 2006, and references therein) – the presentation phase. (4) Pollen is dispersed by different agents, remaining exposed to the environment for different periods – the dispersal phase. (5) Following dispersal, pollen will land on a stigma and, in the case of compatible stigma and suitable conditions, will undergo rehydration and start germination – the pollen–stigma interaction phase (Fig. 1).
Fig. 1.
A semi-diagrammatic scheme of male gametophyte development from the tetrad stage to pollen tube emission, indicating changes in volume (solid line) and water content (dashed line). The scheme does not consider species with tricellular pollen. Vacuoles are in blue, starch is in red and cytoplasmic carbohydrates are in pink. (1) Late tetrad stage. (2) Early microspore stage; small vacuoles are formed. (3) Late vacuolated microspore stage, before the first haploid mitosis; small vacuoles merge into a large one. (4) Early bicellular stage; starch deposition begins, as well as vacuole reduction. (5) Late bicellular stage; vacuoles are absent, starch deposition reaches maximum levels. From stage 5, two possibilities are illustrated (6 and 61). (6) Almost mature pollen grain; starch is completely hydrolysed and carbohydrates are present in the cytoplasm. (61) Almost mature pollen grain; starch is only partially hydrolysed. (7) Mature pollen, of the partially hydrated type (recalcitrant; Nepi et al., 2001), containing cytoplasmic carbohydrates. (71) Mature pollen of the partially dehydrated type (orthodox; Nepi et al., 2001), containing cytoplasmic carbohydrates. (72) Mature recalcitrant pollen with starchy carbohydrate reserves. (73) Mature orthodox pollen with starchy carbohydrate reserves. (8) After landing on the stigma, pollen, independently of the hydration state and type of carbohydrate reserves, rehydrates and small vacuoles are formed. (9) Small vacuoles merge into a large one and a pollen tube is emitted. Starch and cytoplasmic carbohydrates interconvert during presentation and dispersal, enabling control of water loss and water influx, and are consumed (or transformed) during rehydration and germination. Pollen water content (dependent on water absorbed from the anther through the tapetal cells and then through the loculus) starts increasing at the microspore stage, in parallel with the initial increase in volume, up to a few days before flower opening (according to the species; phase 1). Upon beginning the dehydration phase (phase 2), pollen water content starts to decrease, reaching a minimum at maturity. During presentation (phase 3) and dispersal (phase 4), water content adjusts to environmental changes (depending on the species) and will increase sharply during rehydration, upon landing on a compatible stigma (phase 5). This precedes the emission of the pollen tube which grows in the style and will reach the ovule where sperm cells will be delivered for fertilization. It should be noted that the illustrated changes in pollen water content during development are speculative since, to the best of our knowledge, no such measurements have ever been reported. The time scale for water content during the different phases varies widely according to the species and is deeply affected by the environment during dispersal. In general: phase 1 is the longest, lasting from several days to several months, whilst the other phases last from seconds to days. Pollen volume starts increasing significantly at the early microspore stage and continues to increase until partial dehydration. Pollen volume decreases during anther and pollen dehydration and, similar to water content, adapts to environmental conditions during dispersal, increasing during rehydration.
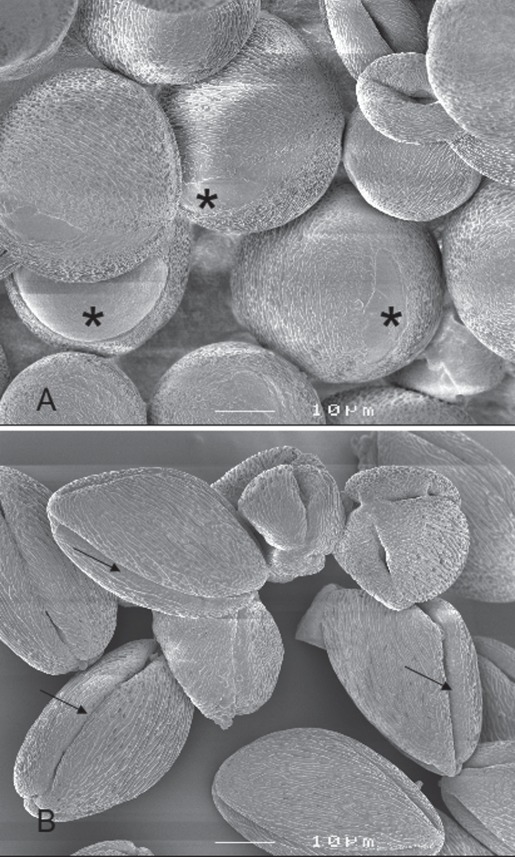
Pollen volume starts increasing during microspore wall formation and continues to increase until dehydration (Pacini, 1994). The increase in volume parallels an increase in water content and vacuolation, part of the space being occupied by vacuoles (Fig. 1, phase 1). At the start of the dehydration phase (phase 2), pollen water content starts to decrease, reaching a minimum at maturity. This causes a decrease in volume, in most cases with a change in pollen shape (Nepi et al., 2001). During presentation and dispersal (phases 3 and 4), water content, though at a low (or even minimal) level, adapts to environmental changes, and will increase sharply during rehydration, upon landing on a compatible stigma (phase 5). Pollen volume may change during dispersal as well, depending on the relative humidity (RH) of the environment (Lisci et al., 1994; Nepi et al., 2001, and references therein). When pollen lands on a compatible stigma, it rehydrates and germinates (Heslop-Harrison, 1987). Wodehouse (1935) termed the changes in pollen volume and shape during dehydration (illustrated in Fig. 2), dispersal and rehydration ‘harmomegathy’; these changes create mechanical stress which must be sustained by the pollen walls, plasma membrane and protoplast (Blackmore and Barnes, 1986).
Fig. 2.
Cryo-scanning electron micrograph of Petunia hybrida pollen after anther opening. (A) Pollen before flower opening. (B) Pollen after flower opening. Pollen grains lose water after flower opening and change in size and shape, from spheroid to ovoid. The furrow area is distended in the closed flower (asterisks) whereas it is folded in the open flower after partial pollen dehydration (arrows). Pictures were taken at the Department of Plant Cytology and Morphology, University of Wageningen, The Netherlands, in 1999 using a JEOL 6300F field emission cryo-SEM.
The aim of this review is to highlight the issue of pollen water status and point out the various mechanisms used by pollen grains during the five phases of their developmental programme to adjust to changes in water content and maintain internal stability. In addition, we argue that pollen grains may also use these mechanisms for coping with stressful environmental conditions. It should be noted that direct data with regard to water content in the anther and pollen are very limited. The review will thus focus on the available data, and relate them to mechanisms used in other plant systems/tissues, highlighting potential homeostasis control points and discussing future research avenues.
WATER STATE AND CHARACTERISTICS
Water has several roles in biological systems: it regulates biological reactions, serves as a fluid medium and stabilizes macromolecular structure. Therefore, removal of water from biological tissues affects their ability to function and may induce deleterious effects (Alpert and Oliver, 2002). Desiccation-tolerant organisms, such as seeds and pollen, are capable of surviving the removal of their cellular water and may also be able to survive in a dry state for extended periods of time (Alpert and Oliver, 2002). In 1986, Burke hypothesized that the seed cytoplasm can enter into a glassy state (Buitink and Leprince, 2004, and references therein). Glass is a thermodynamic liquid which can be defined as a supercooled liquid with an extremely high viscosity (Buitink and Leprince, 2004, and references therein). Intracellular glass, which exhibits slow molecular mobility and high molecular packing, has recently been suggested to be essential for the storage stability of seeds (Buitink and Leprince, 2008). Non-reducing sugars, such as sucrose, are likely to play multifunctional roles during the loss of cellular water. At high water contents, early on in the drying stage, they might act as compatible solutes. Through preferential exclusion, protein unfolding is avoided and membrane disturbance is restricted. Upon further water loss, sugar molecules can replace water at hydrogen-bonding sites to preserve the native protein structure and spacing between phospholipids (Buitink and Leprince, 2004, and references therein). Potts (2001) stressed the importance of both sucrose and trehalose in replacing water around the polar head groups of membranes and in forming glasses in the dry state. Due to their abundance in the cytoplasm, proteins have also been suggested to play a role in glass formation. During maturation, seeds and pollen accumulate both non-reducing sugars and late embryogenesis abundant (LEA) proteins (this issue is further elaborated upon below), and it was therefore suggested that the two types of molecules interact in the formation of a glassy state (Buitink and Leprince, 2004, and references therein).
MICROSPOROGENESIS, MATURATION AND DEHYDRATION (PHASES 1 AND 2)
Pollen grain volume decreases slightly after meiotic cleavage and starts to increase during exine and intine formation (constituting the two walls of the pollen grain); with dehydration, the volume decreases again (Pacini, 1990). Pollen and spores are the only plant cells delimited by two walls with different structures, compositions and functions. These walls are formed centripetally – first the elastic and flexible external exine, and then the internal intine. Exine is lacking, discontinuous or extremely reduced in a small number of plant groups, e.g. seagrasses and certain tropical monocots such as Musaceae, Lauraceae and Zingiberaceae living in environments with high RH (Pacini, 1994, and references therein). Pollen walls are subject to mechanical stress, especially at the apertures, during anther and stigma hydration. During dehydration, the pollen wall folds in on itself to prevent further desiccation (Fig. 2). The structural patterning of the pollen wall – an axially elongated aperture of high compliance and intricate sculpturing – is critical for predictable and reversible folding patterns (Katifori et al., 2010).
Dehydration causes both the anther to dehisce and the pollen grain to become ‘dormant’ (in analogy with seeds), i.e. its biochemical processes function at a low rate. In species without exine, such as seagrasses and some Musaceae, dehydration is reduced or absent (Franchi et al., 2011, and references therein). This emphasizes the importance of the exine in accommodating changes in pollen volume due to uptake and loss of water.
Exine function
Bowman et al. (2007) and Morant et al. (2007) have suggested that a key innovation in the first land plants colonizing the terrestrial environment 600–450 million years ago was acquisition of the ability to generate the sporopollenin polymer to protect haploid spores. Functional and biochemical roles of the exine have been studied using mutants defective in pollen wall development and exine deposition, such as defective in exine formation1 (dex1), ms2, no exine formation1, faceless pollen1 and cer6, as well as the recently characterized acos5 and cyp704B2 mutants (Aarts et al., 1997; Fiebig et al., 2000; Paxson-Sowders et al., 2001; Ariizumi et al., 2003; Kunst and Samuels, 2003; Souza et al., 2009; Li et al., 2010). Jung et al. (2006), for example, characterized a T-DNA insertional mutant in the Wax-deficient anther1 (Wda1) gene of rice (Oryza sativa) which shows significant defects in the biosynthesis of very-long-chain fatty acids in the anther layers. This gene was found to be strongly expressed in the epidermal cells of anthers. Scanning electron microscopy analyses of cells with this mutation showed that epicuticular wax crystals are absent in the outer layer of the anther. The authors reported that the wda1 anthers were severely shrunken by dehydration and easily damaged by physical force, and that microspore development was severely retarded and finally disrupted as a result of defective pollen exine formation in the mutant anthers.
Pollen vacuole function
The plant vacuole acts as a storage compartment. It has roles in turgor regulation, cell signalling and degradation, and it has been shown to accommodate high flux rates of water and small solutes across its membrane (‘tonoplast’; Kaldenhoff and Fischer, 2006), suggesting an important function under conditions of stress (Li et al., 2008). Vacuolation usually occurs from the tetrad stage of pollen development onward (Pacini, 1994). Two vacuolation events occur in Lycopersicum peruvianum and Prunus avium, among many other plants, the first during the microspore stage and the second during the bicellular stage. Only one vacuolation occurs, during the microspore stage, in monocots such as Lolium perenne (Pacini, 1994). Vacuolation in developing pollen seems to follow pollen volume changes as well as amylogenesis (Pacini et al., 2011). Vacuoles may have important functions during dehydration and subsequent rehydration on the stigma, similar to other plant systems where tonoplast water channels (aquaporins) have been shown to play an important role in maintaining water balance under changing environmental conditions (Kaldenhoff and Fischer, 2006).
Uniqueness of the loculus and the locular fluid
The loculus, surrounding and serving as the immediate environment for the developing pollen grains, is formed during meiotic prophase, owing to enlargement of the anther and detachment and separation of meiocytes (Pacini and Franchi, 1983). The dimension of the loculus increases up to pollen grain maturity. It is not known, however, whether the increase in volume of the locular fluid is proportional to the increase in volume of the developing pollen. As reported for Smilax aspera L. plants, during the initial stages of loculus formation, it stores substances containing polysaccharides which can be stained by PAS (periodic acid–Schiff) (Pacini and Franchi, 1983). In Lilium, the locular fluid was shown to store sucrose, glucose and fructose during pollen development (Castro and Clément, 2007) and was suggested to serve as a transitory site for sugar storage. There are different forms of loculi depending on the type of tapetum, pollen dispersal unit and pollen grain size (Pacini, 2010). The presence of locular fluid, which separates the microspores from the cells of the mother plant during a significant period of their development, may contribute to the high sensitivity of the developing pollen grains to the environment.
Roles of the filament and anther transpiration
At the level of the anther, the mechanism leading to dehydration varies from one taxonomic group to the next. It is greatly influenced by environmental factors such as rain, humidity and sunlight, as well as variations in RH with time of day. Dehydration may occur in two different, but not mutually exclusive, ways: active water resorption by other flower organs as in Petunia, or desiccation by passive transpiration as in Tradescantia (Pacini and Franchi, 1984). Most of the anther's water is transported to the expanding filament in Lilium longiflorum, and anther dehiscence is delayed if the filament is tied (Heslop-Harrison et al., 1987). Mechanisms facilitating the evaporation of water are an abscising tissue in the filament hindering liquid inflow, and discontinuties in the anther cuticle, facilitating transpiration (Pacini, 1994, and references therein). Pollen volume decreases due to loss of water, and pollen water content is in equilibrium with the environment during dispersal. The water content of ripe pollen, i.e. when it has reached equilibrium with the environment, generally ranges from 20 to 50 % (Pacini, 1994, and references therein).
Variations in water and carbohydrate content in mature pollen
It should be noted that, as reported for mature seeds (Hay et al., 2010), mature pollen grains derived from the same mother plant, or even from the same flower, are not necessarily uniform. Pollen grains may differ in their water and carbohydrate contents, reflecting the pollen's position inside the loculus and inside the flower, and the consequent effects of the environmental parameters. These variations may be inherent, due to a microenvironment effect of the mother plant during pollen development, e.g. nourishment through the tapetum layer. Examples of different types of microspore–tapetum interactions are presented by Pacini (2010).
PRESENTATION (PHASE 3)
Anther opening
Anther dehiscence involves anther wall rupture at the stomium to allow pollen presentation and release. It is a complex process requiring proper cell differentiation and dehydration. It is agreed that as a general rule, low RH accelerates anther opening, whereas high RH delays or inhibits the process (Linskens and Cresti, 1988; Yates and Sparks, 1993; Lisci et al., 1994). Explosive anther dehiscence in Ricinus communis L. (castor bean) was shown to be influenced by RH (Bianchini and Pacini, 1996), with values >30 % inhibiting anther opening and decreasing the distance the pollen is launched, and a value of 98 % completely preventing anther opening (Bianchini and Pacini, 1996). Matsui et al. (2000) described a mechanism of anther dehiscence related to pollen grain swelling in barley (Hordeum distichum L. emed. Lam.), suggesting the involvement of potassium ions in the swelling. In Allium triquetrum, anther opening occurred at a specific moment of anther development and seemed to be related to dehydration caused by reabsorption of water by contiguous tissues (Carrizo Garcia et al., 2006).
Pollen hydration status
Pollen hydration status at presentation is dependent on its developmental programme and is affected by anther dehydration as well as by the environmental conditions encountered during presentation. Two modes of pollen presentation have been defined: ‘primary pollen presentation’ (Leins, 2000) indicating pollen presented by the anther, and ‘secondary pollen presentation’ indicating pollen adherence to other parts of the flower (Howell et al., 1993; Yeo, 1993; Ladd, 1994; Erbar and Leins, 1995) by virtue of pollenkitt (Pacini, 1996) or other devices (Erbar and Leins, 1995, and references therein). Pollen hydration status is its water content, which may change at anther opening, during presentation (Lisci et al., 1994) or during dispersal. Nepi et al. (2001) defined ‘partially dehydrated pollen’ (PDP) as having <30 % water content, whereas pollen containing >30 % water was termed ‘partially hydrated pollen’ (PHP). By analogy with seeds, PDP has also been defined as orthodox or desiccation tolerant (Tweddle et al., 2003), whereas pollen with a higher water content is defined as recalcitrant or desiccation sensitive (Pacini et al., 2006; Franchi et al., 2011). In this review, we use the terms orthodox and recalcitrant for desiccation-tolerant and -sensitive pollen, respectively. Recalcitrant pollen is almost ready for dispersal when the locular fluid has disappeared. Orthodox pollen must lose water to slow down its metabolism before it is ready for dispersal (Pacini, 1990; Franchi et al., 2002). In Petunia hybrida (possessing orthodox pollen), for example, pollen is still hydrated and spherical when the anther opens and the flower is still closed; pollen dehydration occurs after flower opening (Fig. 2; M. Nepi, pers. obs.). During this process the orthodox pollen, with three pores and/or furrows, for example, changes shape from spherical when hydrated, to ovoid when partially dehydrated (Fig. 2). On the other hand, recalcitrant pollen is usually devoid of furrows and generally spherical in shape, with many, one or no pores (Franchi et al., 2002, 2011); it does not change shape when water is lost, but its volume and diameter decrease (Nepi and Pacini, 1993). However, if the exine and/or intine are thin, they eventually collapse, as commonly seen in grass pollen (Heslop-Harrison, 1979). Most recalcitrant pollen lacks mechanisms for retaining water. If it does not reach a compatible stigma quickly, it rapidly dehydrates and dies (Fig. 3). The molecular mechanisms involved in pollen water homeostasis include water channels that may facilitate water movement, on one hand, and mechanisms that enable it to retain water such as the accumulation of specific sugars and proteins, on the other hand (see details in the section ‘Molecular mechanisms involved in enabling changes in pollen water status’ below).
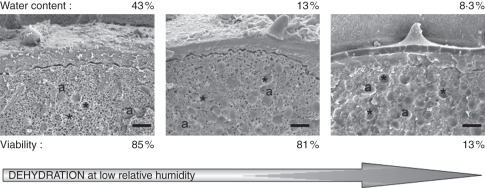
Fig. 3.
Cryo-fractures of Cucurbita pepo pollen observed with a cryo-SEM after freeze-drying (10 min at –90 °C in a vacuum of 5 × 10−8 hPa). The sites containing water appear as vesicle-like structures (dark holes) in the cytoplasm. These vesicles are highly abundant in the cytoplasm of C. pepo pollen from just-opened anthers (water content 43 %). They decrease in pollen dehydrated to 13 % water content and they are no longer evident in pollen dehydrated to 8·5 % water content. Amyloplasts (a) and their imprints (asterisks) are evident in C. pepo pollen. Pollen viability is high at both 43 and 13 % water content, but it decreases drastically upon further dehydration to 8·5 % water content. This degree of dehydration is induced by pollen exposure to 30 % relative humidity for 90 min (see data in Nepi et al., 2010). Pictures were taken at the Department of Plant Cytology and Morphology, University of Wageningen, The Netherlands, in 1999 using a JEOL 6300F field emission cryo-SEM. Scale bar = 2 µm.
Irrespective of pollen hydration status, when anther or pollen dehydration is delayed (naturally or experimentally), pollen of some species will start to emit a pollen tube in the closed anther (Pacini and Franchi, 1982).
Success of orthodox pollen during presentation and the subsequent dispersal phase may be determined by: (a) the ability to keep water content as constant as possible; (b) the ability to adjust turgor pressure with changes in environmental parameters; and (c) the existence of molecular mechanisms that will aid in maintaining (a) and (b).
Ecophysiology
When pollen is dispersed immediately upon anther opening, its flight may be very short – a few centimetres at most and lasting only a few seconds, as in the case of annual grasses. If pollen is retained in the anther by pollenkitt or other means, it may be exposed for periods ranging from a few hours to several weeks, as in orchids, where dispersal depends on pollinator availability (Neiland and Wilcock, 1995). In the case of Cucurbita pepo (a monoecious entomophilous species), the male flower opens at 0600 h and closes at 1100 h (Nepi and Pacini, 1993); uncollected pollen is not exposed again. This may be due to the fact that Cucurbita has recalcitrant pollen which is vulnerable to water loss, even during brief exposure. Corolla closure at the end of anthesis is not limited to Cucurbita: it seems to be typical of species with recalcitrant pollen, such as Convolvulus sp., Mirabilis sp. and Hibiscus sp. (Franchi et al., 2002), and to offer a way of avoiding dispersal of pollen with low viability.
Pollen developmental arrest was demonstrated in hazel, reflecting the dormancy period (Frenguelli et al., 1997). In this case, pollen development lasted for several months (beginning 7–8 months before flowering in late winter or early spring) and variations in pollen volume reflected the beginning and end of the dormancy period (decreased and maximal volume, respectively; Frenguelli et al., 1997).
In the case of Helleborus foetidus and H. bocconei, which have orthodox pollen, the flower may last as long as 40 d, but only a few anthers expose pollen each day (Vesprini et al., 2002). However, the pollen maintains high viability during exposure (lasting for 2 d), despite the fact that night temperatures may fall below 0 °C, with day temperatures of 18–20 °C during the blooming period. This suggests the existence of biochemical mechanisms that keep its viability high with time, by avoiding water loss, water uptake and formation of ice crystals in the cytoplasm (Vesprini et al., 2002), presumably via changes in the relative proportions of soluble carbohydrates. Experiments with Petunia hybrida, Trachycarpus fortunei and Helleborus sp. demonstrated that when water availability decreases – because of low RH which causes dehydration or because of low temperature which induces ice crystal formation – the immediate response is an increase in sucrose (Vesprini et al., 2002; Guarnieri et al., 2006; Nepi et al., 2010). It is also interesting to note that an initial increase in sucrose due to lower water availability is followed by restoration of the quantity of this sugar (M. Nepi, pers. obs.). This suggests that some form of homeostasis maintains a constant sucrose pool following a disturbance.
DISPERSAL (PHASE 4)
Pollen may leave the anther as single grains or en masse (Knox and McConchie, 1986), in units consisting of different numbers of grains. The means for holding the grains together include: (a) common walls; (b) viscous fluids derived from the tapetum or other parts of the anther; or (c) tangling of the grains themselves as in certain marine monocots, or by means of exine filaments or filaments derived from the anther (Pacini, 2000).
During dispersal, pollen grains are exposed to the environment and need to withstand variable conditions of temperature and RH. It has been shown for both seeds and pollen that several physical and chemical changes may occur with time, including decreased activities of enzymes, lipid peroxidation and de-esterification, and Maillard reactions (Buitink et al., 1998, and references therein). It is assumed that the formation of highly viscous intracellular glasses decreases molecular mobility and impedes diffusion within the cytoplasm, thus slowing the deleterious reactions and changes in structure during ageing (Buitink et al., 1998, and references therein) as indicated above in ‘Water state and characteristics’. In the case of pea (Pisum sativum L.) and cattail (Typha latifolia L.) seeds, for example, molecular mobility was found to be inversely correlated with storage stability. Recently, using three species of seeds with different dry matter reserves (elm, safflower and maize with moisture contents ranging from 0·00 to 0·15 g H2O per g dry weight), Zhang et al. (2010) showed that molecular mobility decreases and then increases with seed drying in a pattern resembling that of seed ageing kinetics, thus confirming previous data (Buitink et al., 1998). Aylor (2003) claimed that the relative water content of corn pollen plays an important role in both its viability and flight dynamics in the atmosphere. A model was created for the dynamics of water loss from pollen under a variety of temperature and RH conditions, to predict effective pollen transport distances (Aylor, 2003; Nepi et al., 2010).
In the case of recalcitrant pollen, there is generally a threshold value for pollen water content below which there is an abrupt decrease of pollen viability. For C. pepo and Zea mays, the threshold water content is 13 and 28 %, respectively (Fonseca and Westgate, 2005; Nepi et al., 2010).
Because desiccation tolerance is closely related to pollen viability and longevity, it has a strong influence on reproductive strategy and pollination (Dafni and Firmage, 2000). Species with orthodox pollen may wait a long time for pollination to occur. In contrast, species with recalcitrant pollen rely on rapid transport of their pollen to a compatible stigma, so that it can germinate and fertilize the ovules (Nepi et al., 2010). The issue of pollen and seed desiccation tolerance in relation to dispersal and survival is further elaborated upon by Franchi et al. (2011).
POLLEN–STIGMA INTERACTION (PHASE 5)
This phase begins when the pollen grain lands on and adheres to the stigma of a flower. If conditions are suitable, the pollen grain germinates to produce a pollen tube. The pollen tube invades the stigma and grows through the style toward the ovary, where it enters an ovule, penetrates the embryo sac and releases two sperm cells, one of which fertilizes the egg, while the other fuses with the two polar nuclei of the central cell to form the triploid endosperm. Pollen–pistil interactions involve a continuous exchange of signals between the pollen and the maternal tissue of the pistil (Hiscock and Allen, 2008). Heslop-Harrison (2000) described these events as ‘gates opening’, creating the microenvironment that will enable pollen germination, and illustrating the sequence of events that take place as the female tissues of the stigma, style and ovary mature and prepare for pollination and fertilization. An interesting example is given by Heslop-Harrison (2000) to describe a particular ‘gate opening’ sequence in the case of Trifolium pretense: at maturity, the stigma head secretion has, among other constituents, a sucrose concentration of approx. 35 %, the optimum for pollen hydration and germination, but not for continued tube growth; sucrose concentration in the stylar fluid drops to approx. 12–15 %, the optimum for tube growth but not for germination.
Pollen adhesion occurs only in angiosperms and is a prerequisite for pollen hydration; the opposite holds true for gymnosperms (Nepi et al., 2009). The environment of the landing site determines physical recognition, which enables proper hydration to take place. The increasing percentage of pollen volume from the dry to hydrated state varies between species and depends on the hydration site and the pollen grain itself (Pacini, 1990). The internal factors are: hydration of the intine, selective permeability of the plasma membrane, hydration state of the cytoplasm and the accommodation capacity of the walls. In grasses, fresh mature pollen grains germinate very rapidly on suitably receptive stigmas, with germination in the small-grain cereals taking place in <5 min, and within 1 min for Triticum aestivum ‘Sonora’ (reviewed by Heslop-Harrison, 1979). The rapid transition of the male gametophyte from a state of comparative inactivity in the grain to one of vigorous growth implies a rapid re-establishment of normal metabolism, including the synthetic capacity required to build the pollen tube wall. Activation of the pollen grain depends on rehydration, and this is dependent on the inflow of water from the stigma after attachment of the grain. The speed with which an appropriate water balance is reached will depend on both the capacity of the stigma to support the flow and the requirements of the pollen grain. For mature, germinable pollen, the period from capture to tube emergence is affected by the degree of hydration of the grains at the moment of capture (Heslop-Harrison, 1979). In pistachio (recalcitrant), pollen stored at room temperature only germinates after pre-hydration (Vaknin and Eisikowitch, 2000).
Pollen hydration takes place prior to emission of the pollen tube. A nice example is given by Heslop-Harrison (1979) for rye (Secale cereale L.) pollen, demonstrating water exudation from the aperture before the beginning of tube tip growth; in this particular case, germination took place 2 min and 20 s after contact.
The stigma
Angiosperm stigmas can be classified into two broad categories, wet and dry, depending on whether they possess surface secretions (Heslop-Harrison and Shivanna, 1977; Heslop-Harrison, 1981). As summarized by Edlund et al. (2004), water immediately surrounds grains that land on a wet stigma, but those that land on a dry stigma mobilize their lipid-rich pollen coat to form an interface between the two cell surfaces. This interface is converted into a histochemically distinguishable form thought to promote water flow (Elleman and Dickinson, 1986; Elleman et al., 1992). Water, nutrients and other small molecules are transported rapidly into the grain from the stigma exudate (wet stigmas) or stigma papillae (dry stigmas) by mechanisms that remain unclear. The involvement of water channels in the rapid and regulated water release from the stigma to the pollen has been suggested (Dixit et al., 2001; Swanson et al., 2005). In addition, the stigma cuticle may be uniquely permeable to water traffic (Lolle et al., 1997, 1998; Pruitt et al., 2000). Heslop-Harrison and Heslop-Harrison (1980) showed that the cuticle of maize silk is discontinuous, allowing quick rehydration. Regardless of the transfer mechanism, pollen hydration is often regulated both temporally and spatially. Inappropriate or delayed hydration can have disastrous consequences, leading to premature germination within the anther (Pacini and Franchi, 1982; Johnson and McCormick, 2001) or germination on the wrong surface (Lolle and Cheung, 1993; Lolle et al., 1998).
The outer epidermal cell wall and cuticle present a semi-permeable barrier that maintains the external integrity of the plant and regulates the passage of various classes of molecules into and out of the organism. During vegetative development, the epidermal cells remain relatively inert. During reproductive development and fertilization, however, the epidermis is developmentally more labile and participates in two types of contact-mediated cell interactions: organ fusion and pollen hydration (Pruitt et al., 2000). Pollen grains adhere to the papillar cell (an epidermal derivative) and this interaction triggers the further development of the male gametophyte (Lolle et al., 1998). Pruitt et al. (2000) described the isolation and characterization of a gene, termed FIDDLEHEAD, whose product normally functions in blocking both types of epidermal cell interactions during vegetative development. The gene encodes a protein that is predicted to synthesize long-chain fatty acids. An interesting phenomenon is the promiscuous germination and growth of wild-type pollen on vegetative and non-reproductive floral organs of a fiddlehead mutant (Lolle and Cheung, 1993). These studies indicate that plants normally restrict water flow across the cuticle in all tissues except the stigma, and that the stigma cuticle may be unique in that it can change its water permeability in response to interaction with pollen.
The pollen coat
Disrupting pollen coat lipids or proteins in Brassicaceae species can delay or block pollen hydration. In particular, mutations that impair long-chain lipid synthesis, and consequently proper assembly of the pollen coating, severely reduce the hydration of Arabidopsis pollen, resulting in male sterility (Preuss et al., 1993; Hülskamp et al., 1995). Hydraulic contact can be restored in these mutant grains by the addition of purified triacylglycerides (Wolters-Arts et al., 1998). Mutations that affect pollen coat proteins are less extreme, perhaps because of partial functional redundancy. The lipid-rich stigma exudate of plants with wet stigmas is thought to be at least partially functionally analogous to the pollen coat (Dickinson, 1995). Mutations that eliminate this stigma-secreted matrix cause female sterility, a defect that can be bypassed by adding exogenous lipids (Goldman et al., 1994; Wolters-Arts et al., 1998).
Mutants in lipid biosynthesis indicate a role for lipids in mediating water transfer at the stigma. For instance, the arabidopsis eceriferum (cer) mutants fail to hydrate on the stigma because of decreased lipid content in their pollen coat – a defect that can be overcome by high humidity or the addition of triacylglycerides to the stigma (Preuss et al., 1993; Wolters-Arts et al., 1998; Fiebig et al., 2000).
The arabidopsis pollen coat plays a vital role in mediating the early contact between pollen grain and stigma. It protects pollen grains from excess desiccation after dehiscence, contributes to pollen adhesion to the stigma and, most importantly, facilitates hydration (Edlund et al., 2004). The pollen coat oleosin domain protein GRP17 is required for rapid initiation of hydration on the stigma (Mayfield and Preuss, 2000). Other proteins present in the arabidopsis pollen coat include extracellular lipases, two putative receptor kinases and a potential Ca2+-binding protein (Mayfield et al., 2001). Updegraff et al. (2009) have recently demonstrated that the pollen coat protein extracellular lipase 4 is required for efficient pollen hydration.
MOLECULAR MECHANISMS INVOLVED IN ENABLING CHANGES IN POLLEN WATER STATUS
In the following we describe mechanisms that have been shown to exist in pollen which enable changes in pollen hydration level and protecting cellular components from damage that includes protein denaturation and/or the harmful effects of reactive oxygen species. It should be noted, however, that the use/functionality of these mechanisms for maintaining water homeostasis during each of the different phases of pollen development is not yet established and that, in many cases, their existence in pollen is based upon expression analyses of the respective genes.
Water movement and water channels
Water can cross bilayers due to the lateral dynamics of phospholipids, and large-volume water translocation is facilitated by water channels. Members of this family of membrane channel proteins are called aquaporins and are present in both the plasmalemma and the tonoplast membrane (Losch, 1999). Plant aquaporins are classified into four main sub-families: plasma membrane-intrinsic proteins (PIPs), tonoplast-intrinsic proteins (TIPs), nodulin26-like-intrinsic proteins (NIPs) and small and basic membrane-intrinsic proteins (SIPs) (summarized by Forrest and Bhave, 2007; Soto et al., 2008). Many of the TIPs and PIPs are water channels, although some of the isoforms also transport solutes such as glycerol, urea or boric acid (Forrest and Bhave, 2007; Soto et al., 2008). Soto et al. (2008), hypothesizing that aquaporins might play a significant and important role during pollen rehydration on the stigma, functionally characterized aquaporin genes that are highly and selectively expressed in mature pollen of Arabidopsis thaliana (within the top 10 % of mature pollen-expressed genes). They found two TIP loci, AtTip1;3 and AtTip5;1, confirming data from Bock et al. (2006) who conducted a genome-wide analysis of transporter genes expressed in the male gametophyte at four developmental stages. Using Xenopus oocyte-swelling assays, Soto et al. (2008) established that AtTip1;3 and AtTip5;1 are bifunctional aquaporins with intermediate levels of permeability to water and high permeability to urea. To investigate a putative physiological role for aquaporins in water uptake and pollen tube growth, four well-characterized A. thaliana PIP genes were transiently expressed in lily pollen by particle bombardment (Sommer et al., 2008). Expression of A. thaliana PIPs in lily pollen showed that members of the PIP1 sub-family do not confer enhanced water channel activity, whereas members of the PIP2 sub-family do, suggesting that they may play a role in pollen water transport. Aquaporins of the PIP2 class were also suggested to be required for efficient anther dehydration prior to dehiscence in tobacco (Bots et al., 2005).
Osmoregulation under water stress
Osmoregulation can lower water and osmotic potential by several bars. Solutes accumulated during osmoregulation consist of various carbohydrates, the compatible solute proline and quaternary ammonium compounds.
Proline
The beneficial effect of an increased proline level lies not only in its function as a solute and as a protective molecule for dehydration-sensitive macromolecular surfaces, but also in its ability to reduce the generation of free radicals (Losch, 1999).
Sucrose
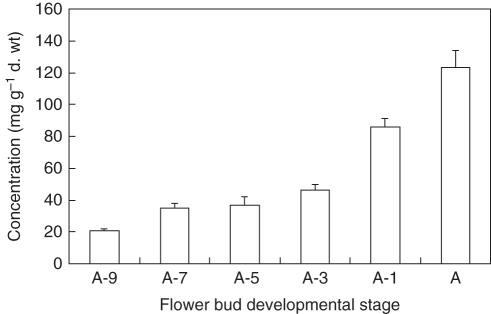
Pollen of some species, e.g. the palms Chamaerops humilis and Trachycarpus fortunei, has a high sucrose content (Speranza et al., 1997; Guarnieri et al., 2006) and survives for many days at high or low RH (Bassani et al., 1994; Pacini et al., 1997). Sucrose also allows pollen to be stored at low temperatures because it prevents the formation of ice crystals; pollen with low sucrose content, such as that from grasses (Speranza et al., 1997), is difficult to store (Barnabas and Rajki, 1981). Other possible functions for sucrose include membrane protection against desiccation (Hoekstra et al., 2001) and providing carbon skeletons during germination for pollen tube wall formation. A system of this type is static because it maintains the turgor pressure constant irrespective of changes in humidity. Nevertheless, the system is probably more complex because sucrose can be hydrolysed to glucose and fructose, doubling turgor pressure. Indeed, variations in the percentages of these sugars have been observed in natural habitats and in the laboratory (Nepi et al., 2001; Vesprini et al., 2002). This enables turgor pressure adjustments in response to variations in temperature and RH during pollen presentation and dispersal. Cytoplasmic polysaccharides and even starch seem to be involved in this process of keeping pollen viability high, at least in Helleborus (Vesprini et al., 2002). The interconversion of carbohydrates seems to be reduced or even absent in species with recalcitrant pollen such as C. pepo and grasses, which have a generally low sucrose content (Speranza et al., 1997). Such pollen soon becomes non-viable, especially if humidity is low (Pacini et al., 1997). Sucrose has been found to accumulate during tomato pollen maturation and dehydration (Fig. 4), suggesting its involvement in desiccation tolerance during pollen water loss.
Fig. 4.
Changes in sucrose concentration in developing pollen grains of tomato ‘Hazera 3017’. Sucrose concentration was analysed according to Hubbard et al. (1990) as described in Firon et al. (2006), at six stages of development, 9, 7, 5, 3, 1 and 0 d before anthesis (A-9 to A-1 and A, respectively). Data represent the average of at least three biological replicates. Vertical bars represent the s.e.
Interestingly, we found that tomato genotypes that exhibit higher sucrose content in mature pollen grains exhibit higher tolerance to heat stress (Firon et al., 2006). In all tested heat-sensitive genotypes, the heat stress conditions (day/night temperatures of 31/25 °C) caused a marked reduction in starch concentration in the developing pollen grains 3 d before anthesis, and a parallel decrease in the total sucrose concentration in the mature pollen, whereas in the tested heat-tolerant genotypes, starch accumulation 3 d before anthesis and sucrose concentration at anthesis were not affected by heat stress (Firon et al., 2006).
Trehalose
The potential role of trehalose as a protective sugar against dehydration (Potts, 2001) should be mentioned, though, to the best of our knowledge, there are no data available regarding its biosynthesis and accumulation during pollen development. Research in this direction has a good chance of contributing to our knowledge of pollen dehydration protective mechanisms.
H+-ATPase activity
It was recently suggested that pollen grain osmoregulation occurs via modulation of plasma membrane H+-ATPase activity by 14-3-3 proteins (Pertl et al., 2010). Increases in both plasma membrane-associated 14-3-3 proteins and plasma membrane H+-ATPase activity were detected in pollen grains challenged by hyperosmolar medium. In Lilium pollen, H+-ATPase activity was shown to be important during the stage at which the dehydrated pollen grains take up water and have to adjust their turgor pressure to the water potential of the surrounding stigma surface (Pertl et al., 2010).
Transporters
Solute import across the pollen plasma membrane, which occurs via proteinaceous transporters, is required to support pollen development as well as for subsequent germination and pollen tube growth. Results summarized by Bock et al. (2006) stress the potential importance of the sucrose transporter SUC1 (At1g71880), a plasma membrane-localized H+/Suc symporter widely expressed in vegetative organs. However, transcriptome results show that it is the only member of the SUC family that is highly expressed at the tricellular pollen stage, during the last phase of pollen tube growth. The plasma membrane proton pump AHA3 (plasma membrane H+-ATPase family), known to reside in phloem companion cells (DeWitt and Sussman, 1995), was found to be selectively expressed in microspores. Only half of the microspores from heterozygous aha3 mutants developed into mature pollen grains. This example suggests a distinct role for the plasma membrane AHA3 in generating a proton gradient to drive uptake of sugars and other nutrients (Robertson et al., 2004) to nourish developing microspores. Schwacke et al. (1999) isolated putative transporters for proline; the uptake and accumulation of proline in developing and germinating pollen in tomato plants correlated with the induction of a pollen-specific member of the proline transporter ProT family, LeProT1. Aside from its presumed role in osmotic adjustment and as a compatible solute that protects proteins, proline may function as a sink for energy and reducing equivalents, as a source of nitrogen upon relief of stress, as a means of reducing acidity and as a radical scavenger (Schwacke et al., 1999, and references therein). Biochemical characterization revealed that in addition to proline, LeProT1 is an efficient transporter of glycine betaine and the stress-induced amino acid γ-aminobutyric acid.
LEA proteins and dehydrins
In orthodox seeds, quiescence is acquired during the later stages of maturation, when about 90 % of the water has been lost by desiccation (Finkelstein et al., 2002). Acquisition of desiccation tolerance is marked by a variety of factors, such as an increase in low molecular weight solutes and the appearance of dehydrins; the D11 family of LEA proteins (Ismail et al., 2010, and references therein). Dehydrins are very rich in glycine residues and they are characterized by highly conserved 15-mer lysine-rich sequences termed K segments. The K segment can form a putative amphipathic α-helical structure, with the potential for both hydrophilic and hydrophobic interactions (Abba et al., 2006, and references therein). Due to this property, dehydrins have a potential chaperone-like function in stabilizing partially denatured proteins or membranes, coating them with a cohesive water layer and preventing their coagulation during desiccation. Indeed, Wolkers et al. (2001) suggested that LEA proteins play a role together with carbohydrates in the formation of a tight hydrogen-bonded network in dehydrated pollen and, possibly, in other anhydrobiotic organisms. Aside from their suggested ion-binding property, LEA proteins would thus have a structural role in that these proteins might serve as anchors in a tight molecular network to provide stability to macromolecular and cellular structures in the cytoplasm of anhydrobiotes in the dry state. In a proteomic study of pollen, there was considerable overlap in some of the major proteins present in mature pollen grains and seeds (Grobei et al., 2009). Notably, both proteomes contained high levels of LEA proteins and chaperones (Grobei et al., 2009), consistent with an expected role for these proteins in conferring relatively stress-tolerant dormant states in both mature pollen grains and seeds (Zinn et al., 2010).
In the cold acclimation process, a large number of Cor (cold-responsive)/Lea genes are transcriptionally activated, and the accumulated proteins and metabolites lead to protection of cell structure integrity and function from freezing damage (Thomashow, 1999). Cor/Lea genes can be regulated by the dehydration-responsive element-binding (DREB) proteins that make up a sub-family of the ethylene-responsive element-binding factor family. The latter contains two main sub-classes, DREB1 and DREB2, involved in two separate signal transduction pathways under low temperature and dehydration, respectively (Agarwal et al., 2006). It is interesting to note that in maturing tomato microspores, several DREB family members (DREB1, DREB2 and DREB3) were found to be expressed, with DREB1 exhibiting heat stress-regulated expression (Frank et al., 2009).
Heat stress proteins (HSPs)
HSPs are assumed to help other proteins maintain or regain their native conformation by stabilizing their partially unfolded states (Kotak et al., 2007). Similar to other organisms, in plants, HSPs are expressed not only in response to stress, but also during various developmental programmes, including pollen maturation, zygotic embryogenesis and seed maturation (zur Nieden et al., 1995; Waters et al., 1996; Wehmeyer and Vierling, 2000).
In seeds, a sub-set of Hsp genes is expressed during seed development (Wehmeyer et al., 1996; Wehmeyer and Vierling, 2000; Hong and Vierling, 2001). These developmentally regulated Hsp genes accumulate late in the maturation phase. The expression of particular isoforms of Hsp genes during seed development suggests that these might have a distinct function during seed maturation and that they are regulated by a defined developmental programme. Arabidopsis plants with a desiccation-intolerant mutant allele of ABI3 (abi3-6; Nambara et al., 1994) were shown to have no detectable Hsp17·4-CI in mature dry green seeds (Wehmeyer and Vierling, 2000). The absence of small HSPs correlates with a desiccation-intolerant phenotype, suggesting that small HSPs might be required for desiccation tolerance in arabidopsis seeds.
Available pollen proteome and transcriptome data (Noir et al., 2005; Volkov et al., 2005; Frank et al., 2009) revealed the expression of several HSPs, including HSP70 and several chaperonins. Expression of HSPs is regulated by heat stress transcription factors (HSFs; Kotak et al., 2007). Indeed, several members of the HSF family were detected in developing tomato microspores/pollen, exhibiting low expression levels under ambient temperature conditions (Frank et al., 2009). A comprehensive analysis of the arabidopsis male gametophyte transcriptome revealed Hsf gene expression, although this family of transcription factors was found to be under-represented in the gametophyte (Honys and Twell, 2003, 2004).
Antioxidants
Studies of Noir et al. (2005) and Holmes-Davis et al. (2005), producing the mature pollen proteome of arabidopsis, indicated the accumulation of several proteins that are involved in the scavenging of reactive oxygen species, including ascorbate peroxidase, several superoxide dismutase proteins, catalase, glutathione peroxidase, glutathione S-transferase and thioredoxin. The presence of these proteins in mature pollen grains may both serve the maturing microspores during the dehydration phase and protect the mature pollen during the subsequent phases of presentation, dispersal and rehydration. High constitutive expression levels of five ascorbate peroxidase genes were recently detected in maturing tomato microspores: two chloroplastic (thylakoid) members, a peroxisomal member and two cytosolic members (Frank et al., 2009), indicating their importance during post-meiotic stages of pollen development, including the dehydration phase.
De Gara et al. (1993) demonstrated long ago that Dasypyrum villosum (a wild species of Triticinae) pollen contains the ascorbic acid synthesis pathway and actively synthesizes this compound. High activity of ascorbate peroxidase and monodehydroascorbate reductase was detected in the mature pollen grains, 10-fold higher than the activity of catalase, suggesting that ascorbate peroxidase may be the main enzyme removing hydrogen peroxide produced during cellular metabolism (De Gara et al., 1993). Sheoran et al. (2009) identified a number of proteins associated with stress responses in mature canola pollen, including catalase, peroxidase, GRP and LEA proteins. Two isoforms of catalase, peroxidase and GRP were found to be upregulated and two isoforms of catalase and LEA proteins were found to be downregulated in germinating pollen. The authors suggested that catalases and peroxidases may have dual roles, in stress tolerance and calcium signalling, during canola pollen germination and tube growth.
CONCLUDING REMARKS
During their developmental programme and functioning, pollen grains undergo remarkable changes in their hydration state – experiencing an increase in volume and water content while being immersed in the locular fluid, followed by a dehydration phase during which a large proportion of the cellular water is removed. Mature, dehydrated pollen can survive in a dry state for extended periods of time and accommodate changes in the RH of the environment to which they are exposed. Landing on a compatible stigma, pollen water content will increase, in accordance with the level of the mature pollen's water content capacity and the ability of the stigma to release water. These drastic changes in water content require structural, physiological and molecular mechanisms that enable adjustments in water level and accommodation of the accompanying changes in volume, membrane and protein environment; such mechanisms must also enable coping with side effects and reactions, such as the production of reactive oxygen species, which may harm the cellular components. It is suggested that pollen grains will use these mechanisms, which have a developmental role, for coping with environmental stress conditions. Additional data are necessary, however, to validate the function of these mechanisms during the various phases of pollen development and under stress conditions. The potential existence of a system that controls pollen water homeostasis is suggested in this review. Data are limited, however, and further research is necessary to identify the components that constitute such a system, namely the ‘water-status sensor’, the ‘messenger(s)’ that deliver the information and the ‘control point’ that processes this information.
LITERATURE CITED
- Aarts MG, Hodge R, Kalantidis K, et al. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. The Plant Journal. 1997;12:615–623. doi: 10.1046/j.1365-313x.1997.00615.x. [DOI] [PubMed] [Google Scholar]
- Abba S, Ghignone S, Bonfante P. A dehydration-inducible gene in the truffle Tuber borchii identifies a novel group of dehydrins. BMC Genomics. 2006;7(39) doi: 10.1186/1471-2164-7-39. http://dx.doi.org/10.1186/1471-2164-7-39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal PK, Agarwal P, Reddy MK, Sopory SK. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Reports. 2006;25:1263–1274. doi: 10.1007/s00299-006-0204-8. [DOI] [PubMed] [Google Scholar]
- Alpert P, Oliver MJ. Drying without dying. In: Black M, Pritchard HW, editors. Dessication and survival in plants. Wallingford, UK: CABI Publishing; 2002. pp. 3–43. [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, et al. A novel male-sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis-sensitive exine. Plant Molecular Biology. 2003;53:107–116. doi: 10.1023/B:PLAN.0000009269.97773.70. [DOI] [PubMed] [Google Scholar]
- Aylor DE. Rate of dehydration of corn (Zea mays L.) pollen in the air. Journal of Experimental Botany. 2003;54:2307–2312. doi: 10.1093/jxb/erg242. [DOI] [PubMed] [Google Scholar]
- Barnabas B, Rajki E. Fertility of deep-frozen maize (Zea mays L.) pollen. Annals of Botany. 1981;48:861–864. [Google Scholar]
- Bassani M, Pacini E, Franchi GG. Humidity stress responses in pollen of anemophilous and entomophilous species. Grana. 1994;33:146–150. [Google Scholar]
- Bianchini M, Pacini E. Explosive anther dehiscence in Ricinus communis L. involves cell wall modifications and relative humidity. International Journal of Plant Sciences. 1996;157:739–745. [Google Scholar]
- Blackmore S, Barnes SH. Pollen and spores: forms and function. London: Academic Press; 1986. Harmomegathic mechanisms in pollen grains; pp. 137–149. [Google Scholar]
- Bock KW, Honys D, Ward JM, et al. Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiology. 2006;140:1151–1168. doi: 10.1104/pp.105.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bots M, Vergeldt F, Wolters-Arts M, Weterings K, van As H, Mariani C. Aquaporins of the PIP2 class are required for efficient anther dehiscence in tobacco. Plant Physiology. 2005;137:1049–1056. doi: 10.1104/pp.104.056408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Floyd SK, Sakakibara K. Green genes – comparative genomics of the green branch of life. Cell. 2007;129:229–234. doi: 10.1016/j.cell.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Buitink J, Leprince O. Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology. 2004;48:215–228. doi: 10.1016/j.cryobiol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Buitink J, Leprince O. Intracellular glasses and seed survival in the dry state. Comptes Rendus Biologies. 2008;331:788–795. doi: 10.1016/j.crvi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Buitink J, Claessens MMAE, Hemminga MA, Hoekstra FA. Influence of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant Physiology. 1998;118:531–541. doi: 10.1104/pp.118.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizo Garcia C, Nepi M, Pacini E. Structural aspects and ecophysiology of anther opening in Allium triquetrum. Annals of Botany. 2006;97:521–527. doi: 10.1093/aob/mcl015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AJ, Clément C. Sucrose and starch catabolism in the anther of Lilium during its development: a comparative study among the anther wall, locular fluid and microspore/pollen fractions. Planta. 2007;225:1573–1582. doi: 10.1007/s00425-006-0443-5. [DOI] [PubMed] [Google Scholar]
- Dafni A, Firmage D. Pollen viability and longevity: practical, ecological and evolutionary implications. In: Dafni A, Hesse M, Pacini E., editors. Pollen and pollination. Vienna: Springer; 2000. pp. 113–132. [Google Scholar]
- De Gara L, Paciolla C, Liso R, Stefani A, Blanco A, Arrigoni O. Ascorbate metabolism in mature pollen grains of Dasypyrum villosum (L.) Borb. during imbibition. Journal of Plant Physiology. 1993;141:405–409. [Google Scholar]
- DeWitt ND, Sussman MR. Immunocytological localization of an epitope-tagged plasma membrane proton pump (H+-ATPase) in phloem companion cells. The Plant Cell. 1995;7:2053–2067. doi: 10.1105/tpc.7.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson H. Dry stigma, water and self-incompatibility in Brassica. Sexual Plant Reproduction. 1995;8:1–10. [Google Scholar]
- Dixit R, Rizzo C, Nasrallah M, Nasrallah J. The brassica MIP-MOD gene encodes a functional water channel that is expressed in the stigma epidermis. Plant Molecular Biology. 2001;45:51–62. doi: 10.1023/a:1006428007826. [DOI] [PubMed] [Google Scholar]
- Edlund AF, Swanson R, Preuss D. Pollen and stigma structure and function: the role of diversity in pollination. The Plant Cell. 2004;16:S84–S97. doi: 10.1105/tpc.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman CJ, Dickinson HG. Pollen–stigma interactions in Brassica. IV. Structural reorganization in the pollen grains during hydration. Journal of Cell Science. 1986;80:141–157. doi: 10.1242/jcs.80.1.141. [DOI] [PubMed] [Google Scholar]
- Elleman CJ, Franklin-Tong V, Dickinson HG. Pollination in species with dry stigmas: the nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytologist. 1992;121:413–424. doi: 10.1111/j.1469-8137.1992.tb02941.x. [DOI] [PubMed] [Google Scholar]
- Erbar C, Leins P. Portioned pollen release and the syndromes of secondary pollen presentation in the Campanulales–Asterales-complex. Flora. 1995;190:323–338. [Google Scholar]
- Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D. Alterations in CER6, a gene identical to CUT1, differentially affect long chain lipid content on the surface of pollen and stems. The Plant Cell. 2000;12:2001–2008. doi: 10.1105/tpc.12.10.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signalling in seeds and seedlings. The Plant Cell. 2002;14:S15–S45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N, Shaked R, Peet MM, et al. Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Scientia Horticulturae. 2006;109:212–217. [Google Scholar]
- Fonseca AE, Westgate ME. Relationship between desiccation and viability of maize pollen. Field Crops Research. 2005;94:114–125. [Google Scholar]
- Forrest KL, Bhave M. Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Functional and Integrative Genomics. 2007;7:263–289. doi: 10.1007/s10142-007-0049-4. [DOI] [PubMed] [Google Scholar]
- Franchi GG, Nepi M, Pacini E. Partially hydrated pollen: taxonomical distribution, ecological and evolutive significance. Plant Systematics and Evolution. 2002;234:211–227. [Google Scholar]
- Franchi GG, Piotto BN, Nepi M, Baskin CC, Baskin JM, Pacini E. Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal and survival. Journal of Experimental Botany. 2011;62:5267–5281. doi: 10.1093/jxb/err154. [DOI] [PubMed] [Google Scholar]
- Frank G, Pressman E, Ophir R, et al. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. Journal of Experimental Botany. 2009;60:3891–3908. doi: 10.1093/jxb/erp234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenguelli G, Ferranti F, Tedeschini E, Andreutti R. Volume changes in the pollen grain of Corylus avellana L. (Corylaceae) during development. Grana. 1997;36:289–292. [Google Scholar]
- Goldman MH, Goldberg RB, Mariani C. Female sterile tobacco plants are produced by stigma-specific cell ablation. EMBO Journal. 1994;13:2976–2984. doi: 10.1002/j.1460-2075.1994.tb06596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobei MA, Qeli E, Brunner E, et al. Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Research. 2009;19:1786–1800. doi: 10.1101/gr.089060.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri M, Speranza A, Nepi M, Artese D, Pacini E. Ripe pollen carbohydrate changes in Trachycarpus fortunei: the effect of relative humidity. Sexual Plant Reproduction. 2006;19:117–124. [Google Scholar]
- Hay FR, Smith RD, Ellis RH, Butler LH. Developmental changes in the germinability, desiccation tolerance, hardseededness, and longevity of individual seeds of Trifolium ambiguum. Annals of Botany. 2010;105:1035–1052. doi: 10.1093/aob/mcq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J. Aspects of the structure, cytochemistry and germination of the pollen of rye (Secale cereale L.) nnals of Botany. 1979;44(Suppl.):S1–S47. [Google Scholar]
- Heslop-Harrison J. Pollen germination and pollen tube growth. International Review of Cytology. 1987;107:1–78. [Google Scholar]
- Heslop-Harrison Y. Stigma characteristics and angiosperm taxonomy. Nordic Journal of Botany. 1981;1:401–420. [Google Scholar]
- Heslop-Harrison Y. Control gates and micro-ecology: the pollen–stigma interaction in perspective. Annals of Botany. 2000;85(Suppl.):S5–S13. [Google Scholar]
- Heslop-Harrison JS, Heslop-Harrison Y. The pollen–stigma interaction in the grasses. 1 Fine-structure and cytochemistry of the stigmas of Hordeum and Secale. Acta Botanica Neerlandica. 1980;29:261–276. [Google Scholar]
- Heslop-Harrison Y, Shivanna KR. The receptive surface of the angiosperm stigma. Annals of Botany. 1977;41:1233–1258. [Google Scholar]
- Heslop-Harrison JS, Heslop-Harrison Y, Reger BJ. Anther-filament extension in Lilium: potassium ion movement and some anatomical features. Annals of Botany. 1987;59:505–515. [Google Scholar]
- Hiscock SJ, Allen AM. Diverse cell signalling pathways regulate pollen–stigma interactions: the search for consensus. New Phytologist. 2008;179:286–317. doi: 10.1111/j.1469-8137.2008.02457.x. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Tetteroo FA, Wolkers WF. Induction of desiccation tolerance in plant somatic embryos: how exclusive is the protective role of sugars? Cryobiology. 2001;43:140–150. doi: 10.1006/cryo.2001.2358. [DOI] [PubMed] [Google Scholar]
- Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S. Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics. 2005;5:4864–4884. doi: 10.1002/pmic.200402011. [DOI] [PubMed] [Google Scholar]
- Hong SW, Vierling E. Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. The Plant Journal. 2001;27:25–35. doi: 10.1046/j.1365-313x.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiology. 2003;132:640–652. doi: 10.1104/pp.103.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biology. 2004;5:R85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GJ, Slater AT, Knox RB. Secondary pollen presentation in angiosperms and its biological significance. Australian Journal of Botany. 1993;41:417–438. [Google Scholar]
- Hubbard NL, Pharr DM, Huber SC. Role of sucrose phosphate synthase in sucrose biosynthesis in ripening bananas and its relationship to the respiratory climacteric. Plant Physiology. 1990;94:201–208. doi: 10.1104/pp.94.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülskamp M, Kopczak SD, Horejsi TF, Kihl BK, Pruitt RE. Identification of genes required for pollen–stigma recognition in Arabidopsis thaliana. The Plant Journal. 1995;8:703–714. doi: 10.1046/j.1365-313x.1995.08050703.x. [DOI] [PubMed] [Google Scholar]
- Ismail FA, Nitsch LM, Wolters-Arts MM, Mariani C, Derksen JW. Semi-viviparous embryo development and dehydrin expression in the mangrove Rhizophora mucronata Lam. Sexual Plant Reproduction. 2010;23:95–103. doi: 10.1007/s00497-009-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, McCormick S. Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiology. 2001;126:685–695. doi: 10.1104/pp.126.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Han MJ, Lee DY, et al. Wax-deficient anther is involved in cuticle and wax production in rice anther walls and is required for pollen development. The Plant Cell. 2006;18:3015–3032. doi: 10.1105/tpc.106.042044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Fischer M. Aquaporins in plants. Acta Physiologica. 2006;187:169–176. doi: 10.1111/j.1748-1716.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- Katifori E, Alben S, Cerda E, Nelson DR, Dumais J. Foldable structures and the natural design of pollen grains. Proceedings of the National Academy of Sciences, USA. 2010;107:7635–7639. doi: 10.1073/pnas.0911223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RB, McConchie CA. Structure and function of compound pollen. In: Blackmore S, Ferguson IK., editors. Pollen and spores: form and function. London: Academic Press; 1986. pp. 265–285. [Google Scholar]
- Kotak S, Vierling E, Bäumlein H, von Koskull-Doring P. A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. The Plant Cell. 2007;19:182–195. doi: 10.1105/tpc.106.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Samuels AL. Biosynthesis and secretion of plant cuticular wax. Progress in Lipid Research. 2003;42:51–80. doi: 10.1016/s0163-7827(02)00045-0. [DOI] [PubMed] [Google Scholar]
- Ladd PG. Pollen presenters in the flowering plants: plants – form and function. Botanical Journal of the Linnean Society. 1994;115:165–195. [Google Scholar]
- Leins P. Blute und Frucht. Aspekte der Morphologie, Entwicklungsgeschichte, Phylogenie, Funktion und Okologie. 2000. Stuttgart: E. Schweizerbart'sche Verlagsbuchhdl. [Google Scholar]
- Li GW, Peng YH, Yu X, Zhang MH, Cai WM, Sun WN, Su WA. Transport functions and expression analysis of vacuolar membrane aquaporins in response to various stresses in rice. Journal of Plant Physiology. 2008;165:1879–1888. doi: 10.1016/j.jplph.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Li H, Pinot F, Sauveplane V, et al. Cytochrome P450 family member CYP704B2 catalyzes the {omega}-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. The Plant Cell. 2010;22:173–190. doi: 10.1105/tpc.109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens HF, Cresti M. The effect of temperature, humidity, and light on the dehiscence of tobacco anthers. Proceedings of the Koninklike Nederlandse Akademie van Wetenschappen. 1988;C91:369–375. [Google Scholar]
- Lisci M, Tanda C, Pacini E. Pollination ecophysiology of Mercurialis annua L. (Euphorbiaceae) an anemophilous species flowering all year round. Annals of Botany. 1994;74:125–135. [Google Scholar]
- Lolle SJ, Cheung AY. Promiscuous germination and growth of wild-type pollen from Arabidopsis and related species on the shoot of the Arabidopsis mutant fiddlehead. Developmental Biology. 1993;155:250–258. doi: 10.1006/dbio.1993.1022. [DOI] [PubMed] [Google Scholar]
- Lolle SJ, Berlyn GP, Engstrom EM, Krolikowski KA, Reiter WD, Pruitt RE. Developmental regulation of cell interactions in the Arabidopsis fiddlehead-1 mutant: a role for the epidermal cell wall and cuticle. Developmental Biology. 1997;189:311–321. doi: 10.1006/dbio.1997.8671. [DOI] [PubMed] [Google Scholar]
- Lolle SJ, Hsu W, Pruitt RE. Genetic analysis of organ fusion in Arabidopsis thaliana. Genetics. 1998;149:607–619. doi: 10.1093/genetics/149.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losch R. Plant water relations. In: Luttge U., editor. Progress in botany, cell biology and physiology. Vol. 60. Berlin: Springer-Verlag; 1999. pp. 193–233. [Google Scholar]
- Matsui T, Omasa K, Horie T. Rapid swelling of pollen grains in the dehiscing anther of two-rowed barley (Hordeum distichum L. emend. Lam.) Annals of Botany. 2000;85:345–350. [Google Scholar]
- Mayfield JA, Preuss D. Rapid initiation of Arabidopsis pollination requires the oleosin-domain protein GRP17. Nature Cell Biology. 2000;2:128–130. doi: 10.1038/35000084. [DOI] [PubMed] [Google Scholar]
- Mayfield JA, Fiebig A, Johnstone SE, Preuss D. Gene families from the Arabidopsis thaliana pollen coat proteome. Science. 2001;292:2482–2485. doi: 10.1126/science.1060972. [DOI] [PubMed] [Google Scholar]
- Morant M, Jorgensen K, Schaller H, et al. CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. The Plant Cell. 2007;19:1473–1487. doi: 10.1105/tpc.106.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Keith K, McCourt P, Naito S. Isolation of an internal deletion mutant of the Arabidopsis thaliana ABI3 gene. Plant and Cell Physiology. 1994;35:509–513. [PubMed] [Google Scholar]
- Neiland MRM, Wilcock CC. Maximization of reproductive success in European orchids under conditions of infrequent pollination. Protoplasma. 1995;187:39–48. [Google Scholar]
- Nepi M, Pacini E. Pollination, pollen viability and pistil receptivity in Cucurbita pepo. Annals of Botany. 1993;72:526–536. [Google Scholar]
- Nepi M, Franchi GG, Pacini E. Pollen hydration status at dispersal: cytophysiological features and strategies. Protoplasma. 2001;216:171–180. doi: 10.1007/BF02673869. [DOI] [PubMed] [Google Scholar]
- Nepi M, von Aderkas P, Wagner R, Mugnaini S, Coulter A, Pacini E. Nectar and pollination drops: how different are they? Annals of Botany. 2009;104:205–219. doi: 10.1093/aob/mcp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepi M, Cresti L, Guarnieri M, Pacini E. Effect of relative humidity on water content, viability and carbohydrate profile of Petunia hybrida and Cucurbita pepo pollen. Plant Systematics and Evolution. 2010;284:57–64. [Google Scholar]
- Noir S, Bräutigam A, Colby T, Schmidt J, Panstruga R. A reference map of the Arabidopsis thaliana mature pollen proteome. Biochemical and Biophysical Research Communications. 2005;337:1257–1266. doi: 10.1016/j.bbrc.2005.09.185. [DOI] [PubMed] [Google Scholar]
- Pacini E. Harmomegathic characters of Pteridophyta spores and Spermatophyta pollen. Plant Systematics and Evolution. 1990;5:S53–S69. [Google Scholar]
- Pacini E. Cell biology of anther and pollen development. In: Williams EG, Clarke AE, Knox RB., editors. Genetic control of self-incompatibility and reproductive development in flowering plants. Dordrecht: Kluwer Academic Publishers; 1994. pp. 289–308. [Google Scholar]
- Pacini E. Tapetum types in the Compositae: form and function. In: Hind DJH, Beentije H, Pope GV, editors. Proceedings of the International Compositae Conference. Royal Botanic Gardens Kew. 1996. pp. 21–28. [Google Scholar]
- Pacini E. From anther and pollen ripening to pollen presentation. Plant Systematics and Evolution. 2000;22:19–43. [Google Scholar]
- Pacini E. Relationship between tapetum, loculus, and pollen during development. International Journal of Plant Sciences. 2010;171:1–11. [Google Scholar]
- Pacini E, Franchi GG. Germination of pollen inside anthers in some non-cleistogamous species. Caryologia. 1982;35:205–215. [Google Scholar]
- Pacini E, Franchi GG. Pollen grain development in Smilax aspera L. and possible function of the loculus. In: Mulcahy DL, Ottaviano E, editors. Pollen biology and implications for plant breeding. New York: Elsevier; 1983. pp. 183–190. [Google Scholar]
- Pacini E, Franchi GG. Harmomegathy un problema aperto e misconosciuto. Giornale Botanico Italiano. 1984;118:271–282. [Google Scholar]
- Pacini E, Franchi GG, Lisci M, Nepi M. Pollen viability related to type of pollination in six angiosperm species. Annals of Botany. 1997;80:83–87. [Google Scholar]
- Pacini E, Guarnieri M, Nepi M. Pollen carbohydrates and water content during development, presentation, and dispersal: a short review. Protoplasma. 2006;228:73–77. doi: 10.1007/s00709-006-0169-z. [DOI] [PubMed] [Google Scholar]
- Pacini E, Jacquard C, Clément C. Pollen vacuoles and their significance. Planta. 2011;234:217–227. doi: 10.1007/s00425-011-1462-4. [DOI] [PubMed] [Google Scholar]
- Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA. DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiology. 2001;127:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Pertl H, Pockl M, Blaschke C, Obermeyer G. Osmoregulation in Lilium pollen grains occurs via modulation of the plasma membrane H+ ATPase activity by 14-3-3 proteins. Plant Physiology. 2010;154:1921–1928. doi: 10.1104/pp.110.165696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts M. Desiccation tolerance: a simple process? Trends in Microbiology. 2001;9:553–559. doi: 10.1016/s0966-842x(01)02231-4. [DOI] [PubMed] [Google Scholar]
- Preuss D, Lemieux B, Yen G, Davis RW. A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes and Development. 1993;7:974–985. doi: 10.1101/gad.7.6.974. [DOI] [PubMed] [Google Scholar]
- Pruitt RE, Vielle-Calzada JP, Ploense SE, Grossniklaus U, Lolle SJ. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proceedings of the National Academy of Sciences, USA. 2000;97:1311–1316. doi: 10.1073/pnas.97.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson WR, Clark K, Young JC, Sussman MR. An Arabidopsis thaliana plasma membrane proton pump is essential for pollen development. Genetics. 2004;168:1677–1687. doi: 10.1534/genetics.104.032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke R, Grallath S, Breitkreuz KE, et al. LeProT1, a transporter for proline, glycine betaine, and γ-amino butyric acid in tomato pollen. The Plant Cell. 1999;11:377–392. doi: 10.1105/tpc.11.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran IS, Ross AR, Olson DJ, Sawhney VK. Differential expression of proteins in the wild type and 7B-1 male-sterile mutant anthers of tomato (Solanum lycopersicum): a proteomic analysis. Journal of Proteomics. 2009;71:624–636. doi: 10.1016/j.jprot.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Sommer A, Geist B, Da Ines O, Gehwolf R, Schäffner AR, Obermeyer G. Ectopic expression of Arabidopsis thaliana plasma membrane intrinsic protein 2 aquaporins in lily pollen increases the plasma membrane water permeability of grain but not of tube protoplasts. New Phytologist. 2008;180:787–797. doi: 10.1111/j.1469-8137.2008.02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto G, Alleva K, Mazzella MA, Amodeo G, Muschietti JP. AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Letters. 2008;582:4077–4082. doi: 10.1016/j.febslet.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Souza CA, Kim SS, Koch S, et al. A novel fatty acyl-CoA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. The Plant Cell. 2009;21:507–525. doi: 10.1105/tpc.108.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza A, Calzoni GL, Pacini E. Occurrence of mono- or disaccharides and polysaccharide reserves in mature pollen grains. Sexual Plant Reproduction. 1997;10:110–115. [Google Scholar]
- Swanson R, Clark T, Preuss D. Expression profiling of Arabidopsis stigma tissue identifies stigma-specific genes. Sexual Plant Reproduction. 2005;18:163–171. [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Tweddle JC, Dickie JB, Baskin CC, Baskin JM. Ecological aspects of seed desiccation sensitivity. Journal of Ecology. 2003;91:294–304. [Google Scholar]
- Updegraff EP, Zhao F, Preuss D. The extracellular lipase EXL4 is required for efficient hydration of Arabidopsis pollen. Sexual Plant Reproduction. 2009;22:197–204. doi: 10.1007/s00497-009-0104-5. [DOI] [PubMed] [Google Scholar]
- Vaknin Y, Eisikowitch D. Effects of short-term storage on germinability of pistachio pollen. Plant Breeding. 2000;119:347–350. [Google Scholar]
- Vesprini JL, Nepi M, Cresti L, Guarnieri M, Pacini E. Changes in cytoplasmic carbohydrate content during Helleborus pollen presentation. Grana. 2002;41:16–20. [Google Scholar]
- Volkov RA, Panchuk, Schoffl F. Small heat shock proteins are differentially regulated during pollen development and following heat stress in tobacco. Plant Molecular Biology. 2005;57:487–502. doi: 10.1007/s11103-005-0339-y. [DOI] [PubMed] [Google Scholar]
- Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. Journal of Experimental Botany. 1996;47:325–338. [Google Scholar]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E. Synthesis of small heat shock proteins is part of the developmental program of late seed maturation. Plant Physiology. 1996;112:747–757. doi: 10.1104/pp.112.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer N, Vierling E. The expression of sHsps in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiology. 2000;122:189–198. doi: 10.1104/pp.122.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodehouse RP. Pollen grains. New York: McGraw-Hill; 1935. [Google Scholar]
- Wolkers WF, McCready S, Brandt WF, Lindsey GG, Hoekstra FA. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochimica et Biophysica Acta. 2001;1544:196–206. doi: 10.1016/s0167-4838(00)00220-x. [DOI] [PubMed] [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C. Lipids are required for directional pollen-tube growth. Nature. 1998;392:818–821. doi: 10.1038/33929. [DOI] [PubMed] [Google Scholar]
- Yates IE, Sparks D. Environmental regulation of anther dehiscence and pollen germination in pecan. Journal of the American Society for Horticultural Science. 1993;118:699–706. [Google Scholar]
- Yeo P. Secondary pollen presentation: form, function and evolution. Plant Systematics and Evolution Supplementum. Wien, Austria: Springer-Verlag; 1993. [Google Scholar]
- Zhang M, Zhuo JJ, Wang X, Wu S, Wang XF. Optimizing seed water content: relevance to storage stability and molecular mobility. Journal of Integrative Plant Biology. 2010;52:324–331. doi: 10.1111/j.1744-7909.2010.00916.x. [DOI] [PubMed] [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany. 2010;61:1959–1968. doi: 10.1093/jxb/erq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Nieden U, Neumann D, Bucka A, Nover L. Tissue-specific localization of heat-stress proteins during embryo development. Planta. 1995;196:530–538. [Google Scholar]