Abstract
Extracellular nucleotides and nucleosides promote a vast range of physiological responses, via activation of cell surface purinergic receptors. Virtually all tissues and cell types exhibit regulated release of ATP, which, in many cases, is accompanied by the release of uridine nucleotides. Given the relevance of extracellular nucleotide/nucleoside-evoked responses, understanding how ATP and other nucleotides are released from cells is an important physiological question. By facilitating the entry of cytosolic nucleotides into the secretory pathway, recently identified vesicular nucleotide and nucleotide–sugar transporters contribute to the exocytotic release of ATP and UDP-sugars not only from endocrine/exocrine tissues, but also from cell types in which secretory granules have not been biochemically characterized. In addition, plasma membrane connexin hemichannels, pannexin channels, and less-well molecularly defined ATP conducting anion channels have been shown to contribute to the release of ATP (and UTP) under a variety of conditions.
Keywords: ATP release, Extracellular nucleotides, UDP-sugars, Exocytosis, VNUT, Connexins, Pannexins
Introduction
At least 19 nucleotide/nucleoside-activated cell surface purinergic receptors exist in the human genome, and remarkably broad and varied physiological responses occur downstream of purinergic receptor activation [1]. Purinergic receptors comprise three subfamilies (Table 1): (1) ATP-gated ion channel P2X receptors [2]; (2) G protein-coupled P2Y receptors, which are activated by ATP, ADP, UTP, UDP, and UDP-sugars [3]; and (3) G protein-coupled adenosine receptors [4]. Responses to nucleotides and nucleosides are observed in the CNS and all peripheral tissues, indicating that nucleotide release underlies many important physiological processes. Virtually all tissues and cell types exhibit regulated release of ATP, which, in many cases is accompanied by the release of UTP and UDP-sugars (reviewed in [5–10]). Ubiquitously distributed cell surface and secreted ecto-enzymes control nucleotide actions by catalyzing their breakdown and interconversion and providing a mechanism for the formation of ADP, UDP, and adenosine [5, 11–13].
Table 1.
Agonists acting at functionally defined purinergic receptors (the most potent naturally occurring agonists acting on human P2Y, P2X, and adenosine receptors are indicated)
| Agonist | Receptor |
|---|---|
| ATP | P2X1-P2X7, P2Y2, P2Y11 |
| ADP | P2Y1, P2Y12, P2Y13 |
| UTP | P2Y2, P2Y4 |
| UDP | P2Y6, P2Y14 |
| UDP-glucose | P2Y14 |
| Adenosine | A1, A2A, A2B, A3 |
Given the relevance of extracellular nucleotide/nucleoside-evoked responses, understanding how ATP and other nucleotides are released from cells is an important physiological question. The initial observation that ATP is stored within (and released from) secretory granules in neuroexcitatory tissues [14] led to the perception that nucleotide release occurs, predominantly, via exocytotic pathways. However, conductive/transport mechanisms of nucleotide release also exist (and sometimes co-exist with exocytotic mechanisms) in many tissues and cell types. Progress in identifying components of the vesicular and conductive nucleotide release mechanisms is discussed in this review article.
Cellular release of nucleotides from the secretory pathway
Exocytotic ATP release from neuroendocrine and exocrine cells
It has been long known that ATP, neurotransmitters, and other extracellular mediators are packed in specialized granules in neuroendocrine tissues and circulating platelets and released from cells via regulated exocytosis [14–23]. For example, catecholamines, serotonin, and ATP are transported and co-stored in chromaffin granules, using the electrochemical potential (Δψ) and a pH gradient provided by the V-type H+-ATPase (V/H+-ATPase) of the granule membrane [19, 20, 24–27]. Stimulation of chromaffin cells by preganglionic sympathetic neurons results in granule transport along the filaments of the cytoskeleton network to the subplasma membrane compartment, fusion of the granule with the plasma membrane, and release of contents (e.g., ATP) into the extracellular space, in a Ca2+-dependent manner [28]. In addition, release of ATP has been associated with regulated exocytosis of specialized granules in pancreatic acinar cells, goblet epithelial cells, mast cells, pancreatic β islets, and other exocrine/endocrine tissues [13].
The process of regulated exocytosis encompasses the coordinated action of the SNARE [soluble N-ethyl maleimide-sensitive factor attachment protein (SNAP) receptor] family of proteins (Rothman, 1994). Accordingly, vesicle associated v-SNARE proteins pair with corresponding members of the t-SNARE family of proteins located on the target membrane to determine the specificity of vesicle targeting, docking, and fusion. Major components of the SNARE machinery include the t-SNAREs syntaxin and SNAP-25 and the v-SNARE synaptobrevin, also known as VAMP. The synaptic fusion complex is initiated by formation of a ternary (synaptobrevin, syntaxin, SNAP-25) complex, but additional proteins, including Rabs and Muncs, function at steps up-stream of the SNARE complex formation. During the late step of neurotransmitter release, syntaxin/SNAP 25 binds to the vesicle protein synaptotagmin, a Ca2+ sensor, triggering fusion of the presynaptic vesicle with the plasma membrane [29–33].
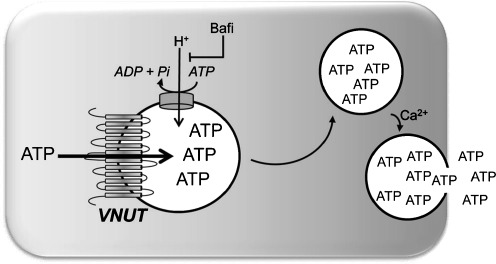
In many cells, evidence for exocytotic mechanisms of ATP release have been largely based on the inhibitory effect of Ca2+ chelators (e.g., BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid]) and reagents that disrupt the secretory pathway, e.g., cytoskeletal venoms (cytochalasins, nocodazole), Golgi inhibitors (brefeldin A), and tetanus neurotoxin (which cleaves synaptobrevin/VAMPII). However, these reagents do not discern between vesicular ATP release and vesicular insertion of ATP transporters/channels to the plasma membrane. An additional (although indirect) probe for the involvement of secretory pathways in the release of ATP is bafilomycin A1, a well-characterized inhibitor of the V/H+-ATPase that provides the electrochemical gradient for the uptake of ATP (and other molecules) into vesicles/granules [26] (Fig. 1).
Fig. 1.
Vesicular nucleotide transporter. Schematic representation of SLC17A9/VNUT displaying its predicted 12 transmembrane-spanning domains. VNUT transports ATP to the lumen of secretory granules, using the electrochemical gradient provided by the bafilomycin A1 (Bafi)-sensitive proton pump V-ATPase. In specialized tissues, ATP containing granules are secreted via Ca2+-regulated exocytosis
The molecular identification of the transporter that transfers cytosolic ATP into secretory granules has provided a more conclusive approach to associate ATP release with vesicle/granule exocytosis. Solute carrier (SLC) 17A9, a member of the SLC17 family of ion transporters [34–36], was recently de-orphaned and characterized as a vesicular nucleotide transporter (thereafter named VNUT) that contributes to the release of ATP from a variety of tissues. SLC17A9 is predicted to encode a 430 residue-long protein with 12 transmembrane domains and N and C termini located in the cytosol (Fig. 1) [37]. Sawada et al. elegantly demonstrated that liposomes reconstituted with purified, recombinant SLC17A9 exhibited pharmacological and biochemical properties similar to the ATP transporter endogenously expressed in chromaffin cells, e.g., Δψ-driven Cl−-dependent ATP transport activity that was inhibited by 4,4′-diisothiocyanostilbene-2,2′-disulfonate (DIDS) [37]. Furthermore, strong SLC17A9 immunoreactivity that co-localized with synaptotagmin (a secretory granule marker) was observed in chromaffin-like PC12 cells. Knocking down SLC17A9 by small interfering RNA (siRNA) decreased KCl-triggered ATP release in PC12 cells [37]. In situ hybridization studies indicated that SLC17A9 transcripts are widely expressed in the brain, with particularly high levels of expression in regions of the hippocampus known to display purinergic neurotransmission [35].
Relatively high levels of VNUT mRNA and/or VNUT immunoreactivity are present in the stomach, intestine, liver, lung, skeletal muscle, thyroid, spleen, blood cells, chemosensory epithelial cells, and keratinocytes [35, 37–40], suggesting that VNUT-mediated nucleotide entry into the secretory pathway is not restricted to the brain and neuroendocrine tissues. Indeed, VNUT has emerged as a widely expressed nucleotide transporter responsible for the uptake of ATP into secretory granules and vesicles, consequently, contributing to the release of ATP (and likely other nucleotides) from the secretory pathway in a variety of physiologically relevant conditions.
In the pancreatic acini, cholinergic stimulation causes release of ATP onto the lumen [23], potentially leading to up-regulation of secretin-evoked bicarbonate and fluid secretion in pancreatic ducts [41]. The fact that (1) ATP was released concomitantly with digestive enzymes during stimulation of the pancreatic acini, and (2) zymogen granules (ZG) can be fluorescently labelled with quinacrine (which labels ATP-rich compartments, acid compartments, and secretory organelles [42–46]) and MANT-ATP, suggested that ATP is stored into (and released from) ZG [23]. This hypothesis was recently confirmed by directly measuring ATP uptake into isolated ZG [47]. ATP transport to ZG exhibited kinetics and pharmacological properties consistent with VNUT-mediated transport. That is, ATP uptake was inhibited by bafiloimycin A1 and stimulated by Cl−, and exhibited Km values, pH dependence, and susceptibility to inhibitors similar to VNUT [47]. Western blot analysis indicated strong VNUT immunoreactivity in isolated zymogen granules [47].
In the airways, extracellular nucleotides are key components of the mucociliary clearance machinery that traps and removes microorganisms from the lung. Nucleotides/nucleosides within the airway surface liquid promote mucin secretion, via activation of P2Y2 receptors in mucous (goblet) cells, and stimulate ciliary beating and mucin hydration via A2B and P2Y2 receptor activation in ciliated cells [48]. Since goblet cells lack ion transport activities necessary for the proper hydration of released mucins, understanding the mechanisms by which mucin-secreting cells signal toward ciliated cells to promote water transport into the airway lumen is highly relevant. Recent studies demonstrated that mucin secretion provides a pathway for ATP release. Real-time confocal microscopic analyses of goblet-like Calu-3 cells revealed that mucin granules labeled with the fluorophore FM 1–43 (a probe for vesicle exocytosis) or quinacrine underwent Ca2+-regulated exocytosis, exhibiting kinetics overlapping with that of ATP release. Ca2+-promoted ATP release was markedly reduced by bafilomycin A1 [49]. Primary cultures of human bronchial epithelial (HBE) cells, which were induced to develop goblet cell metaplasia by infection with respiratory syncytial virus or treatment with interleukin-13, displayed enhanced mucin secretion, which was accompanied by increased rates of ATP release. Intracellular calcium chelation or disruption of the secretory pathway decreased both mucin secretion and ATP release in goblet cell metaplastic HBE cell cultures [50]. Furthermore, mucin granules isolated via density gradients were enriched with ATP, ADP, and AMP. A marked increase of extracellular ADP/AMP accumulation accompanied that of ATP on Calu-3 cells stimulated with the mucin secretagogue thrombin [51]. These findings, together with the observation that SLC17A9/VNUT transcripts are abundantly expressed in Calu-3 and goblet cell-rich HBE cell cultures (Sesma J and Lazarowski E, unpublished), support the hypothesis that goblet cells utilize a mechanism of ATP storage in mucin granules similar to that described above for zymogen and chromaffin granules.
The potential involvement of VNUT in the uptake of nucleotides into specialized granules in cells in which ATP release via Ca2+-regulated exocytosis has been documented, e.g., mucin granules in goblet cells (see above), insulin granules in pancreatic β cells [52–56], histamine granules in mast cells [22, 57–59], and dense core granules in platelets [18] has yet to be assessed.
VNUT-dependent ATP release from orphan vesicles
VNUT has emerged as a useful tool to assess the involvement of the secretory pathway in the release of ATP from tissues and cell types in which specialized granules have not been biochemically characterized. Activation of T cell receptors (TCR) results in rapid and robust release of ATP from lymphocytes, leading to P2X receptor-evoked responses, e.g., cell proliferation [60–63]. Initial studies suggested that TRC-promoted ATP release encompasses a conductive pathway (see Pannexins, further below), but a recent report by Tokunaga et al. [38] suggested that the secretory pathway represents an additional source of ATP release in activated T cells. TCR-activated T lymphocytes and T cell-derived Jurkat lymphoma cells displayed robust Ca2+-dependent ATP release that was reduced (although not abolished) by inhibitors of vesicle trafficking/exocytosis and by bafilomycin A1. Knocking down SLC17A9 (via shRNA) markedly reduced TCR-evoked ATP release from Jurkat cells. ATP release from cholangiocytes contributes to bile formation and stimulation of biliary secretion. Hypotonic cell swelling of human Mz-ChA-1 cholangiocarcinoma cells resulted in abrupt increase in exocytosis, which was accompanied by increased rates of ATP release [64]. Cholangiocytes loaded with quinacrine exhibited a subpopulation of fluorescently labeled small (<0.99 μm) vesicles susceptible to hypotonic cell swelling-promoted exocytosis. VNUT siRNA decreased the number of quinacrine-labeled small vesicles and reduced cell swelling-evoked ATP release [65].
In sum, T lymphocytes and cholangiocytes store and release ATP via Ca2+-regulated exocytotic mechanisms similar to those described above with secretory cells. It will now be important to assess the contribution of VNUT to the release of ATP in other tissues in which evidence of exocytotic release of ATP has been reported, e.g., astrocytes [66–70], hepatocytes [64, 71, 72], alveolar A549 and intestinal 407 epithelial cells [73–77], osteoblasts [78], and esophageal keratinocytes [40]. It will be also important to biochemically and functionally identify the ATP-rich compartment(s) contributing to VNUT-dependent ATP release from non-endocrine/exocrine cell types exhibiting vesicular release of ATP.
Release of UDP-sugars from the secretory pathway
The identification of the P2Y14-R as a Gi-coupled receptor that is potently activated by UDP-glucose and other UDP-sugars [79] suggested that, in addition to their vital role in metabolic processes, nucleotide–sugars are released from cells to play extracellular signaling roles. P2Y14-R is functionally expressed in brain glia and peripheral leucocytes, including neutrophils, lymphocytes, and mast cells [80–83], suggesting that UDP-sugar release contributes to inflammatory responses. Quantification of endogenous UDP-glucose in extracellular solutions indicated that UDP-glucose release accompanies the release of ATP under resting and stimulated conditions in a number of cells [49, 84, 85]. Similarly to ATP, enhanced release of UDP-glucose has been associated with mucin secretion from airway epithelial goblet cells [49, 50]. Notably, relatively high concentrations of UDP-glucose (i.e., capable of promoting P2Y14 receptor activation) were observed in lung secretions from patients with cystic fibrosis (CF), a chronic lung disease characterized by progressive goblet cell metaplasia, mucus plugs, and neutrophil inflammation [86].
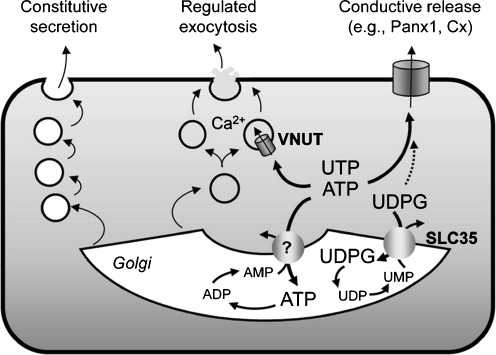
UDP-sugars are abundantly present in the lumen of the ER and Golgi apparatus, serving as sugar donors in glycosylation reactions. The entry of UDP-sugars to the ER/Golgi is mediated by nucleotide–sugar transporters that utilize luminal UMP as antiporter substrate. UDP-sugar/UMP translocators belong to the family of SLC35 ER/Golgi nucleotide–sugar transporters [87]. Since UDP-sugars entering the lumen of the ER/Golgi are not transported back to the cytosol, they may be retained within vesicles and exocytoticaly released as co-cargo with exported glycoproteins [8]. Sesma et al. tested this hypothesis by examining the effect of removing/overexpressing endoplasmic reticulum (ER)/Golgi-resident UDP-sugar transporters on the release of their cognate substrate. Deletion of the YEA4 gene, which encodes the UDP-N-acetylglucosamine transporter1 in yeast, resulted in decreased release of this UDP-sugar; complementing the mutant strain with the WT YEA4 gene re-established the UDP-N-acetylglucosamine release function [86]. Furthermore, human bronchial epithelial 16HBE14o− cells stably overexpressing SLC35D2 (a human Golgi UDP-N-acetylglucosamine transporter also known as HFRC1) displayed increased rate of brefeldin A-sensitive mucosal release of UDP-N-acetylglucosamine [86]. These results indicate that, by regulating the entry of nucleotides to the ER/Golgi, SLC35 UDP-sugar transporters contribute to the cellular release of their cognate substrates via the constitutive pathway (Fig. 2). It is worth noting that ER/Golgi ATP/AMP trasnslocator activities have been described [88, 89], but the molecular identity of the putative ER/Golgi ATP transporter (different from VNUT) has remained elusive (Fig. 2).
Fig. 2.
Potential pathways for nucleotide release. Several candidate ATP conducting channels, including Panx1 and connexins (Cx) efflux cytosolic ATP and UTP out of the cells. VNUT transports ATP into dense-core granules and vesicles competent for Ca2+ regulated exocytosis. UDP-glucose (UDPG) and possibly ATP, enter the secretory pathway via ER/Golgi-resident SLC35-like transporters, using UMP and AMP as antiporter substrates, respectively. ER/Golgi nucleotides serving in glycosylation and energy-driven reactions and their luminal metabolites are released as residual cargo molecules via the constitutive secretory pathway
Conductive release of nucleotides
In many cells, nucleotide release occurs in the absence of apparent vesicle exocytosis and is not or only partially affected by bafilomycin A1 and by inhibitors of vesicle trafficking/fusion. The multidrug resistance (MDR)-1 protein, the CF transmembrane conductance regulator (CFTR) Cl− channel, and the mitochondrial voltage-dependent anion channel-1 (VDAC-1) were initially proposed as ATP release pathways or facilitators of ATP release in various cell types, but the involvement of these proteins in an ATP release function could not be corroborated by a number of studies [90–96]; for a critical review on these proposed pathways, see [8, 97].
More recently, two classes of plasma membrane channels have been associated with an ATP conductive activity: (1) Cl− channels such as maxi anion channels, volume-regulated ion channels, and tweety; and (2) pore forming connexins, pannexins, and P2X7 receptors.
ATP conducting Cl− channels
An ATP-conductive large conductance anion channel was first described in murine mammary C127i cells and subsequently found in a variety of tissues and cell types, including cardiomyocytes, astrocytes, and kidney macula densa (reviewed in [97, 98]). Maxi anion channels exhibit large single-channel conductance of 300–400 pS, have a wide pore (effective radius of ~1.3 nm), discriminate between Na+ and halides, and allow the passage of small organic anions, including signaling molecules such as glutamate, ATP, and UTP [97–100]. Maxi anion channels are activated by osmotic cell swelling, ischemia/hypoxia, and in response to excision of the membrane patch, and are inhibited by Gd3+ and by a number of anion channel blockers of relative selectivity, such as DIDS, NPPB [5-nitro-2-(3-phenylpropylamino)benzoic acid], SITS (4-acetamido-4′-isothiocyanatostilben-2,2′-disulfonate), and DPC (diphenylamine-2-carboxylate), but not by glybenclamide [98]. The involvement of maxi ion channels in the cellular release of ATP is supported by the observation that cells exhibiting maxi ion channel activity display osmotic cell swelling-evoked release of ATP, in a SITS-, NPPB-, and Gd3+-sensitive manner [98]. The molecular identity of the maxi anion channel is not known.
Volume-regulated anion channels (VRAC) are predicted to be permeable to organic anions, including amino acids and ATP [97, 101–104]. VRAC Cl− channel activity is activated in response to osmotic cell swelling and inhibited by a broad spectrum of reagents, including glibenclamide, verapamil, tamoxifen, fluoxetine, nordihydroguaiaretic acid, dideoxyforskolin, niflumic acid, quinine, NPPB, DIDS, and SITS [101, 105]. VRAC inhibitors suppressed ATP release in aortic endothelial cells [102] and 1321N1 astrocytoma cells [105], but not in Intestine 407 cells [106] or alveolar or airway epithelial cells [73]. Recently, bradykinin-stimulated astrocytes exhibited VRAC-like activity that was associated with the release of glutamate but not ATP [107]. The rather low selectivity of the reagents used to inhibit VRAC activity and the fact that the molecular identity of VRAC is not known have prevented unambiguously assessing the putative ATP release activity of this channel.
TTYH1, TTYH2, and TTYH3, the human homologues to the Drosophila flightless gene tweety are predicted to encode large-conductance Cl− channels with properties similar to the maxi anion channel described above [108]. The potential contribution of TTYH1 and TTYH2 to ATP release has not been investigated. However, two splice variants of TTHY1 (TTYH1-SV and TTYH1-E) failed to produce maxi anion channel currents in excised patches when expressed in null cells [98]. Interestingly, TTHY3 exhibits Ca2+-activated Cl− channel activity [108], and has been recently implied in the release of ATP from chemo-attractant-stimulated neutrophils; knocking down TTHY3 via shRNA markedly decreased ATP release from HL60 cells stimulated with the formyl-bacterial peptide fMLP [109].
Connexins
Connexin (Cx) subunits are the building blocks of vertebrate gap junction channels [110–113]. More than 20 human connexin isoforms have been identified, with predicted size of individual species ranging from 23 to 62 kDa and, accordingly, referred as to Cxn, where n denotes the molecular weight (e.g., Cx30, Cx43). Connexin subunits display a predicted structure of four transmembrane domains, with N and C termini located in the cytosol (however, recent X-ray structure analysis of Cx26 suggested that the N terminus of this proteins is embedded within the plasma membrane [114]). Hemichannel assemblies composed of connexin subunits are known as connexons. Connexons from two adjacent cells align and bind to each other, forming an intercellular gap that allows the passage of small cytosolic molecules between cells. In addition, some connexons may localize to non-junctional regions of the plasma membrane, thus forming functional plasma membrane hemichannels that are not involved in cell contact. Most hemichannels exhibit permeability to ATP and small dyes (e.g., propidium and ethidium iodide, carboxyfluorescein, YoPro, and Lucifer Yellow). Opening of connexins can be induced by membrane depolarization, typically in the 40–60 mV range. Connexin hemichannels are non-selectively inhibited by α-glycyrrhetinic acid, flufenamic acid, carbenoxolone (20–100 μM range), alkanols, and synthetic peptides that mimic segments of connexin extracellular loops [110, 115]. Some connexin hemichannels are activated by lowering the extracellular Ca2+ concentration, likely due to Ca2+-induced conformational changes leading to pore closure [116].
The involvement of connexin hemichannels in the release of ATP was first suggested by Nedergaard and co-workers, who observed that C6 rat glioma cells expressing human Cx32 or Cx43 (but not WT C6 cells), displayed increased release of ATP in response to extracellular Ca2+ removal [117]. Lowering the extracellular Ca2+ concentration promoted the uptake of the hemichannel probe propidium iodide, as well as ATP release, in astrocytes, endothelial cells, bronchial epithelial cells, and Cx43-overexpressing C6 (Cx43-C6) cells [118]. Cx43-C6 cells exhibited ATP release events (assessed by real-time bioluminescence imaging) that temporally coincided with hemichannel opening; excised Cx43-expressing patches displayed ATP conductance [119]. Romanello et al. [120] suggested that the involvement of Cx43 in the release of ATP triggered by lowering the extracellular Ca2+ concentration may be cell specific, since Cx43 overexpression did not affect this response in human HOBIT osteoblastic cells.
De Vuyst et al. [121, 122] reported that controlled increase of intracellular Ca2+ resulted in enhanced ATP release and propidium iodide uptake in Cx32- and Cx43-overexpressing (but not WT) cells. Based on this and other considerations, these authors proposed that intracellular Ca2+ triggers connexin hemichannel opening via multiple signaling steps, including Ca2+/calmodulin-dependent pathways [121, 122].
Connexin subtype-specific knockdown approaches have been recently shown to reduce the release of ATP from various cell types. For example, cultured glomerular endothelial cells displayed ATP release-dependent Ca2+ waves in response to mechanical stress. Both ATP release and Ca2+ wave propagation were markedly reduced by Cx40 siRNA, suggesting that interendothelial calcium signaling in the juxtaglomerular vasculature is mediated by Cx40-dependent ATP release and subsequent activation of purinergic receptors [123]. ATP release from cerebellar granule neurons exposed to depolarizing conditions was reduced after knocking down connexin 36 expression via siRNA [124].
Connexin-mediated ATP release has been linked to (patho)physiological responses in a variety of tissues. Using an in vitro model of hypoxia in the ventral surface of the medulla oblongata (VMS), Huckstepp et al. illustrated that CO2-triggered ATP release was accompanied by the uptake of hemichannel dyes and inhibited by connexin hemichannel inhibitors. Cx26 is expressed in astrocytes in areas of VMS displaying CO2-dependent dye uptake, suggesting that Cx26-mediated release of ATP contributes to central respiratory chemosensitivity ([125] and see comment in [126]). The involvement of connexin hemichannels in the release of ATP from astrocytes during ischemia and epilepsy has been recently reviewed [127].
Connexin-deficient mice have been useful tools to associate connexin-mediated ATP release with specific phenotypes. In the inner ear, ATP-evoked Ca2+ wave propagation has been linked to noise-induced hearing loss and development of hair cell-afferent synapses. Ca2+ wave propagation in cochlear organotypic cells reflects hemichannel opening-mediated ATP release [128]. Cochlear organotypic cultures from Cx26 (tissue-targeted)- or Cx30-deficient (but not pannexin 1-deficient) mice exhibited reduced ATP release, impaired Ca2+ wave propagation, and reduced dye efflux (from calcein-loaded cultures), suggesting that Cx26 and Cx30 operate as ATP conduits in the inner ear [128].
Cx30-deficient mice exhibited impaired ATP release from epithelial cells in the distal nephron. Microperfused cortical collecting ducts from WT mice displayed flow- and hypotonicity-evoked ATP release (from intercalated cells), which were impaired in Cx30−/− animals [129]. Urine output and urinary Na+ secretion in response to increased arterial pressure (following ligating the distal aorta) were greater in WT relative to Cx30−/− mice. Cx30−/− mice exhibited salt-sensitive elevation in mean arterial pressure. The data suggest that ATP release via Cx30 controls salt and water reabsorption at the collecting ducts, regulating pressure natriuresis and diuresis [129]. In another study, increasing Na+ intake resulted in enhanced urinary ATP secretion, which was robust in WT mice but modest in Cx30−/− animals. Conversely, epithelial Na+ channel (ENaC) activity was greater in Cx30-deffcient animals, likely reflecting decreased ATP release-promoted P2Y2 receptor-evoked ENaC inhibition [130].
ATP release in response to inflammatory mediators is a fundamental mechanism required for neutrophil activation and immune defense [109, 131–133]. Neutrophils isolated from Cx43 knockout mice exhibited impaired release of ATP in response to leukotriene B4 [132]. In the same study, ATP release from human neutrophils exposed to the formyl peptide fMLP was inhibited by connexin inhibitors and Cx43 blocking peptides [132]. However, the involvement of Cx43 in the release of ATP from human neutrophils has been recently challenged [109].
Phenotypes associated with connexin gene deletion or knockdown should be interpreted with caution. Cx43 may form complexes with membrane receptors, cell signaling molecules, scaffolding proteins, cytoskeleton components, and other proteins independently of Cx43 hemichannel activity [134–138]. Deletion of genes encoding Cx32, Cx36, or Cx43 affected the transcription of unrelated genes [139, 140]. Nevertheless, physiological, pharmacological, and genetic evidence support the hypothesis that connexin hemichannels contribute to ATP release in a variety of cell types.
Pannexins
Pannexins comprise three gene products, pannexin 1 (Panx1), Panx2, and Panx3. Pannexins are not related to connexins, but pannexin and connexin proteins share a predicted four transmembrane spanning topology. Like connexins, six2 pannexin subunits are predicted to form a channel (pannexon), are permeable to small dyes, and exhibit ATP conductance [110]. Unlike connexins, pannexons in mammalian cells do not readily assemble into the plaque-like ensembles that typify gap junctions; glycosylation of pannexin subunits likely prevents the docking between pannexons on adjacent cells [142, 143]. Pannexin gating is not affected by external Ca2+ [144]. In addition, while connexins open at supraphysiological depolarization conditions (>+40 mV), Panx1 opens at normal resting potentials [110, 145]. Pannexin inhibitors (of relative selectivity) include carbenoxolone (<10 μM), DIDS, NPPB, and probenecid [146, 147], as well as peptides mimicking segments of the extracellular loops of Panx1 [115, 146, 148].
Dahl and co-workers provided initial evidence that Panx 1 functions as an ATP channel by illustrating that expression of human Panx1 in Xenopus oocytes resulted in large unitary conductance channel activity that exhibited ATP permeability [149]. Conditions favoring Panx1 channel opening (high K+) enhanced ATP release from Panx1-expressing oocytes [149]. This group also showed that human erythrocytes exhibited mechanosensitive large conductance channel activity consistent with Panx1, and that red cells exposed to either high K+ or hypotonic stress displayed enhanced ATP release and dye uptake, in a carbenoxolone-sensitive manner [150]. A number of studies followed indicating that pharmacological inhibitors of Panx1 reduced ATP release in a broad range of cells, including lung epithelial cells [151–153], hypoxic red cells [154–156], bovine ciliary epithelial cells [157], retina and trabecular meshwork (TM5) cells [158, 159], skeletal muscle [160], taste bud cells [161], T lymphocytes [61, 62, 162], and human neutrophils [109]. Panx1 knockdown (siRNA or shRNA) reduced nucleotide release in hypotonic-stress-stimulated airway epithelial cells [151, 152], stretched cardiomyocytes [163], apoptotic T lymphocytes [162] and T cells undergoing HIV infection [164], TM5 cells [159], and astrocytes [165]. Conversely, overexpression of Panx1 resulted in enhanced release of ATP from apoptotic Jurkat cells [162] and hypotonically swollen human embryonic kidney (HEK) 293 cells [159]. Panx1 siRNA and Panx1-GFP overexpression reduced and increased, respectively, phenylephrine-induced constriction of thoracodorsal resistance arteries, a response considered to be mediated by released ATP [166].
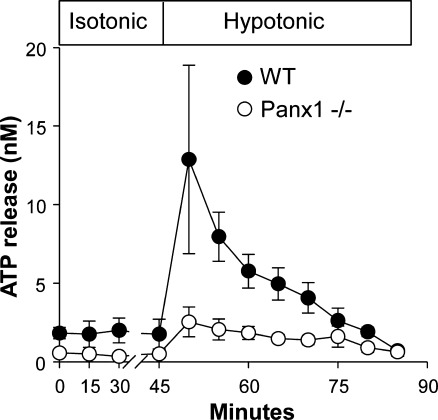
The involvement of Panx1 in ATP release in airway epithelia, erythrocytes, and thymocytes was further illustrated using Panx1−/− mouse models. Primary cultures of human airway epithelial cells exhibited hypotonic cell swelling-promoted ATP release and propidium iodide uptake with overlapping kinetics [152] and sensitivity to Panx1 inhibitors and Panx1 shRNAs [151, 152]. Utilizing a perfusion approach to assess ATP levels in tracheal luminal secretions under controlled flow conditions, Seminario-Vidal et al. showed that ATP release from WT tracheas increased up to sixfold following a brief exposure to hypotonicity. In contrast, Panx1−/− animals exhibited impaired hypotonicity-evoked ATP release (Fig. 3 and [152]). Furthermore, primary cultures of murine tracheal epithelial cells from Panx1−/− mice exhibited both impaired hypotonic stress-evoked dye uptake [152] and decreased ATP release (Okada S. and Lazarowski E.R, unpublished).
Fig. 3.
Reduced ATP release from Panx1−/− tracheas. Tracheas excised from WT and Panx1−/− littermates were perfused with isotonic buffer for 45 min and then exposed to hypotonic solutions (from [152])
Qiu et al. [167] reported that murine red blood cells release ATP when exposed to hypotonic K+ solutions, and that this release was reduced in cells isolated from Panx1−/− mice. Interestingly, ATP release pathways additional to Panx1 are present in murine erythrocytes since (1) significant residual ATP release was observed in Panx1−/− animals challenged with hypotonic K+, and (2) iloprost-promoted ATP release from erythrocytes was not different between wild-type and Panx1−/− mice.
Ravichandran and co-workers demonstrated that apoptotic T-lymphocytes release ATP and UTP as “find me” signals to recruit phagocytes via activation of P2Y2 receptors in monocytes and macrophages [168]. They also showed that ATP and UTP release from apoptotic T cells was markedly reduced by Panx1 inhibitors and Panx1 siRNA ([162] and see below). Qu et al. verified and expanded these observations by demonstrating that thymocytes from Panx1−/− mice displayed deficient dye uptake and impaired ATP release during early stages of apoptosis (induced by dexamethasone). Consistent with the involvement of Panx1 in the release of nucleotide “find me” signals, supernatants from dexamethasone-treated WT (but not Panx1−/−) thymocytes promoted macrophage chemotaxis [169].
Regulation of Panx1-mediated ATP release in non-apoptotic cells
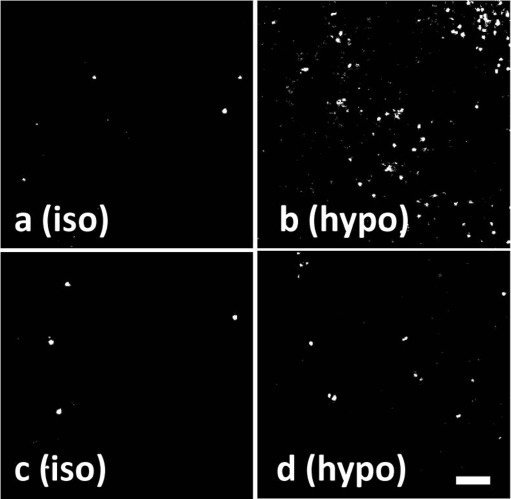
A body of evidence supports the involvement of Panx1 in the release of ATP from living cells under a broad spectrum of physiologically relevant conditions. It could be argued that opening of such as a large, non-selective channel as Panx1 may collapse the ion gradients, resulting in cell death. Therefore, an important question is whether physiological (non-apoptotic) responses associated with Panx1 pore opening in mammalian living cells reflect reversible processes. Measurements of dye uptake in cells undergoing Panx1-mediated ATP release are consistent with the “reversible” hypothesis. That is, airway epithelial cells exposed to hypotonicity exhibited enhanced propidium iodide uptake only if the dye was added concurrently with the hypotonic challenge, i.e., no changes in dye uptake were observed if the dye was added to cells after isotonic conditions were restored [152] (Fig. 4). Thus, opening of the propidium iodide permeable channel/pore during hypotonic cell swelling reflected a transient (i.e., regulated) phenomenon rather than irreversible plasma membrane damage.
Fig. 4.
Dye uptake in human bronchial epithelial cells reflects a reversible phenomenon. Representative images of propidium iodide uptake in primary cultures of human bronchial epithelial cells that were incubated for 5 min with isotonic (iso) or hypotonic (hypo) solutions in the presence of propidium iodide (a, b) or in its absence (c, d). Isotonicity was restored in d, and propidium iodide subsequently added to c and d for additional 5 min (from [152])
Recent studies suggest clues on potential mechanisms that may control Panx1-mediated responses under non-lytic conditions:
Cytosolic Ca2+
Co-expressing Panx1 with P2Y1 or P2Y2 receptors conferred ATP-evoked Panx1 currents to Xenopus oocytes, a response that was also observed in Panx1-expressing oocytes stimulated with Ca2+ ionophores. In the same study, application of calcium to the cytoplasmic face of excised inside-out membrane patches from murine Panx1-expressing oocytes resulted in channel activity that ceased upon calcium removal. These results led to hypothesize that Panx1 activity is sensitive to changes in cytosolic Ca2+ levels [170]. Consistent with this hypothesis, activation of the Ca2+ mobilizing ryanodine receptor with caffeine or administration of P2Y or P2X receptor agonists resulted in Panx1-like large conductance channel activity in cardiac myocytes [171]. However, Panx1 sensitivity to cytosolic Ca2+ changes may be a cell-specific phenomenon. Studies with HEK cells overexpressing Panx1 showed that Panx1 currents were not affected by BAPTA-AM or by dialyzing the cells with either low or high Ca2+ solutions [146]. Recent studies with human lung epithelial cells also indicated no correlation between Ca2+ signaling and Panx1-associated ATP release/dye uptake respsones. That is, Ca2+ signaling (triggered with ionomycin or P2Y2 receptor activation) was not sufficient to promote ATP release or propidium iodide uptake in these cells [50, 152].
ATP as an allosteric inhibitor of Panx1
Based on the observation that submillimolar concentrations of extracellular ATP blocked Panx1-associated currents in Xenopus oocytes [172] and HEK cells [146], Qiu and Dahl proposed that ATP release acts as negative feedback on Panx1, binding to and preventing deleterious, long-lasting opening of the channel [172]. By performing mutational analysis, these authors identified R75 in the putative first extracellular loop of Panx1 to be critical for ATP inhibition of currents in oocytes expressing murine Panx1. Inhibition of Panx1 by extracellular ATP may be important in atrial myocytes, which display large conductance cation channel activity that resembles Panx1 and is inhibited by 2 mM ATP [171].
Redox potentials and protein-protein interactions
The potassium channel subunit Kvβ3, was identified as a potential interaction partner of the C-terminus of Panx1 using an Escherichia coli two-hybrid system. Interestingly, administration of the reducing agent tris(2-carboxyethyl) phosphine (TCEP) to Panx1-expressing oocytes attenuated Panx1 current amplitude, but the effect of TCEP was negligible in oocytes co-expressing Panx1 with Kvβ3 [173]. Panx1 and Kvβ3 co-distribute within discrete areas of the hippocampus and cerebellum, and they co-precipitated and co-localized when co-expressed as recombinant proteins in Neuro2A neuroblastoma cells [173]. Based on these observations, it could be speculated that the electrophysiological properties of Panx1 are affected by changes in redox potentials and that Kvβ3 modulates the susceptibility of Panx1 to inhibition by reducing conditions. Cysteine residues within the putative cytosolic domains of Panx1 seemed to be not involved in the inhibition of the channel by TCEP [174]. Independently of Kvβ3, a cysteine to serine substitution at the C terminal position of Panx1 (Panx1-C346S) resulted in constitutively leaky channels in both injected oocytes and transfected Neuro2A cells, suggesting that cysteine 346 is important in regulating Panx1 channel properties in living cells [174].
Laird and co-workers have illustrated that Panx1 co-immunoprecipitated with β-actin and that the carboxyl terminal tail of Panx1 directly interacts with F actin [175], suggesting that the cytoskeleton plays an important role in the assembly and/or functional properties of Panx1.
A recent study showed that Panx1 natively expressed in thoracodorsal resistance arteries was pulled-down by an anti-α1 adrenergic receptor antibody, and that phenylephrine promoted constrictive responses that were attributed to Panx1-mediated ATP released [166]. The mechanistic details of these findings remain to be elucidated.
Rho signaling
Studies from our lab indicated that Panx1-mediated ATP release and dye uptake in airway epithelial cells was associated with Rho activation and myosin light change (MLC) phosphorylation. Specifically, cells exposed to the protease-activated receptor (PAR) agonist thrombin or hypotonic cell swelling displayed increased RhoA-GTP formation, which was accompanied by MLC phosphorylation, and carbenoxolone-sensitive propidium iodide uptake and ATP release. Disrupting Rho signaling (via RhoA dominant negative mutants or Rho kinase inhibitors) or incubating the cells with MLC kinase inhibitors markedly decreased MLC phosphorylation, propidium iodide uptake, and ATP release evoked by thrombin or hypotonicity [152, 153]. Given the involvement of Rho/Rho kinase in cytoskeleton rearrangements, one speculation would be that Rho activation facilitates Panx1 translocation to the plasma membrane and/or its interaction with regulatory proteins.
In conclusion, a number of published studies support the notion that Panx1 is involved in the regulated release of ATP from viable mammalian cells. However, how tight control of Panx1 pore gating is ensured to prevent collapses of the ion gradients and cell death remains incompletely defined. An alternative possibility is that Panx1 interacts with a different ATP/dye channel or transporter, as suggested [77, 148].
Panx1 and cell death
Panx1 activation has been associated with cell death in ischemic hippocampal and cortical neurons [176], aberrant bursting in N-methyl-D-aspartate receptor (NMDAR)-stimulated pyramidal neurons [177], and spontaneous and stretch-triggered action potentials and arrhythmogenic activities in cardiac myocytes [163, 171]. It has been also suggested that Panx1 forms a large pore contributing to cell death during prolonged activation of the P2X7 receptor [148, 178]. As mentioned above, apoptotic lymphocytes (anti-Fas-exposed T cells) release ATP and UTP. Nucleotide release from apoptotic T cells was inhibited by a cell-permeable pan-caspase inhibitor [168] and by carbenoxolone, probenecid, and Panx1 knockdown. Conversely, Panx1-overexpression enhanced the release of nucleotides from apoptotic Jurkat cells [162]. Whole cell patch-clamp recording of single-cell channel activity indicated the presence Panx1-dependent carbenoxolone-sensitive currents in apoptotic (but not control, “living”) cells. In agreement with these findings, thymocytes isolated from Panx1−/− mice displayed impaired release of ATP in response to the apoptotic drug dexamethasone, as mentioned above [169]. A caspase-cleavage site within the C terminus of Panx1 (DVVD at residues 376–379) was crucial for the induction of Panx1-associated responses during apoptosis. That is, mutating aspartic acid to either alanine or glutamic acid rendered Panx1 resistant to caspase, resulting in the loss of currents, impaired dye uptake, and reduced ATP release in apoptotic cells. Conversely, a truncation mutant that mimics the cleavage of Panx1 at DVVD 376–379 exhibited enhanced dye uptake and current–voltage relationship (in non-apoptotic cells) that resembles that of activated WT Panx1, suggesting that removal of the C terminus of Panx1 resulted in constitutively active Panx1 [162]. Thus, the C terminus may function as a dominant-negative domain within Panx1. Removal of the caspase cleavage site in the C terminus of Panx1 is necessary for the induction of Panx1-mediated plasma membrane permeability during apoptosis.
Pannexin 3
Unlike widely distributed Panx1, Panx3 expression is restricted to a few tissues, including the skin, cochlea, and developing cartilage and bone [142, 179, 180]. Although Panx3 has been shown to be expressed as a glycoprotein at the cell surface and form dye permeable pores [142, 179], little is known about the functional properties of plasma membrane Panx3. A recent study indicated that Panx3-transfected ATDC5 chondrocytes and undifferentiated C2C12A osteoblasts displayed enhanced KGlu-promoted ATP release, which was reduced by a Panx3 antibody and a Panx3 mimicking peptide [180, 181]. Suppression of endogenous Panx3 by shRNA inhibited ATP release in differentiated C2C12 cells [181]. Further research is required to delineate in more detail the potential contribution of Panx3 to ATP release.
P2X7 Receptor
P2X7 receptor activation results in large pore formation that allows the passage of melecules up to 900 Da [182, 183], and facilitates the release of ATP [184, 185]. Since the P2X7 receptor is associated with Panx1 [148], Panx1 may have been the actual conduit for ATP release in response to P2X7 receptor activation. However, Qu et al. [169] recently demonstrated that macrophages from Panx1−/− mice exhibited P2X7 receptor-dependent uptake of the 375 Da dye Yo-Pro-1, which was indistinguishable from wild type (WT) mice, indicating that in these cells, P2X7 receptor activation results in formation of pores that are, at least, the size of Yo-Pro-1. Whether the Panx1-independent, P2X7 receptor-associated pore allows the efflux of cytosolic ATP was not examined. Given the relatively high concentration of extracellular ATP needed to activate the P2X7 receptor [169], the relevance of ATP-promoted P2X7 receptor activation-dependent ATP release is unclear.
TRPV4 as a mechano/osmotic sensor upstream of ATP release
The TRPV4 channel is a widely expressed cation channel (relatively selective for Ca2+ and to lesser extent Na+) that acts as a sensor of various physical stimuli such as heat, osmotic/shear stresses, and stretch [186–188]. Several lines of evidence suggest that TRPV4 transduces physical stimuli into ATP release. For example, Mochizuki et al. illustrated that TRPV4 senses distension of the bladder urothelium, which is converted to an ATP signal in the micturition reflex pathway during urine storage. These authors demonstrated that urothelial cell stretching caused a marked release of ATP, which was abrogated in the TRPV4 knockout mouse [189]. Similarly, Gevaert et al. [190] demonstrated that intravesical stretch-evoked ATP release was reduced in isolated whole bladders from TRPV4−/− mice, relative to WT animals. In the thick ascending limb of the renal medulla, TRPV4 siRNA and TRPV4 inhibitors reduced ATP release in response to osmotic stress [191]. In a recent study with primary cultures of esophageal keratinocytes, heat-evoked Ca2+-dependent release of ATP was reduced in cells from TRPV4−/− mice [40]. The fact that in these studies ATP release was associated with TRPV4-dependent Ca2+ elevation suggests that TRPV4 transduced physical stimuli into Ca2+-dependent ATP release, e.g., via Ca2+-regulated exocytosis. Consistent with this hypothesis, esophageal keratinocytes express VNUT and exhibit Ca2+-dependent ATP release in response to the TRPV4 activator GSK1016790A, a response that was inhibited by brefeldin A and abrogated in TRPV4−/− keratinocytes [40].
A TRPV4-dependent mechanism that involves Panx1-mediated ATP release has been recently proposed. In human airway epithelial cells, TRPV4 shRNA and the highly selective TRPV4 inhibitor HC67047 reduced hypotonic stress-evoked ATP and propidium iodide uptake. Notably, HC67047 also abolished RhoA activation in hypotonicity-challenged cells, suggesting that TRPV4 channels transduce hypotonic cell swelling into Rho activation, upstream, of Panx1 channel activation [152].
Summary
The last few years have witnessed important advances in the identification and characterization of key components of the nucleotide release mechanisms that contribute to the variety of physiological responses triggered by extracellular ATP, ADP, adenosine, UTP, UDP, and UDP-sugars. The occurrence of exocytotic ATP release is firmly supported by the demonstration that secretory granules isolated from exocrine/neuroendocrine tissues store ATP. The observation that ATP release from cells exhibiting vesicle/granule exocytosis is negatively affected by knocking down the vesicular ATP transporter SLC17A9/VNUT has provided further insights into the molecular processes that control ATP entry into secretory granules and vesicles. The finding that VNUT displays UTP transport activity, in addition to ATP, suggests that the secretory pathway could also be a source of released uridine nucleotides. A separate class of SLC transporters (SLC35 nucleotide–sugar transporters) are expressed in the ER and Golgi apparatus and translocate UDP-sugars from the cytosol to these organelles, using UMP as luminal antiporter substrate. Golgi-resident SLC35D2 and other nucleotide–sugar translocators contribute to the cellular release of UDP-sugars, via the constitutive secretory pathway.
In addition to exocytotic pathways, less well-characterized conductive mechanisms of nucleotide release exist. They include members of the connexin family of gap junction hemichannels, non-junctional pannexin channels, and molecularly elusive ATP conducting anion channels. Important challenges to the field are the elucidation of mechanisms regulating connexin and pannexin channel opening/closing activities in living cells and the molecular identification of maxi anion channels and volume-regulated anion channels. The finding that the C terminus of Panx1 contains a proteolysis-sensitive inhibitory domain suggests an additional degree of complexity in the regulation of this channel in apoptotic cells.
Acknowledgments
We thank Lisa Brown for editorial assistance of the manuscript. Supported by National Institute of Health grant P01-HL034322.
Conflict of interest statement
The author has no potential conflict of interest.
Glossary
- SLC
Solute carrier
- VNUT
Vesicular nucleotide transporter
- Panx
Pannexin
- Cx
Connexin
- siRNA
Small interfering RNA
- shRNA
Short hairpin RNA
- BAPTA
1,2-Bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- DIDS
4,4′-Diisothiocyanostilbene-2,2′-disulfonate
- NBPP
5-Nitro-2-(3-phenylpropylamino)benzoic acid
- fMLP
Formyl-Met-Leu-Phe
Footnotes
References
- 1.Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 3.Kügelgen I, Harden TK. Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv Pharmacol. 2011;61:373–415. doi: 10.1016/B978-0-12-385526-8.00012-6. [DOI] [PubMed] [Google Scholar]
- 4.Gessi S, Merighi S, Varani K, Borea PA. Adenosine receptors in health and disease. Adv Pharmacol. 2011;61:41–75. doi: 10.1016/B978-0-12-385526-8.00002-3. [DOI] [PubMed] [Google Scholar]
- 5.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Corriden R, Insel PA. Basal release of ATP: an autocrine–paracrine mechanism for cell regulation. Sci Signal. 2010;3:re1–re25. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 9.Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM. Is ATP a signaling agent in plants? Plant Physiol. 2003;133:456–461. doi: 10.1104/pp.103.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chara O, Espelt MV, Krumschnabel G, Schwarzbaum PJ. Regulatory volume decrease and P receptor signaling in fish cells: mechanisms, physiology, and modeling approaches. J Exp Zool A Ecol Genet Physiol. 2011;315:175–202. doi: 10.1002/jez.662. [DOI] [PubMed] [Google Scholar]
- 11.Kukulski F, Levesque SA, Sevigny J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv Pharmacol. 2011;61:263–299. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 12.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol. 2011;61:221–261. doi: 10.1016/B978-0-12-385526-8.00008-4. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann H. ATP and acetylcholine, equal brethren. Neurochem Int. 2008;52:634–648. doi: 10.1016/j.neuint.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 16.Evans RJ, Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol. 1992;106:242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 18.Dean GE, Fishkes H, Nelson PJ, Rudnick G. The hydrogen ion-pumping adenosine triphosphatase of platelet dense granule membrane. Differences from F1F0- and phosphoenzyme-type ATPases. J Biol Chem. 1984;259:9569–9574. [PubMed] [Google Scholar]
- 19.Gualix J, Abal M, Pintor J, Garcia-Carmona F, Miras-Portugal MT. Nucleotide vesicular transporter of bovine chromaffin granules. Evidence for a mnemonic regulation. J Biol Chem. 1996;271:1957–1965. doi: 10.1074/jbc.271.4.1957. [DOI] [PubMed] [Google Scholar]
- 20.Gualix J, Pintor J, Miras-Portugal MT. Characterization of nucleotide transport into rat brain synaptic vesicles. J Neurochem. 1999;73:1098–1104. doi: 10.1046/j.1471-4159.1999.0731098.x. [DOI] [PubMed] [Google Scholar]
- 21.Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- 22.Anderson P, Rohlich P, Slorach SA, Uvnas B. Morphology and storage properties of rat mast cell granules isolated by different methods. Acta Physiol Scand. 1974;91:145–153. doi: 10.1111/j.1748-1716.1974.tb05670.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276:32925–32932. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- 24.Aberer W, Kostron H, Huber E, Winkler H. A characterization of the nucleotide uptake of chromaffin granules of bovine adrenal medulla. Biochem J. 1978;172:353–360. doi: 10.1042/bj1720353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1:65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]
- 26.Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts—synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–17138. doi: 10.1074/jbc.271.29.17132. [DOI] [PubMed] [Google Scholar]
- 27.Hanada H, Moriyama Y, Maeda M, Futai M. Kinetic studies of chromaffin granule H+-ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun. 1990;170:873–878. doi: 10.1016/0006-291x(90)92172-v. [DOI] [PubMed] [Google Scholar]
- 28.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 29.Chapman ER, An S, Barton N, Jahn R. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994;269:27427–27432. [PubMed] [Google Scholar]
- 30.Li JY, Jahn R, Dahlstrom A. Axonal transport and targeting of the t-SNAREs SNAP-25 and syntaxin 1 in the peripheral nervous system. Eur J Cell Biol. 1996;70:12–22. [PubMed] [Google Scholar]
- 31.Rettig J, Neher E. Emerging roles of presynaptic proteins in Ca++-triggered exocytosis. Science. 2002;298:781–785. doi: 10.1126/science.1075375. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34:599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 33.Chaineau M, Danglot L, Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 2009;583:3817–3826. doi: 10.1016/j.febslet.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflugers Arch. 2004;447:629–635. doi: 10.1007/s00424-003-1087-y. [DOI] [PubMed] [Google Scholar]
- 35.Sreedharan S, Shaik JH, Olszewski PK, Levine AS, Schioth HB, Fredriksson R. Glutamate, aspartate and nucleotide transporters in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression. BMC Genomics. 2010;11:17. doi: 10.1186/1471-2164-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fredriksson R, Nordstrom KJ, Stephansson O, Hagglund MG, Schioth HB. The solute carrier (SLC) complement of the human genome: phylogenetic classification reveals four major families. FEBS Lett. 2008;582:3811–3816. doi: 10.1016/j.febslet.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–5. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 40.Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589:3471–3482. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4:237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chander A, Johnson RG, Reicherter J, Fisher AB. Lung lamellar bodies maintain an acidic internal pH. J Biol Chem. 1986;261:6126–6131. [PubMed] [Google Scholar]
- 43.Costa JL, Fay DD, Kirk KL. Quinacrine and basic amines in human platelets: subcellular compartmentation and effects on serotonin. Res Commun Chem Pathol Pharmacol. 1984;43:25–42. [PubMed] [Google Scholar]
- 44.Di A, Krupa B, Bindokas VP, Chen Y, Brown ME, Palfrey HC, Naren AP, Kirk KL, Nelson DJ. Quantal release of free radicals during exocytosis of phagosomes. Nat Cell Biol. 2002;4:279–285. doi: 10.1038/ncb771. [DOI] [PubMed] [Google Scholar]
- 45.Kolber MA, Henkart PA. Quantitation of secretion by rat basophilic leukemia cells by measurements of quinacrine uptake. Biochim Biophys Acta. 1988;939:459–466. doi: 10.1016/0005-2736(88)90092-2. [DOI] [PubMed] [Google Scholar]
- 46.Goren MB, Swendsen CL, Fiscus J, Miranti C. Fluorescent markers for studying phagosome-lysosome fusion. J Leukoc Biol. 1984;36:273–292. doi: 10.1002/jlb.36.3.273. [DOI] [PubMed] [Google Scholar]
- 47.Haanes KA, Novak I. ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J. 2010;429:303–311. doi: 10.1042/BJ20091337. [DOI] [PubMed] [Google Scholar]
- 48.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9:262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreda SM, Okada SF, Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC. Coupled nucleotide and mucin hypersecretion from goblet cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol. 2011;45:253–260. doi: 10.1165/rcmb.2010-0253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreda SM, Seminario-Vidal L, Heusden CA, O'Neal W, Jones L, Boucher RC, Lazarowski ER. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol. 2010;588:2255–2267. doi: 10.1113/jphysiol.2009.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leitner JW, Sussman KE, Vatter AE, Schneider FH. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975;96:662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- 53.Hutton JC, Penn EJ, Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210:297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Detimary P, Jonas JC, Henquin JC. Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology. 1996;137:4671–4676. doi: 10.1210/endo.137.11.8895332. [DOI] [PubMed] [Google Scholar]
- 55.Obermuller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- 56.Karanauskaite J, Hoppa MB, Braun M, Galvanovskis J, Rorsman P. Quantal ATP release in rat beta-cells by exocytosis of insulin-containing LDCVs. Pflugers Arch. 2009;458:389–401. doi: 10.1007/s00424-008-0610-6. [DOI] [PubMed] [Google Scholar]
- 57.Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2010;6:3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 59.Uvnas B. The molecular basis for the storage and release of histamine in rat mast cell granules. Life Sci. 1974;14:2355–2366. doi: 10.1016/0024-3205(74)90131-3. [DOI] [PubMed] [Google Scholar]
- 60.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 62.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukimoto M, Tokunaga A, Harada H, Kojima S. Blockade of murine T cell activation by antagonists of P2Y6 and P2X7 receptors. Biochem Biophys Res Commun. 2009;384:512–518. doi: 10.1016/j.bbrc.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Gatof D, Kilic G, Fitz JG. Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G538–G546. doi: 10.1152/ajpgi.00355.2003. [DOI] [PubMed] [Google Scholar]
- 65.Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 67.Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 69.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 70.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feranchak AP, Lewis MA, Kresge C, Sathe M, Bugde A, Luby-Phelps K, Antich PP, Fitz JG. Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem. 2010;285:8138–8147. doi: 10.1074/jbc.M109.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dolovcak S, Waldrop SL, Fitz JG, Kilic G. 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) stimulates cellular ATP release through exocytosis of ATP-enriched vesicles. J Biol Chem. 2009;284:33894–33903. doi: 10.1074/jbc.M109.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boudreault F, Grygorczyk R. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol. 2004;561:499–513. doi: 10.1113/jphysiol.2004.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Groulx N, Boudreault F, Orlov SN, Grygorczyk R. Membrane reserves and hypotonic cell swelling. J Membr Biol. 2006;214:43–56. doi: 10.1007/s00232-006-0080-8. [DOI] [PubMed] [Google Scholar]
- 75.Tatur S, Groulx N, Orlov SN, Grygorczyk R. Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides. J Physiol. 2007;584:419–435. doi: 10.1113/jphysiol.2007.133314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wijk T, Tomassen SF, Houtsmuller AB, Jonge HR, Tilly BC. Increased vesicle recycling in response to osmotic cell swelling. Cause and consequence of hypotonicity-provoked ATP release. J Biol Chem. 2003;278:40020–40025. doi: 10.1074/jbc.M307603200. [DOI] [PubMed] [Google Scholar]
- 77.Akopova I, Tatur S, Grygorczyk M, Luchowski R, Gryczynski I, Gryczynski Z, Borejdo J, Grygorczyk R. Imaging exocytosis of ATP-containing vesicles with TIRF 1 microscopy in lung epithelial A549 cells. Purinergic Signal. 2012;8:59–70. doi: 10.1007/s11302-011-9259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orriss IR, Knight GE, Utting JC, Taylor SE, Burnstock G, Arnett TR. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol. 2009;220:155–162. doi: 10.1002/jcp.21745. [DOI] [PubMed] [Google Scholar]
- 79.Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- 80.Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res. 2003;118:10–23. doi: 10.1016/s0169-328x(03)00330-9. [DOI] [PubMed] [Google Scholar]
- 81.Scrivens M, Dickenson JM. Functional expression of the P2Y(14) receptor in human neutrophils. Eur J Pharmacol. 2006;543:166–173. doi: 10.1016/j.ejphar.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 82.Gao ZG, Ding Y, Jacobson KA. UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol. 2010;79:873–879. doi: 10.1016/j.bcp.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sesma JI, Lazarowski ER, Harden TK (2011) UDP-glucose promotes Rho activation in human neutrophils. FASEB J 25:751.16 (Abstr.)
- 84.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- 85.Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol. 2008;153:1528–1537. doi: 10.1038/sj.bjp.0707692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sesma JI, Esther CR, Jr, Kreda SM, Jones L, O'Neal W, Nishihara S, Nicholas RA, Lazarowski ER. ER/golgi nucelotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem. 2009;284:12572–12583. doi: 10.1074/jbc.M806759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishida N, Kawakita M. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35) Pflugers Arch. 2004;447:768–775. doi: 10.1007/s00424-003-1093-0. [DOI] [PubMed] [Google Scholar]
- 88.Guillen E, Hirschberg CB. Transport of adenosine triphosphate into endoplasmic reticulum proteoliposomes. Biochemistry. 1995;34:5472–5476. doi: 10.1021/bi00016a018. [DOI] [PubMed] [Google Scholar]
- 89.Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- 90.Schwiebert EM. ABC transporter-facilitated ATP conductive transport. Am J Physiol. 1999;276:C1–C8. doi: 10.1152/ajpcell.1999.276.1.C1. [DOI] [PubMed] [Google Scholar]
- 91.Roman RM, Lomri N, Braunstein G, Feranchak AP, Simeoni LA, Davison AK, Mechetner E, Schwiebert EM, Fitz JG. Evidence for multidrug resistance-1 P-glycoprotein-dependent regulation of cellular ATP permeability. J Membr Biol. 2001;183:165–173. doi: 10.1007/s00232-001-0064-7. [DOI] [PubMed] [Google Scholar]
- 92.Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- 93.Grygorczyk R, Tabcharani JA, Hanrahan JW. CFTR channels expressed in CHO cells do not have detectable ATP conductance. J Membr Biol. 1996;151:139–148. doi: 10.1007/s002329900065. [DOI] [PubMed] [Google Scholar]
- 94.Watt WC, Lazarowski ER, Boucher RC. Cystic fibrosis transmembrane regulator-independent release of ATP—its implications for the regulation of P2Y(2) receptors in airway epithelia. J Biol Chem. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- 95.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol. 2004;124:513–526. doi: 10.1085/jgp.200409154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sabirov R, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1:311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sabirov RZ, Okada Y. The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity. J Physiol Sci. 2009;59:3–21. doi: 10.1007/s12576-008-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sabirov RZ, Okada Y. Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys J. 2004;87:1672–1685. doi: 10.1529/biophysj.104.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 102.Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol. 2002;119:511–520. doi: 10.1085/jgp.20028540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Okada Y. Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am J Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- 104.Okada Y, Sato K, Numata T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol. 2009;587:2141–2149. doi: 10.1113/jphysiol.2008.165076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blum AE, Walsh BC, Dubyak GR. Extracellular osmolarity modulates G protein-coupled receptor-dependent ATP release from 1321N1 astrocytoma cells. Am J Physiol Cell Physiol. 2010;298:C386–C396. doi: 10.1152/ajpcell.00430.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y. Swelling-induced, CFTR-independent ATP release from a human epithelial cell line. J Gen Physiol. 1999;114:525–533. doi: 10.1085/jgp.114.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu HT, Akita T, Shimizu T, Sabirov RZ, Okada Y. Bradykinin-induced astrocyte-neuron signalling: glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J Physiol. 2009;587:2197–2209. doi: 10.1113/jphysiol.2008.165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suzuki M, Mizuno A. A novel human Cl(−) channel family related to Drosophila flightless locus. J Biol Chem. 2004;279:22461–22468. doi: 10.1074/jbc.M313813200. [DOI] [PubMed] [Google Scholar]
- 109.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scemes E, Spray DC, Meda P. Connexins, pannexins, innexins: novel roles of “hemi-channels”. Pflugers Arch. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.D'hondt C, Ponsaerts R, De SH, Vinken M, De VE, De BM, Wang N, Rogiers V, Leybaert L, Himpens B, Bultynck G. Pannexin channels in ATP release and beyond: an unexpected rendezvous at the endoplasmic reticulum. Cell Signal. 2011;23:305–316. doi: 10.1016/j.cellsig.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 112.Thompson RJ, MacVicar BA. Connexin and pannexin hemichannels of neurons and astrocytes. Channels (Austin) 2008;2:81–86. doi: 10.4161/chan.2.2.6003. [DOI] [PubMed] [Google Scholar]
- 113.D'hondt C, Ponsaerts R, De SH, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? BioEssays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- 114.Nakagawa S, Maeda S, Tsukihara T. Structural and functional studies of gap junction channels. Curr Opin Struct Biol. 2010;20:423–430. doi: 10.1016/j.sbi.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 115.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 116.Muller DJ, Hand GM, Engel A, Sosinsky GE. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002;21:3598–3607. doi: 10.1093/emboj/cdf365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Romanello M, Pani B, Bicego M, D'Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun. 2001;289:1275–1281. doi: 10.1006/bbrc.2001.6124. [DOI] [PubMed] [Google Scholar]
- 121.Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2005;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vuyst E, Wang N, Decrock E, Bock M, Vinken M, Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 123.Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1769–R1776. doi: 10.1152/ajpregu.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schock SC, Leblanc D, Hakim AM, Thompson CS. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun. 2008;368:138–144. doi: 10.1016/j.bbrc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 125.Huckstepp RT, Id BR, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Funk GD. The ‘connexin’ between astrocytes, ATP and central respiratory chemoreception. J Physiol. 2010;588:4335–4337. doi: 10.1113/jphysiol.2010.200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009;20:1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem. 2011;286:1054–1060. doi: 10.1074/jbc.M110.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med. 2008;18:103–107. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 133.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 134.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 135.Singh D, Lampe PD. Identification of connexin-43 interacting proteins. Cell Commun Adhes. 2003;10:215–220. doi: 10.1080/cac.10.4-6.215.220. [DOI] [PubMed] [Google Scholar]
- 136.Gilleron J, Fiorini C, Carette D, Avondet C, Falk MM, Segretain D, Pointis G. Molecular reorganization of Cx43, Zo-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J Cell Sci. 2008;121:4069–4078. doi: 10.1242/jcs.033373. [DOI] [PubMed] [Google Scholar]
- 137.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1. Cancer Res. 2010;70:4222–4232. doi: 10.1158/0008-5472.CAN-09-3281. [DOI] [PubMed] [Google Scholar]
- 138.Scemes E. Modulation of astrocyte P2Y1 receptors by the carboxyl terminal domain of the gap junction protein Cx43. Glia. 2008;56:145–153. doi: 10.1002/glia.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spray DC, Iacobas DA. Organizational principles of the connexin-related brain transcriptome. J Membr Biol. 2007;218:39–47. doi: 10.1007/s00232-007-9049-5. [DOI] [PubMed] [Google Scholar]
- 140.Iacobas DA, Iacobas S, Urban-Maldonado M, Scemes E, Spray DC. Similar transcriptomic alterations in Cx43 knockdown and knockout astrocytes. Cell Commun Adhes. 2008;15:195–206. doi: 10.1080/15419060802014222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–24431. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 143.Boassa D, Qiu F, Dahl G, Sosinsky G. Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun Adhes. 2008;15:119–132. doi: 10.1080/15419060802013885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 145.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 146.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 150.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, Heusden CA, Zhu Y, Jones LC, O'Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seminario-Vidal L, Kreda S, Jones L, O'Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of Rho- and Ca2+-dependent signaling pathways. J Biol Chem. 2009;284:20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced atp release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med. 2011;39:2478–2486. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forsyth AM, Wan J, Owrutsky PD, Abkarian M, Stone HA. Multiscale approach to link red blood cell dynamics, shear viscosity, and ATP release. Proc Natl Acad Sci U S A. 2011;108:10986–10991. doi: 10.1073/pnas.1101315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol. 2010;299:C1308–C1317. doi: 10.1152/ajpcell.00333.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reigada D, Lu W, Zhang M, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannels. Neuroscience. 2008;157:396–404. doi: 10.1016/j.neuroscience.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Mitchell CH, Civan MM (2011) Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol (in press) [DOI] [PMC free article] [PubMed]
- 160.Buvinic S, Almarza G, Bustamante M, Casas M, Lopez J, Riquelme M, Saez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem. 2009;284:34490–34505. doi: 10.1074/jbc.M109.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, Ide T, Suzuki K, Inoue K, Nagao T, Kurose H. P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 2008;27:3104–3115. doi: 10.1038/emboj.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seror C, Melki MT, Subra F, Raza SQ, Bras M, Saidi H, Nardacci R, Voisin L, Paoletti A, Law F, Martins I, Amendola A, Abdul-Sater AA, Ciccosanti F, Delelis O, Niedergang F, Thierry S, Said-Sadier N, Lamaze C, Metivier D, Estaquier J, Fimia GM, Falasca L, Casetti R, Modjtahedi N, Kanellopoulos J, Mouscadet JF, Ojcius DM, Piacentini M, Gougeon ML, Kroemer G, Perfettini JL. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med. 2011;208:1823–1834. doi: 10.1084/jem.20101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates {alpha}1-adrenergic receptor-mediated vasoconstriction. Circ Res. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qiu F, Wang J, Spray DC, Scemes E, Dahl G. Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett. 2011;585:3430–3435. doi: 10.1016/j.febslet.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011;186:6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- 170.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 171.Kienitz MC, Bender K, Dermietzel R, Pott L, Zoidl G. Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J Biol Chem. 2011;286:290–298. doi: 10.1074/jbc.M110.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–C255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bunse S, Locovei S, Schmidt M, Qiu F, Zoidl G, Dahl G, Dermietzel R. The potassium channel subunit Kvbeta3 interacts with pannexin 1 and attenuates its sensitivity to changes in redox potentials. FEBS J. 2009;276:6258–6270. doi: 10.1111/j.1742-4658.2009.07334.x. [DOI] [PubMed] [Google Scholar]
- 174.Bunse S, Schmidt M, Prochnow N, Zoidl G, Dermietzel R. Intracellular cysteine 346 is essentially involved in regulating Panx1 channel activity. J Biol Chem. 2010;285:38444–38452. doi: 10.1074/jbc.M110.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bhalla-Gehi R, Penuela S, Churko JM, Shao Q, Laird DW. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J Biol Chem. 2010;285:9147–9160. doi: 10.1074/jbc.M109.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 177.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 178.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Penuela S, Celetti SJ, Bhalla R, Shao Q, Laird DW. Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell Commun Adhes. 2008;15:133–142. doi: 10.1080/15419060802014115. [DOI] [PubMed] [Google Scholar]
- 180.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, Vega S, Fukumoto S, Yamada Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285:18948–18958. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol. 2011;193:1257–1274. doi: 10.1083/jcb.201101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 183.Virginio C, MacKenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol. 1999;519(Pt 2):335–346. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Virgilio F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16:3659–3665. doi: 10.1091/mbc.E05-03-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 188.Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 189.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Leuven F, Voets T, Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol. 2008;295:F1090–F1095. doi: 10.1152/ajprenal.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]