Abstract
Pulmonary arterial hypertension (PAH) is a devastating disease, and no effective treatments are available. Hypoxia-induced pulmonary artery remodeling, including smooth muscle cell proliferation, contributes to PAH, but the exact mechanisms underlying this abnormal process are largely undefined. The forkhead box M1 (FoxM1) transcription factor regulates cancer cell growth by modulating gene expression critical for cell cycle progression. Here, we report for the first time, to the best of our knowledge, a novel function of FoxM1 in the hypoxia-stimulated proliferation of human pulmonary artery smooth muscle cells (HPASMCs). Exposure to hypoxia caused a marked up-regulation of FoxM1 gene expression, mainly at the transcription level, and this induction correlated with HPASMC cell proliferation. The knockdown of FoxM1 inhibited the hypoxia-stimulated proliferation of HPASMCs. We found that the knockdown of HIF-2α, but not HIF-1α, diminished FoxM1 induction in response to hypoxia. However, the knockdown of FoxM1 did not alter expression levels of HIF-2α or HIF-1α, suggesting that HIF-2α is an upstream regulator of FoxM1. Furthermore, the knockdown of FoxM1 prevented the hypoxia-induced expression of aurora A kinase and cyclin D1. Collectively, our results suggest that hypoxia induces FoxM1 gene expression in an HIF-2α–dependent pathway, thereby promoting HPASMC proliferation.
Keywords: cell proliferation, FoxM1, HIF, hypoxia, pulmonary artery smooth muscle cells
Clinical Relevance
Our results provide direct evidence that forkhead box M1 (FoxM1) plays a critical role in the hypoxia-induced proliferation of human pulmonary artery smooth muscle cells. Therapies targeting FoxM1 may provide novel insights into the treatment of this disease.
Pulmonary artery hypertension (PAH) is a devastating vasculopathy that results in decreased pulmonary perfusion, hypoxemia, and right-sided heart failure (1). A limited understanding of the basic cellular mechanisms governing this disease process has led to few therapeutic options and continued morbidity and mortality (2). Hypoxia is a well-known stimulus for the development of pulmonary hypertension (3). Acute hypoxia induces vasoconstriction in the pulmonary vasculature, whereas chronic hypoxia promotes pulmonary arterial wall remodeling via the induction of cell proliferation in all three layers, and particularly in the smooth muscle cells of the tunica media (4, 5).
Hypoxia-inducible factors (HIFs) are members of the basic helix-loop-helix/PAS family (bHLH-PAS) family of proteins that play a significant role in oxygen homeostasis and cellular adaptation to hypoxia by binding to the hypoxia response elements in the promoter or enhancer region of the target genes (6–8). The HIF is a heterodimer consisting of α and β subunits, and the regulation of HIF is primarily mediated by the stabilization of α subunits during low O2 concentrations (6, 7). Both HIF-1 and HIF-2 were implicated in hypoxia-mediated pulmonary hypertension. However, the signaling mechanisms involved in the hypoxia-induced proliferation of pulmonary artery smooth muscle cells (PASMCs) have not been fully elucidated (9, 10).
Forkhead box M1 (FoxM1) is a transcription factor that regulates cell cycle progression, and it has been implicated in cancer cell lines (11–13). In response to proliferative signaling, it translocates to the nucleus and promotes G1/S and G2/M transitions, leading to the progression of mitosis via its downstream targets (14). Recent evidence suggests that the FoxM1 promoter contains HIF response elements, and that hypoxia induces FoxM1 in some cancer cell lines (11–13, 15). In the lung, FoxM1 was reported to be required for normal pulmonary vascular development (16, 17). The loss of FoxM1 also attenuates epithelial and endothelial repair after lung injury (16–19). The lungs of Rosa26-FoxM1B mice exhibit earlier nuclear localization of FoxM1 with its downstream targets, and increased cell proliferation, in response to butylated hydroxytoluene–induced lung injury (20). However, whether FoxM1 affects PASMC proliferation, and whether FoxM1 plays a role in the pathogenesis of PAH, remain unknown.
In this study, we test the hypothesis that FoxM1 is critical for hypoxia-induced pulmonary artery smooth muscle proliferation. Our studies suggest that hypoxia induces FoxM1 in human pulmonary artery smooth muscle cells (HPASMCs), and FoxM1 is required for the hypoxia-induced proliferation of HPASMCs. Furthermore, we show that the induction of FoxM1 is dependent on HIF-2α. This pathway may provide a novel therapeutic target for the treatment of PAH.
Materials and Methods
Cell Culture
HPASMCs (Lonza, Basel, Switzerland) were subcultured in SmGM-2 BulletKit media (Lonza) at 37°C in a humidified HEPA Class 100 CO2 Incubator (Thermo Scientific, Waltham, MA). Passages 6–10 were used for all experiments. Hypoxia (3% O2) was achieved in the incubator by flowing nitrogen gas into it, and the oxygen concentration within was monitored continuously with an oxygen sensor.
Small Interfering RNA Suppression of HIF-1α, HIF-2α, and FoxM1
HPASMCs were plated on 60-mm dishes and transfected with small interfering RNA (siRNA) and Lipofectamine 2000 (Invitrogen, Carlsbad, CA) at approximately 60–70% confluence, following the manufacturer's protocol. Scrambled siRNA (siNeg) was used as negative controls. After transfection, cells were treated with complete media and incubated overnight.
Luciferase Assay
Firefly luciferase constructs FoxM1-Luc (containing the FoxM1 proximal promoter) and CDx6-Luc (containing six copies of the FoxM1-binding elements of the cyclin B1 promoter) were cotransfected into HPASMCs, along with a Renilla luciferase reporter construct (internal control). Cells were exposed to normoxia or hypoxia for 24 hours. Relative luciferase activity was measured using the GloMax-Multi Detection System (Promega, Madison, WI).
Western Blot Analysis
Cells were lysed in modified radioimmunoprecipitation assay (mRIPA) lysis buffer with a protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO), and protein quantification and Western blot analysis were performed according to standard procedures. Details are provided in the online supplement. Antibodies included FoxM1 (Santa Cruz Biotechnology, Santa Cruz, CA), proliferating cell nuclear antigen (PCNA; Protein Tech, Chicago, IL), α-tubulin (Sigma Aldrich), and anti-T7 tag antibody (Novagen, Gibbstown, NJ).
Cell Proliferation Assays
Cell proliferation was determined by Western blotting for PCNA, a bromodeoxyuridine (BrdU) incorporation assay, and cell counting. The BrdU incorporation assay was performed using a cell proliferation assay kit (Calbiochem, San Diego, CA). For cell counting, cells were plated at the same density. After experimental procedures, cells were trypsinized and resuspended, and the density of cells was counted with a hemocytometer.
Quantitative Real-Time RT-PCR
Total RNA was isolated using the RNeasy Minikit (Qiagen, Valencia, CA),and quantified with a Nanodrop 2000 spectrophotometer (Thermo-Fisher Scientific, Wilmington, DE). RNA was transcribed to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). PCR was performed with a Step One Plus Real-Time PCR System, using the QuantiTect SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA). Primers were synthesized by Integrated DNA Technologies (Coralville, IA) for the following genes: FoxM1, HIF-1α, HIF-2α, cyclin D1, aurora A kinase, and ribosomal protein L19 (RPL19). RPL19 was used as an internal control. Primer sequences are provided in the online supplement.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Results are expressed as mean ± SEM from at least three experiments (each performed in replicates). We used t tests and ANOVA to compare two and three groups, respectively. P < 0.05 was considered statistically significant.
Results
Hypoxia Induces HPASMC Proliferation and FoxM1 Expression
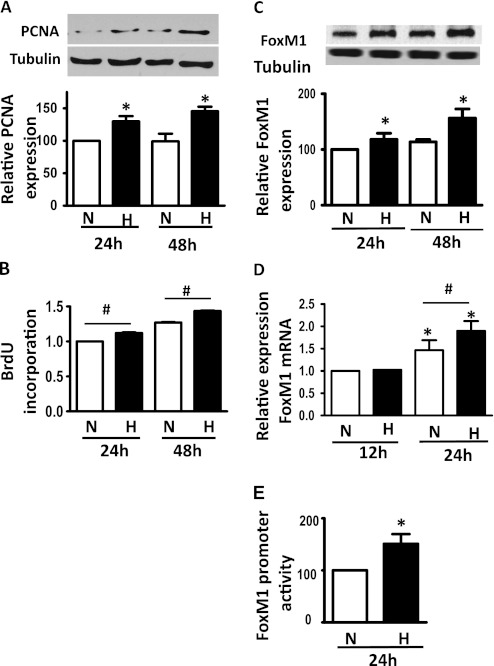
To determine whether hypoxia induces HPASMC proliferation, we exposed HPASMCs to hypoxia (3% O2) or normoxia (21% O2) for 24 or 48 hours, and measured the protein expression of PCNA or BrdU incorporation into cells. As shown in Figures 1A and 1B, hypoxia significantly induced PCNA protein expression and increased BrdU incorporation as early as 24 hours of exposure to hypoxia, suggesting that hypoxia promotes HPASMC proliferation. We then determined the protein expression of FoxM1 in cells exposed to normoxia or hypoxia for 24 and 48 hours, and found that hypoxia induced FoxM1 protein expression and mRNA levels as early as 24 hours of incubation (Figures 1C and 1D). Furthermore, hypoxia induced a 50% increase in FoxM1 promoter activity, as measured by an assay using a luciferase construct driven by FoxM1 promoter (Figure 1E). Together, these findings indicate that hypoxia induces HPASMC proliferation and FoxM1 expression.
Figure 1.
Hypoxia increases the proliferation of human pulmonary artery smooth muscle cells (HPASMCs), and induces the expression of forkhead box M1 (FoxM1). Cells were plated at an equal density and exposed to normoxia (N) or hypoxia (H) for 12, 24, or 48 hours (h). Cell lysates and total RNA were used to perform Western blot analyses, probing for the protein expression of proliferating cell nuclear antigen (PCNA) (A) and FoxM1 (C), and to perform quantitative RT-PCR for the measurement of FoxM1 mRNA levels (D). Cell proliferation was measured by the incorporation of bromodeoxyuridine (BrdU) at 24 and 48 hours (B). (E) FoxM1 promoter activity was measured by a Luciferase reporter assay at 24 hours of normoxia or hypoxia. Representative blots are shown, and the gray density of the blots was quantified and is presented in the graphs. Data are expressed as mean ± SEM (n = 3). *P < 0.05. #Significantly different between normoxic and hypoxic groups.
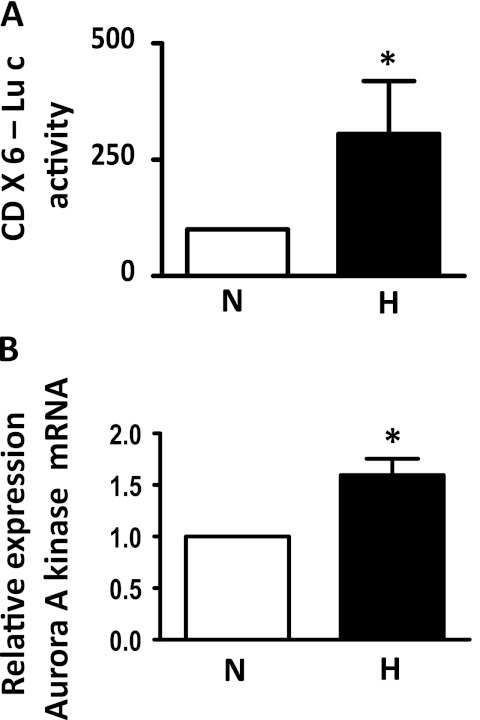
Hypoxia Increases FoxM1 Activity and Induces the Expression of a FoxM1 Downstream Target
FoxM1 is a transcription factor regulating many downstream targets, including cyclin B and aurora A Kinase (21, 22). To determine whether hypoxia increases FoxM1 activity in HPASMCs, we cotransfected cells with CDx6-Luc (a reporter construct driven by six copies of the FoxM1 binding site of the cyclin B promoter) with a Renilla Luciferase reporter construct, followed by exposure to normoxia or hypoxia for 24 hours. As shown in Figure 2A, exposure to hypoxia induced a 2.6-fold increase in CDx6-Luc activity, suggesting that hypoxia increases FoxM1 transcriptional activity in HPASMCs. We also measured the expression levels of a FoxM1 downstream target aurora A kinase after exposure to normoxia or hypoxia, and found that hypoxia induced aurora A kinase mRNA concentrations (Figure 2B), confirming the elevated FoxM1 activity under hypoxic conditions.
Figure 2.
Hypoxia induces FoxM1 target gene expression. (A) Relative luciferase activity was measured after cotransfection of HPASMCs with the firefly luciferase construct CDX6-LUC (containing six copies of the FoxM1-binding elements of the cyclin B1 promoter) and Renilla luciferase reporter plasmid, and exposure to normoxia (N) or hypoxia (H) for 24 hours. (B) Cells were exposed to normoxia or hypoxia, and the lysate was used to measure aurora A kinase mRNA levels by quantitative RT-PCR. Data are expressed as mean ± SEM (n = 3). *P < 0.05.
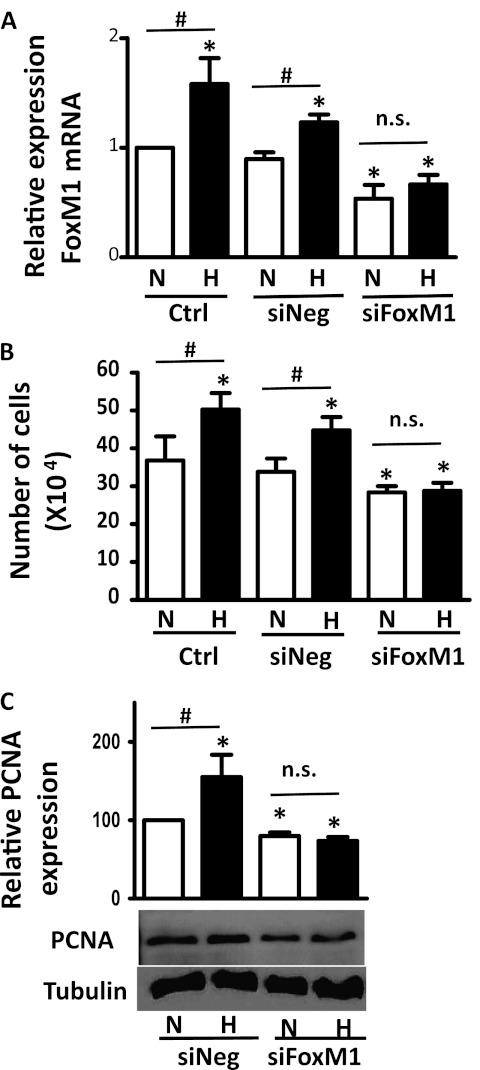
FoxM1 Is Necessary for the Hypoxia-Induced Proliferation of HPASMCs
To determine whether FoxM1 is necessary for the hypoxia-induced proliferation of HPASMCs, we suppressed FoxM1 expression by siRNA, and exposed cells to normoxia or hypoxia, followed by measurements of cell proliferation. As shown in Figure 3A, FoxM1 specific siRNA successfully knocked down FoxM1 mRNA expression by 50% under both normoxic and hypoxic conditions. In addition, the suppression of FoxM1 prevented hypoxia-mediated increases in cell numbers (Figure 3B) and the induction of PCNA (Figure 3C), suggesting that FoxM1 is critical for the hypoxia-induced proliferation of HPASMCs.
Figure 3.
FoxM1 is required for the hypoxia-stimulated proliferation of HPASMCs. HPASMCs were plated and either left untreated or were transfected with small interfering RNA (siRNA) against FoxM1 (siFoxM1) or scrambled siRNA (siNeg). Forty-eight hours after exposure to normoxia (N) or hypoxia (H), total RNA was extracted, and FoxM1 mRNA levels were determined by quantitative RT-PCR (A), total cell numbers were counted with a hemocytometer (B), and the protein expression of PCNA was measured by Western blot analysis (C). Data were normalized to the normoxic control (N/Ctrl, A and B) or N/siNeg (C), and are expressed as mean ± SEM (n = 6 in A, 3 in B, and 3 in C). *P < 0.05. #Significantly different between normoxic and hypoxic groups. n.s., no significance.
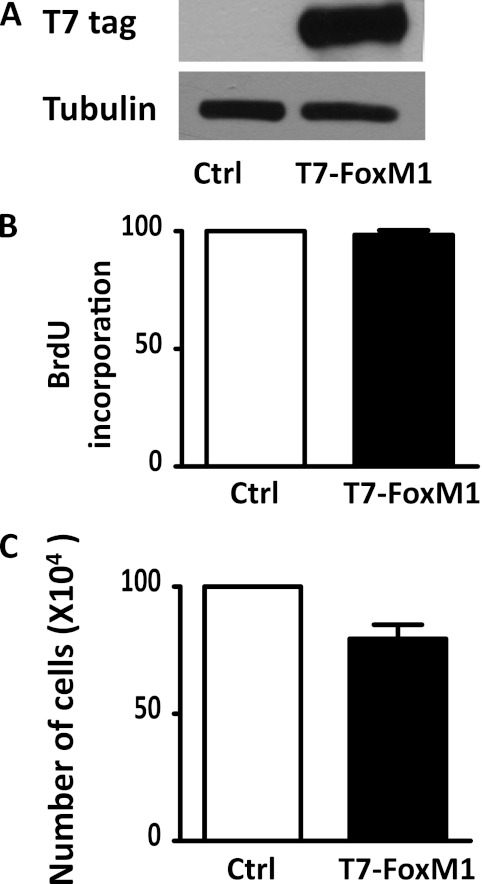
Overexpression of FoxM1 Is Not Sufficient to Induce HPASMC Proliferation in Normoxia
To determine whether FoxM1 was sufficient to induce cell proliferation in HPASMCs, we overexpressed FoxM1 in HPASMCs by transfecting the cells with a plasmid encoding T7-FoxM1. Cells were then incubated in normoxia for 48 hours, and BrdU incorporation and cell numbers were determined. As shown in Figure 4A, FoxM1 was overexpressed in HPASMCs after transfection, and the overexpression of FoxM1 did not increase BrdU incorporation or cell numbers (Figures 4B and 4C), suggesting that FoxM1 is not sufficient to induce HPASMC proliferation under normal conditions.
Figure 4.
Overexpression of FoxM1 is insufficient to induce HPASMC proliferation under normal conditions. HPASMCs were plated at equal density and transfected with a plasmid encoding T7-FoxM1 or an empty vector plasmid. Forty-eight hours after transfection, exogenous FoxM1 expression was determined by Western blot analysis with a T7 tag antibody (A), cell proliferation was measured by BrdU incorporation (B), and total cell numbers were counted using a hemocytometer. Data are expressed as mean ± SEM (n = 3). Ctrl, control.
FoxM1 Expression Is Regulated by HIF-1α under Normoxic Conditions, whereas HIF-2α Is Critical for FoxM1 Induction during Hypoxia
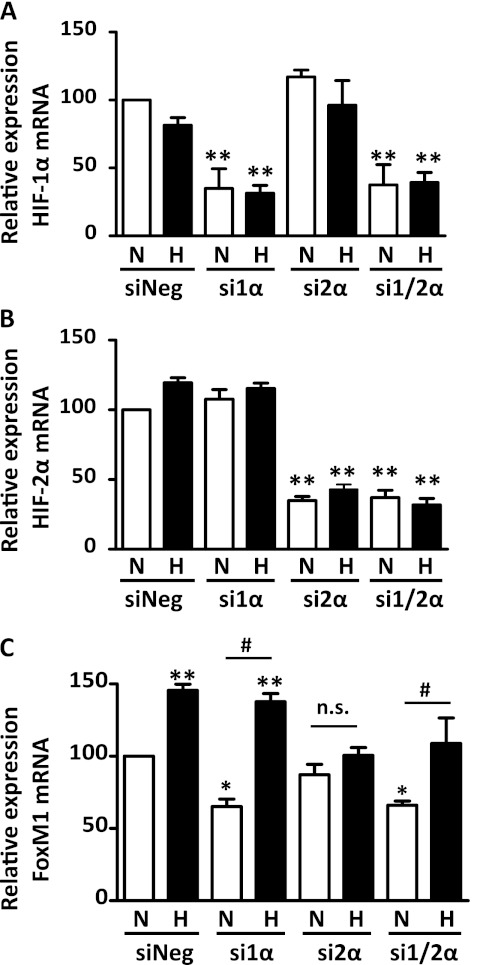
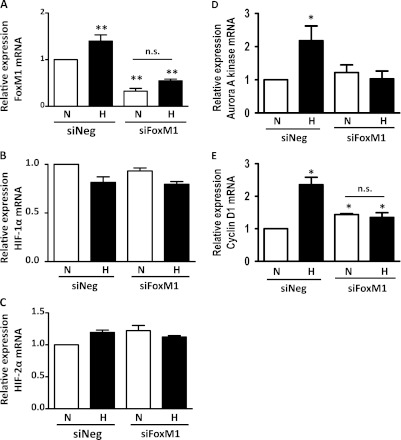
Because HIF is a master regulator of the cellular adaptation to hypoxia, we investigated whether hypoxia induces FoxM1 expression via the HIF pathway in HPASMCs. We suppressed the expression of HIF-1α and HIF-2α via their cognate siRNA, and exposed cells to normoxia or hypoxia, followed by the measurement of mRNA concentrations of HIF-1α, HIF-2α, and FoxM1. As shown in Figures 5A and 5B, these siRNAs specifically inhibited mRNA concentrations of HIF-1α and HIF-2α. Furthermore, we found that the knockdown of HIF-1α reduced the expression of FoxM1 mRNA under normal conditions, whereas the loss of HIF-2α prevented the hypoxia-mediated induction of FoxM1 (Figure 5C), suggesting that HIF-1α and HIF-2α have differential roles in the regulation of FoxM1. In addition, we suppressed FoxM1 using siRNA, and determined the effects of FoxM1 on HIF-1α and HIF-2α mRNA expression. As shown in Figures 6B and 6C, the knockdown of FoxM1 exerted little effect on the mRNA expression levels of HIF-1α and HIF-2α, further confirming that HIF is an upstream regulator of FoxM1.
Figure 5.
HIF-2α is required for the hypoxia-stimulated expression of FoxM1, whereas HIF-1α participates in FoxM1 regulation during normal conditions. HPASMCs were plated at equal density and transfected with siRNA against HIF-1α and/or HIF-2α (si1α and si2α, respectively) or scrambled siRNA (siNeg), and then exposed to normoxia or hypoxia for 24 hours. mRNA levels of HIF-1α (A), HIF-2α (B), and FoxM1 (C) were measured by quantitative RT-PCR. Results are shown as percent change over the group in which cells were transfected with negative control siRNA and exposed to normoxia (N/siNeg). Data are expressed as mean ± SEM (n = 4 in A, 9 in B, and 9 in C). *P < 0.05. **P < 0.01. #Significantly different between normoxic and hypoxic groups. n.s., no significance.
Figure 6.
Suppression of FoxM1 does not alter mRNA expression of HIF-1α or HIF-2α, but reduces the induction of aurora A kinase and cyclin D1 during hypoxia. HPASMCs were plated at equal density and transfected with FoxM1-specific siRNA (siFoxM1). Twenty-four hours after exposure to normoxia or hypoxia, mRNA levels of FoxM1 (A), HIF-1α (B), HIF-2α (C), aurora A kinase (D), and cyclin D1 (E) were determined by quantitative RT-PCR. Results are shown as percent change over the group in which cells were transfected with negative control siRNA and exposed to normoxia (N/siNeg). Data are expressed as mean ± SEM (n = 3in A, D, and E, 4 in B, and 6 in C). *P < 0.05. **P < 0.01. n.s., no significance.
FoxM1 Regulates Aurora A Kinase and Cyclin D1 Expression under Hypoxic Conditions
To determine the mechanism of FoxM1-induced cell proliferation, we suppressed the expression of FoxM1 by siRNA transfection, exposed cells to normoxia or hypoxia, and measured mRNA levels of aurora A kinase and cyclin D1, two genes known to promote cell cycle progression. As shown in Figures 6D and 6E, the suppression of FoxM1 prevented the hypoxia-induced expression of aurora A kinase and cyclin D1, suggesting that during hypoxia, FoxM1 may promote HPASMC proliferation via the induction of aurora A kinase and cyclin D1.
Discussion
Although FoxM1 was implicated in the unregulated growth of cancer cells, this is the first study, to our knowledge, to demonstrate that FoxM1 plays a critical role in the hypoxia-induced proliferation of PASMCs. We report here that induction of FoxM1 is necessary for the hypoxia-induced proliferation of PASMCs. Furthermore, our results suggest that HIF-2α is critical for FoxM1 induction during hypoxia.
Hypoxia has been established to induce HPASMC proliferation (Figures 1A and 1B) (4, 23), but the exact mechanisms involved in this process are largely undefined. We show that during hypoxia-induced cell proliferation, FoxM1, a transcription factor participating in cell cycle progression, is up-regulated at both the mRNA and protein levels (Figures 1C and 1D). Moreover, hypoxia increases the promoter activity of FoxM1, suggesting that hypoxia induces FoxM1 gene expression (Figure 1E). Because FoxM1 is a transcription factor, we reason that the induction of FoxM1 will result in its elevated activity and the up-regulation of its downstream targets. Indeed, hypoxia increases FoxM1 transcriptional activity and induces its downstream target Aurora A kinase gene expression (Figure 2). These data are consistent with previous reports that FoxM1 is active or induced in proliferative conditions, whereas it is quiescent in terminally differentiated cells (12, 15, 24, 25). Under normal conditions, PASMCs are differentiated, and during hypoxia or PAH, HPASMCs display a de-differentiated and proliferative phenotype (4, 23, 26). Thus, elevated concentrations of FoxM1 are associated with the proliferative phenotype of HPASMCs during hypoxia. Together, these findings confirm the notion that FoxM1 promotes cell proliferation and may play a role in the pathogenesis of PAH.
To further validate its role in the proliferation of HPASMCs, we silenced or overexpressed FoxM1 in HPASMCs. As shown in Figure 3, the knockdown of FoxM1 significantly prevents hypoxia-induced proliferation, whereas it slightly decreases cell proliferation during normoxia, suggesting that the inhibition of FoxM1 selectively prevents the growth of actively dividing cells but not quiescent cells. In addition, the overexpression of FoxM1 is not sufficient to induce the proliferation of HPASMCs during normoxia (Figure 4). Together, our results provide direct evidence that FoxM1 plays a differential role in proliferative and differentiated cells. Thus, drugs targeting FoxM1 to treat patients with PAH may have fewer side effects.
Because HIF is a known oxygen sensor and was implicated in PAH, we sought to determine whether HIF is the upstream regulator of FoxM1. We show that although HIF-1α is required for FoxM1 expression under normoxic conditions, HIF-2α is essential for the induction of FoxM1 during hypoxia, suggesting that FoxM1 expression is tightly regulated by HIF isoforms. This finding that both HIF isoforms regulate FoxM1 is important, because both HIF-1α and HIF-2α were implicated in the pathogenesis of PAH, and the reduction in HIF-1α or HIF-2α is protective against hypoxia-induced PAH (9, 10). Although both isoforms share some common target genes and functions, they vary in tissue distribution and response to different degrees of O2 concentration (9). For example, HIF-1α is universally expressed in all tissues types, whereas the expression of HIF-2α is more restricted in certain types of cells (27–30). Thus, we speculate that HIF-1α regulates basal FoxM1 expression in HPASMCs to maintain their normal proliferative potential, whereas HIF-2α is responsible for the induced proliferating phenotype. The knockdown of FoxM1 does not alter HIF expression, indicating that HIF is truly the upstream regulator of FoxM1.
To address how FoxM1 contributes to the hypoxia-induced proliferation of HPASMCs, we measured the expression levels of aurora A kinase and cyclin D1, two downstream targets that are known to regulate the cell cycle. We found that during hypoxia, aurora A kinase and cyclin D1 are up-regulated, and the knockdown of FoxM1 prevents their induction, suggesting that FoxM1 may promote HPASMC proliferation through aurora A kinase and cyclin D1. Further studies using animal models are warranted to understand more fully the significance of FoxM1 in the development of PAH, and to elucidate the molecular mechanisms of FoxM1 function.
Taken together, our results demonstrate for the first time, to the best of our knowledge, that the hypoxia-induced activation of FoxM1 is necessary for the proliferation of HPASMCs, and HIF-2α mediates this process. This finding is of particular significance, given the temporospatial regulation of FoxM1 by HIF. Because the induction of FoxM1 is a critical step in the hypoxia-induced proliferation of HPASMCs, therapies targeting FoxM1 may provide novel insights into the treatment of this disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Sekhar Reddy, Dr. Evgeny Pilipenko, Dr. Dev Singh, Dr. Joy Sarkar, and Laura Bach for helpful discussions and technical assistance.
Footnotes
This work was supported by National Heart, Lung and Blood Institute grants RO1HL059435 and RO1HL075187 (J.U.R.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0128OC on October 27, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Lechtzin N, Chami H, Girgis RE, et al. Outcomes of hospitalization for right heart failure in pulmonary arterial hypertension. Eur Respir J 2011;38:359–367 [DOI] [PubMed] [Google Scholar]
- 2.Gomberg-Maitland M, Dufton C, Oudiz RJ, Benza RL. Compelling evidence of long-term outcomes in pulmonary arterial hypertension? A clinical perspective. J Am Coll Cardiol 2011;57:1053–1061 [DOI] [PubMed] [Google Scholar]
- 3.Fishman AP. Hypoxia on the pulmonary circulation: how and where it acts. Circ Res 1976;38:221–231 [DOI] [PubMed] [Google Scholar]
- 4.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 2006;99:675–691 [DOI] [PubMed] [Google Scholar]
- 5.Kato M, Staub NC. Response of small pulmonary arteries to unilobar hypoxia and hypercapnia. Circ Res 1966;19:426–440 [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106 [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–732 [DOI] [PubMed] [Google Scholar]
- 8.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006;441:437–443 [DOI] [PubMed] [Google Scholar]
- 9.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 1999;103:691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor–2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 2003;111:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, Major ML, Gusarova GA, Yoder HM, Costa RH, et al. The forkhead box M1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res 2006;66:2153–2161 [DOI] [PubMed] [Google Scholar]
- 12.Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, Lau LF, Costa RH, Raychaudhuri P. FoxM1: a critical regulator of oxidative stress during oncogenesis. EMBO J 2009;28:2908–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu QF, Liu C, Tai MH, Liu D, Lei L, Wang RT, Tian M, Lu Y. Knockdown of FoxM1 by siRNA interference decreases cell proliferation, induces cell cycle arrest and inhibits cell invasion in MHCC-97H cells in vitro. Acta Pharmacol Sin 2010;31:361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang IC, Chen YJ, Hughes DE, Ackerson T, Major ML, Kalinichenko VV, Costa RH, Raychaudhuri P, Tyner AL, Lau LF. FoxM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness. J Biol Chem 2008;283:20770–20778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia LM, Huang WJ, Wang B, Liu M, Zhang Q, Yan W, Zhu Q, Luo M, Zhou ZZ, Tian DA. Transcriptional up-regulation of FoxM1 in response to hypoxia is mediated by HIF-1. J Cell Biochem 2009;106:247–256 [DOI] [PubMed] [Google Scholar]
- 16.Kalin TV, Wang IC, Meliton L, Zhang Y, Wert SE, Ren X, Snyder J, Bell SM, Graf L, Jr, Whitsett JA, et al. Forkhead box M1 transcription factor is required for perinatal lung function. Proc Natl Acad Sci USA 2008;105:19330–19335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box M1 transcription factor is essential for embryonic development of pulmonary vasculature. J Biol Chem 2005;280:22278–22286 [DOI] [PubMed] [Google Scholar]
- 18.Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest 2006;116:2333–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ustiyan V, Wang IC, Ren X, Zhang Y, Snyder J, Xu Y, Wert SE, Lessard JL, Kalin TV, Kalinichenko VV. Forkhead box M1 transcriptional factor is required for smooth muscle cells during embryonic development of blood vessels and esophagus. Dev Biol 2009;336:266–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem 2003;278:37888–37894 [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The forkhead box M1B transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci USA 2002;99:16881–16886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, Yoder HM, Kiyokawa H, Kaestner KH, Costa RH. The mouse forkhead box M1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev Biol 2004;276:74–88 [DOI] [PubMed] [Google Scholar]
- 23.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J 2007;30:364–372 [DOI] [PubMed] [Google Scholar]
- 24.Krupczak-Hollis K, Wang X, Dennewitz MB, Costa RH. Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box M1B. Hepatology 2003;38:1552–1562 [DOI] [PubMed] [Google Scholar]
- 25.Leung TW, Lin SS, Tsang AC, Tong CS, Ching JC, Leung WY, Gimlich R, Wong GG, Yao KM. Over-expression of FoxM1 stimulates cyclin B1 expression. FEBS Lett 2001;507:59–66 [DOI] [PubMed] [Google Scholar]
- 26.Schultz K, Fanburg BL, Beasley D. Hypoxia and hypoxia-inducible factor–1alpha promote growth factor–induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 2006;290:H2528–H2534 [DOI] [PubMed] [Google Scholar]
- 27.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest 2007;117:862–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 2003;23:9361–9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandel NS, Simon MC. Hypoxia-inducible factor: roles in development, physiology, and disease. Cell Death Differ 2008;15:619–620 [DOI] [PubMed] [Google Scholar]
- 30.Patel SA, Simon MC. Biology of hypoxia-inducible factor–2alpha in development and disease. Cell Death Differ 2008;15:628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.