Abstract
Goldfish retinas were examined for changes in the labeling pattern of protein during regeneration of retinal ganglion cell axons following unilateral optic nerve crush. At various times after optic nerve crush the normal retinas were incubated in vitro with [3H] methionine and retinas from the opposite side of the treated fish were inculbated with [35S] methionine. The incubated retinas were combined and a soluble protein fraction was isolated and separated by sodium dodecyl sulfate-urea polyacrylamide gel electrophoresis. A band on the gel showing an elevated 35S to 3H ratio appeared at 5 days following optic nerve crush, increased to a maximum at 15 days, and was barely detectable at 45 days, during which time vision is known to return. On the basis of several criteria, the protein fraction showing the increased incorporation of methionine in retina after optic nerve crush appears to be of the tubulin class.
Full text
PDF
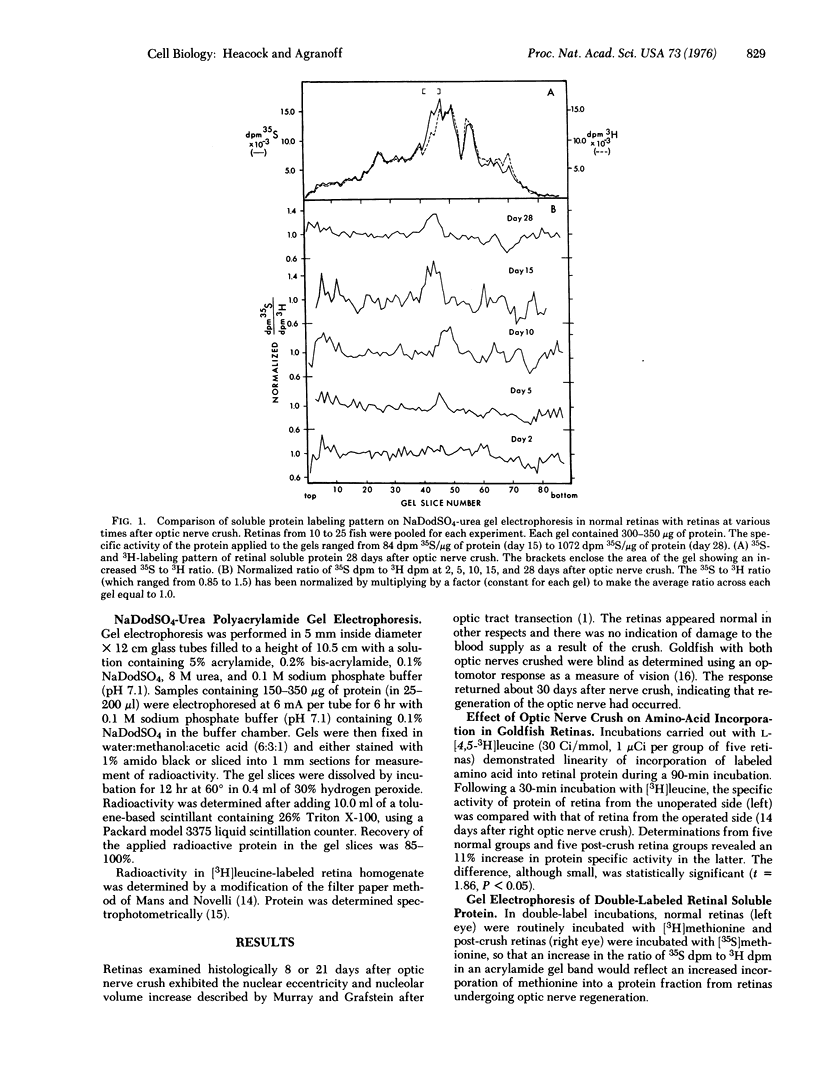
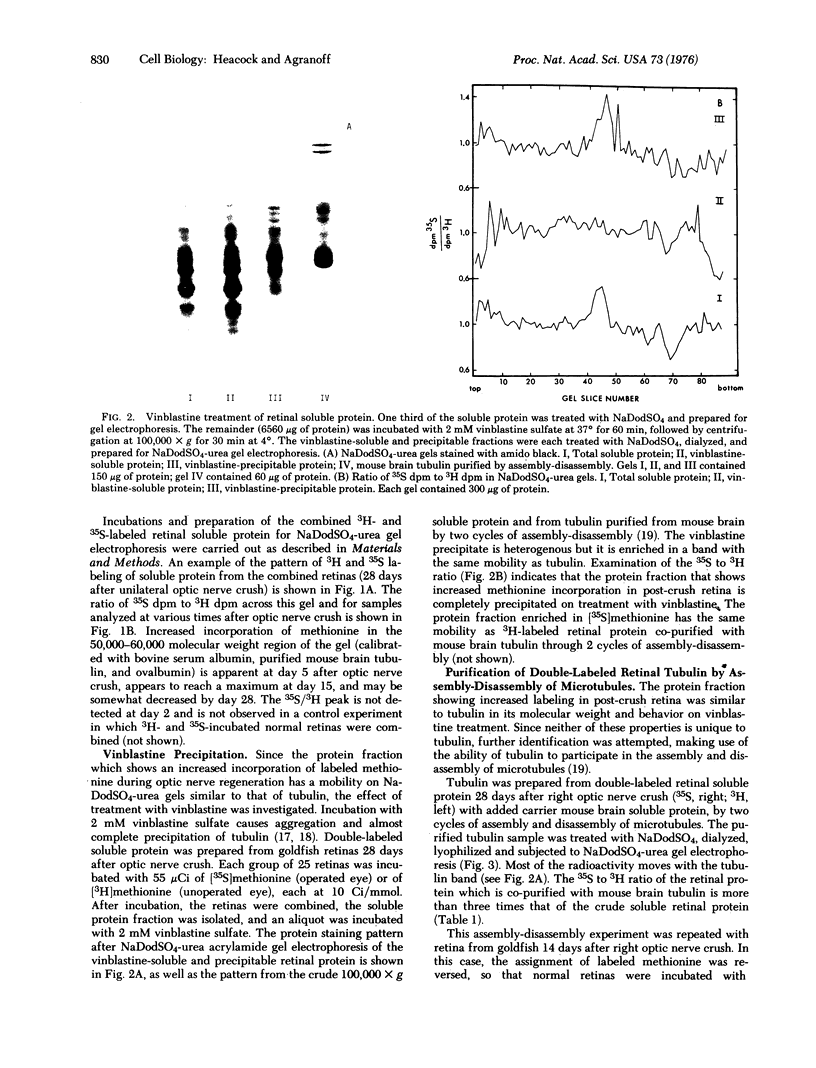
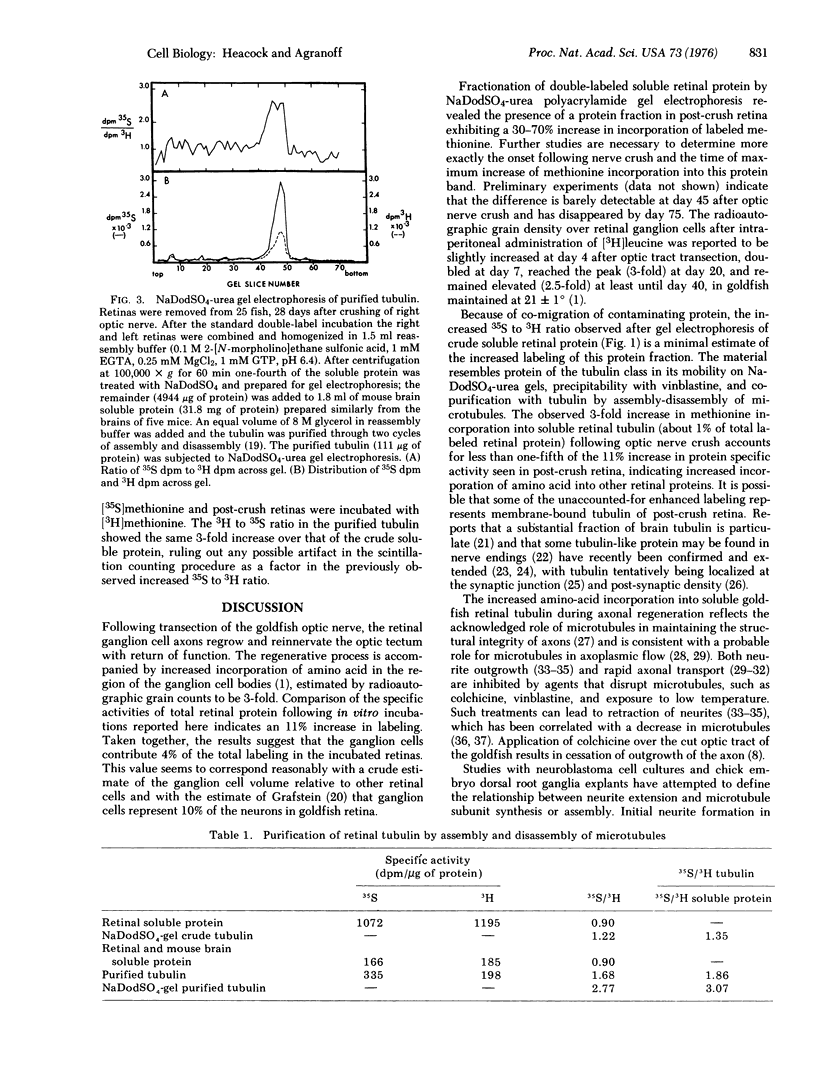

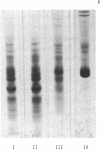
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRATTGARD S. O., HYDEN H., SJOSTRAND J. Incorporation of orotic acid-14c and lysine-14c in regenerating single nerve cells. Nature. 1958 Sep 20;182(4638):801–802. doi: 10.1038/182801b0. [DOI] [PubMed] [Google Scholar]
- Blitz A. L., Fine R. E. Muscle-like contractile proteins and tubulin in synaptosomes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4472–4476. doi: 10.1073/pnas.71.11.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt A. H., Lund R. D. Vinblastine-induced blockage of orthograde and retrograde axonal transport of protein in retinal ganglion cells. Exp Neurol. 1974 Nov;45(2):288–297. doi: 10.1016/0014-4886(74)90119-8. [DOI] [PubMed] [Google Scholar]
- Crothers S. D., McCluer R. H. Effect of colchicine on the delayed appearance of labelled protein into synaptosomal soluble proteins. J Neurochem. 1975 Feb;24(2):209–214. doi: 10.1111/j.1471-4159.1975.tb11866.x. [DOI] [PubMed] [Google Scholar]
- Daniels M. P. Colchicine inhibition of nerve fiber formation in vitro. J Cell Biol. 1972 Apr;53(1):164–176. doi: 10.1083/jcb.53.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. P. Fine structural changes in neurons and nerve fibers associated with colchicine inhibition of nerve fiber formation in vitro. J Cell Biol. 1973 Aug;58(2):463–470. doi: 10.1083/jcb.58.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. The role of microtubules in the growth and stabilization of nerve fibers. Ann N Y Acad Sci. 1975 Jun 30;253:535–544. doi: 10.1111/j.1749-6632.1975.tb19227.x. [DOI] [PubMed] [Google Scholar]
- Dunlop D. S., Van Elden W., Lajtha A. Measurements of rates of protein synthesis in rat brain slices. J Neurochem. 1974 May;22(5):821–830. doi: 10.1111/j.1471-4159.1974.tb04300.x. [DOI] [PubMed] [Google Scholar]
- Feit H., Barondes S. H. Colchicine-binding activity in particulate fractions of mouse brain. J Neurochem. 1970 Sep;17(9):1355–1364. doi: 10.1111/j.1471-4159.1970.tb06870.x. [DOI] [PubMed] [Google Scholar]
- Feit H., Dutton G. R., Barondes S. H., Shelanski M. L. Microtubule protein. Identification in and transport to nerve endings. J Cell Biol. 1971 Oct;51(1):138–147. doi: 10.1083/jcb.51.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizell M., Sjöstrand J. The axonal transport of slowly migrating (3H)leucine labelled proteins and the regeneration rate in regenerating hypoglossal and vagus nerves of the rabbit. Brain Res. 1974 Dec 6;81(2):267–283. doi: 10.1016/0006-8993(74)90941-x. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S., McEwen B. S. Effects of temperature on axonal transport and turnover of protein in goldfish optic system. Exp Neurol. 1972 Jan;34(1):158–170. doi: 10.1016/0014-4886(72)90196-3. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Murray M. Transport of protein in goldfish optic nerve during regeneration. Exp Neurol. 1969 Dec;25(4):494–508. doi: 10.1016/0014-4886(69)90093-4. [DOI] [PubMed] [Google Scholar]
- Grafstein B. Role of slow axonal transport in nerve regeneration. Acta Neuropathol. 1971;5(Suppl):144–152. doi: 10.1007/978-3-642-47449-1_19. [DOI] [PubMed] [Google Scholar]
- Hier D. B., Arnason B. G., Young M. Studies on the mechanism of action of nerve growth factor. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2268–2272. doi: 10.1073/pnas.69.8.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. O., Hansson H. A., Sjöstrand J. Effect of colchicine on axonal transport and morphology of retinal ganglion cells. Z Zellforsch Mikrosk Anat. 1971;115(2):265–283. doi: 10.1007/BF00391128. [DOI] [PubMed] [Google Scholar]
- Kolber A. R., Goldstein M. N., Moore B. W. Effect of nerve growth factor on the expression of colchicine-binding activity and 14-3-2 protein in an established line of human neuroblastoma. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4203–4207. doi: 10.1073/pnas.71.10.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornguth S. E., Sunderland E. Isolation and partial characterization of a tubulin-like protein from human and swine synaptosomal membranes. Biochim Biophys Acta. 1975 May 30;393(1):100–114. doi: 10.1016/0005-2795(75)90220-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MIANI N., RIZZOLI A., BUCCIANTE G. Metabolic and chemical changes in regenerating neurons. II. In vitro rate of incorporation of amino acids into proteins of the nerve cell perikaryon of the C.8 spinal ganglion of rabbit. J Neurochem. 1961 Jul;7:161–173. doi: 10.1111/j.1471-4159.1961.tb13500.x. [DOI] [PubMed] [Google Scholar]
- Morgan J. L., Seeds N. W. Tubulin constancy during morphological differentiation of mouse neuroblastoma cells. J Cell Biol. 1975 Oct;67(1):136–145. doi: 10.1083/jcb.67.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M., Grafstein B. Changes in the morphology and amino acid incorporation of regenerating goldfish optic neurons. Exp Neurol. 1969 Apr;23(4):544–560. doi: 10.1016/0014-4886(69)90124-1. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Carlson K., Klebe R., Ruddle F., Rosenbaum J. Isolation of microtubule protein from cultured mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Jan;65(1):129–136. doi: 10.1073/pnas.65.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., McClure W. O. Inhibition of axoplasmic transport by colchicine, podophyllotoxin, and vinblastine: an effect on microtubules. Ann N Y Acad Sci. 1975 Jun 30;253:517–527. doi: 10.1111/j.1749-6632.1975.tb19225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. L., Porter K. R. The response of ventral horn neurons to axonal transection. J Cell Biol. 1972 Apr;53(1):24–37. doi: 10.1083/jcb.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES A., FORD D., RHINES R. COMPARATIVE UPTAKE OF DL-LYSINE-H3 BY NORMAL AND REGENERATIVE HYPOGLOSSAL NERVE CELLS IN EUTHYROID, HYPOTHYROID AND HYPERTHYROID MALE RATS. Exp Neurol. 1964 Sep;10:251–263. doi: 10.1016/0014-4886(64)90066-4. [DOI] [PubMed] [Google Scholar]
- Roisen F. J., Braden W. G., Friedman J. Neurite development in vitro: III. The effects of several derivatives of cyclic AMP, colchicine, and colcemid. Ann N Y Acad Sci. 1975 Jun 30;253:545–561. doi: 10.1111/j.1749-6632.1975.tb19228.x. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D., Jr, Gutmann E., Horsky P. Regeneration in spinal neurons: proteosynthesis following nerve growth factor administration. Science. 1966 May 6;152(3723):787–788. doi: 10.1126/science.152.3723.787. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cameron B. F. Morphological evidence for the participation of microtubules in axonal transport. Ann N Y Acad Sci. 1975 Jun 30;253:472–506. doi: 10.1111/j.1749-6632.1975.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Watson W. E. An autoradiographic study of the incorporation of nucleic-acid precursors by neurones and glia during nerve regeneration. J Physiol. 1965 Oct;180(4):741–753. doi: 10.1113/jphysiol.1965.sp007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard M., Cowan W. M., Vagelos P. R. The polypeptide composition of intra-axonally transported proteins: evidence for four transport velocities. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2183–2187. doi: 10.1073/pnas.71.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Bryan J., Ruby A., Mazia D. Precipitation of proteins by vinblastine and calcium ions. Proc Natl Acad Sci U S A. 1970 Jul;66(3):807–814. doi: 10.1073/pnas.66.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Axon growth: roles of microfilaments and microtubules. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1206–1212. doi: 10.1073/pnas.66.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]