Abstract
Introduction
Brain-derived neurotrophic factor (BDNF) Val66Met genotype has been associated with neurobehavioral deficits. To examine its relevance for addiction, we examined BDNF genotype differences in drug–seeking behavior.
Methods
Heroin-dependent volunteers (N=128) completed an interview that assessed past-month naturalistic drug seeking/use behaviors.
Results
In African Americans (N=74), the Met allele was uncommon (carrier frequency 6.8%); thus, analyses focused on European Americans (N=54), in whom the Met allele was common (carrier frequency 37.0%). In their natural setting, Met carriers (n=20) reported more time– and cost–intensive heroin–seeking and more cigarette use than Val homozygotes (n=34). BDNF Val66Met genotype predicted 18.4% of variance in ‘weekly heroin investment’ (purchasing time × amount × frequency).
Conclusions
These data suggest the BDNF Met allele may confer a ‘preferred drug–invested’ phenotype, resistant to moderating effects of higher drug prices and non-drug reinforcement. These preliminary hypothesis-generating findings require replication, but are consistent with preclinical data that demonstrate neurotrophic influence in drug reinforcement. Whether this genotype is relevant to other abused substances besides opioids or nicotine, or treatment response, remains to be determined.
Keywords: Heroin, Opioid, Drug-seeking phenotypes, BDNF Val66Met genotype, Cigarette use
Genetic association studies of addictive disorders typically attempt to identify polymorphisms underlying initial vulnerability (e.g. Enoch et al, 2009; Saccone et al, 2009; Yuferov et al, 2010). Yet genetic influences may operate at various stages of the addiction cycle (Li and Burmeister, 2009; Khokhar et al, 2010). Little is known about which biologically plausible functional genotypes alter persistence of addictive behaviors. Improved knowledge could help predict resistance to, or benefit from treatments. Unlike studies of vulnerability, genetic studies of addictive persistence target individuals who are already drug-dependent, because the goal is to understand genotypic and phenotypic heterogeneity within the clinical population.
Substance use disorders are complex syndromes that are not ideally suited for genetic studies (Wong and Schumann, 2008). Phenotype selection requires a targeted approach (Gottesman and Gould, 2003; Ducci and Goldman, 2008; Lerman et al, 2009). Assessing an intermediate phenotype (versus a broader phenotype such as a nosological condition) is preferable because a circumscribed measure will tend to be more reliable (which improves power to find associations with the genotype) and perhaps more closely related to genetic underpinnings than a multifactor syndrome. In the present research, we selected drug seeking behavior (intermediate phenotype) by heroin-dependent, out-of-treatment volunteers because: (1) individual drug seeking patterns are periodic (within days) and stable (between days), due to physical dependence and motivation to avoid opioid withdrawal signs/symptoms (Koob and Le Moal, 2001); and (2) this characteristic pattern enables investigation to focus on predictive validity, i.e. whether genetic heterogeneity within this group explains phenotypic variance. Our approach emphasizes the drug user’s habitual purchasing costs (time and money), which capture behavioral investment in this drug-seeking repertoire. We control for enabling environmental factors (e.g. income, drug cost and supply), which are presumably orthogonal to genetic influence. Finally, because cigarette smoking is highly prevalent among heroin-dependent individuals, we examined whether genotypic effect is limited to opioid seeking behavior or may also apply to nicotine-reinforced behavior (as cigarette smoking also follows a highly period and stable pattern), i.e. behavioral specificity.
Brain-derived neurotrophic factor (BDNF) is the most widely distributed neurotrophin and is involved in neurogenesis, differentiation, survival and synaptic plasticity (Lu, 2003; Lipsky and Marini, 2007; Russo et al, 2009). BDNF secretion is activity-dependent – e.g. increased by cognition and exercise, and decreased by stressors – and modulates neurotransmission in dopamine, glutamate, GABA and serotonin systems (e.g. Goggi et al, 2002; Carvalho et al, 2008). The human BDNF gene encodes a 247 amino acid pre-protein (proBDNF) that is cleaved to form an evolutionarily conserved 120 amino acid mature protein (Maisonpierre et al, 1991). A single nucleotide polymorphism (rs6265) results in methionine substitution for valine at codon 66 (Val66Met). The evolutionarily recent, less-frequent Met allele alters the proBDNF protein sequence, which disrupts trafficking and results in less activity-dependent BDNF secretion without affecting the mature BDNF sequence (Egan et al, 2003; Lu, 2003; Chen et al, 2004).
As might be expected from its neurotrophic physiological influence, the BDNF Val66Met genotype has unsurprisingly been associated with pleiotropic effects. Met allele carriers exhibit several reliable intermediate phenotypes: reduced gray matter volume in the hippocampus (Pezawas et al, 2004; Szeszko et al, 2005; Bueller et al, 2006; Frodl et al, 2007 [European/Caucasian]) and dorsolateral prefrontal cortex (e.g. Hariri et al, 2003; Pezawas et al, 2004), and impaired hippocampal-dependent memory function (Egan et al, 2003; Hariri et al, 2003 [Caucasian]). In the realm of substance use, the BDNF 66Met allele has been associated with headache-related overuse of non-opioid analgesics (Di Lorenzo et al, 2008), increased risk of nicotine dependence (Lang et al, 2007 [German]) and earlier onset of alcohol dependence (Matsushita et al, 2004 [Japanese]), but decreased risk of dependence on heroin (Cheng et al, 2005 [Han Chinese]) and protection against post-treatment alcohol relapse (Wojnar et al, 2009 [Polish]). Met allele frequency and informativeness varies significantly by ancestry, with highest prevalence in Asians, moderate prevalence for Europeans, and lowest among American Indians and individuals of African descent (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=rs6265; Petryshen et al, 2010). Accordingly, association studies should test phenotypic relationships with the BDNF Val66Met genotype within clearly defined ancestral groups.
In addition to evidence above that the Val66Met genotype has neurobiological and clinical relevance, findings from animal studies further suggest that BDNF physiology influences opioid dependence behaviors. Chronic opioid exposure alters BDNF/TrkB receptor-mediated dopamine function in the ventral tegmental area (VTA; Bolanos and Nestler, 2004; Russo et al, 2009) and its projection to the nucleus accumbens (NAc), the key neural circuitry underlying opioid reinforcement. Experimental infusions of BDNF directly into the VTA produce drug seeking (Lu et al, 2004) and biochemical changes (Berhow et al, 1995; Sklair-Tavron et al, 1996; Vargas-Perez et al, 2009). Discontinuation of chronic opioid exposure leads to increased BDNF mRNA expression in brain regions underlying physical dependence and drug seeking (Numan et al, 1998; Hatami et al, 2007). BDNF mRNA expression in prefrontal cortex is upregulated following exposure to psychostimulants and morphine, but to a lesser extent with nicotine (Le Foll et al, 2005).
Given that the BDNF 66Met allele has been linked to impaired hippocampal and frontal-cortical morphology and learning/memory problems, and thus impaired behavioral flexibility, we theorized the Met allele might confer resistance to environmentally- or pharmacologically induced changes in drug seeking/use. If true, then the Met allele could have opposite effects at different stages of addiction, i.e. protecting against initial vulnerability (thus explaining counterintuitive results by Cheng et al, 2005) while making it more difficult to modify chronic drug seeking/use (i.e. harder to unlearn). Thus, we asked: Do heroin-dependent Met allele carriers exhibit greater drug seeking behavior in the context of non-drug environmental alternatives? Our aim was to determine the extent of BDNF Val66Met associations with behavioral investment in opioid seeking.
MATERIALS AND METHODS
Participants
This investigation encompasses three source studies approved by Investigational Review Boards at Wayne State University and the University of Michigan (for methodological details, see Greenwald and Hursh, 2006; Greenwald and Steinmiller, 2009; Greenwald, 2010), and conducted in accordance with the Declaration of Helsinki. Certificates of confidentiality were obtained from the National Institute on Drug Abuse. Male and female volunteers, 18–55 years old, were recruited from the Detroit area using newspaper ads and word-of-mouth. Ethnicity and race were not exclusion factors but, due to low frequency of other racial/ethnic groups, only African-American (AA) and European-American (EA) subjects were included in the present data analyses. Those identifying themselves as heroin-dependent and not seeking treatment were instructed to call for a telephone interview.
The screening process included informed consent, providing demographic data, comprehensive substance use and medical histories, and an interview lasting 20–30 min that was used to obtain specific data about past-month income, drug purchasing and use factors (see below). All participants reported current daily heroin use, provided a urine sample that was positive for opioids (>300 ng/ml), and were diagnosed with current Opioid Dependence based on clinician interview using DSM-IV criteria. Urine samples were also tested for methadone, cocaine, benzodiazepines, cannabinoids and barbiturates. Volunteers had to provide an alcohol-free breath sample (< .002%). Participants were paid $30 for completing the first screening visit, and those who continued in screening toward qualifying for laboratory based studies could earn $25 more over two subsequent screening visits.
Genotyping
The Golden Gate drug addiction Illumina panel (Hodgkinson et al, 2008) was used to genotype blood samples provided by each participant. Whole blood (6 ml per subject) was collected into EDTA tubes and DNA was extracted using Qiagene kit (formerly Gentra Puregene kit).
Due to the relatively rare BDNF rs6265 Met/Met genotype (see Table 1), all analyses contrasted Met carriers (Met/Met + Met/Val) with Val homozygotes. To examine the specificity of the BDNF rs6265 polymorphism, four BDNF variants with relatively high minor allele frequencies (MAFs > .25) located in the 3′ untranslated (UTR) region (rs1519480, rs7124442, rs7934165, and rs11030121) were also included in the analyses. All of these 3′UTR SNPs were in linkage disequilibrium (LD) with the functional SNP (rs6265), and all were in LD with each other. Furthermore, none of the 3′UTR SNPs was significantly related to the phenotypes tested here. For this reason, these 3′UTR SNPs are not mentioned further.
Table 1.
BDNF Val/Met Genotype Distributions and Allele Frequencies (% within Group [row])
| Genotype (N) | Met/Met | Met/Val | Val/Val | Minor allele | Major allele |
|---|---|---|---|---|---|
|
| |||||
| rs6265 | A/A | A/G | G/G | A (Met) | G (Val) |
| Black (74) | 0 (0.0) | 5 (6.8) | 69 (93.2) | 5 (3.4) | 143 (96.6) |
| White (54) | 3 (5.6) | 17 (31.5) | 34 (63.0) | 23 (21.3) | 85 (78.7) |
| Overall (128) | 3 (2.3) | 22 (17.2) | 103 (80.5) | 28 (10.9) | 228 (89.1) |
Phenotyping Measures
Phenotypes were derived from a semi-structured interview that was previously validated with heroin abusers (Roddy and Greenwald, 2009; Roddy et al, 2011). Participants were asked a series of interrelated questions to ascertain past-month sources and amounts of income (legal and illegal), heroin price, estimated purity, all drug and non-drug expenditures, drug-acquisitive behaviors (e.g. purchase time, amount spent, purchasing frequency), and heroin consumption (e.g. bags per day, including the distribution of use throughout the day). To examine the behavioral specificity of BDNF genotype on drug seeking, we also ascertained daily cigarette use, alcohol use, and illegal drug use as part of a comprehensive substance use history questionnaire.
Data Analyses
BDNF Val66Met genotype and allelic distributions were computed for each ancestral group (Table 1). Genotype frequencies were tested for Hardy-Weinberg equilibrium using a web-based calculator (http://scienceforall.org/2010/06/20/hardy-weinberg-equilibrium-calculator/). Analyses were conducted using SPSS v.19. BDNF genotype comparisons on categorical variables (other BDNF genotypes, race, gender, route of heroin use [injection vs. non-injection], ever overdosed on heroin, and lifetime psychiatric diagnoses) were performed using chi-square tests. BDNF genotype comparisons for continuous variables were conducted using one-way Analyses of Variance (Table 2). Descriptive statistics are presented as means ± 1 standard deviation (SD). For all analyses, the criterion for null hypothesis rejection was set at nominal P < 0.05.
Table 2.
Characteristics of European American Participants (N = 54), by BDNF Val66Met Genotype
| Measure |
Met carrier N = 20 |
Val/Val N = 34 |
Effect size partial η2 (power) | χ2 [1,54] or F[1,53] (P=) |
|---|---|---|---|---|
| Demographics | ||||
| Gender (% male) | 70 | 71 | 0.01 (.964) | |
| Education (years) | 12.2 (0.8) | 12.5 (0.5) | .018 (.16) | 0.94 (.336) |
| Estimated IQ | 107.1 (7.6) | 109.0 (10.4) | .010 (.11) | 0.50 (.481) |
| History of Heroin Use | ||||
| Duration of regular use (years) | 19.8 (10.9) | 15.6 (9.5) | .040 (.30) | 2.17 (.147) |
| # Times tried to quit | 19.8 (29.5) | 14.4 (24.5) | .010 (.11) | 0.54 (.468) |
| Ever overdosed (%) | 45 | 44 | 0.01 (.950) | |
| Current Heroin Use | ||||
| Injection use (%) | 100 | 88 | 2.54 (.111) | |
| # Suppliers | 3.2 (2.0) | 3.1 (1.4) | .000 (.05) | 0.01 (.945) |
| Heroin unit price ($) | 10.00 (2.58) | 9.74 (3.81) | .001 (.06) | 0.08 (.784) |
| Purchase time (min) | 81.5 (66.7) | 52.1 (60.8) | .111 (.71) § | 6.52 (.014) |
| Unit purchase amount ($) | 46.50 (20.01) | 31.77 (21.21) | .135 (.80) § | 8.12 (.006) |
| # Weekly purchases | 11.9 (6.3) | 14.2 (9.8) | .010 (.11) § | 0.51 (.478) |
| Daily use (# bags) | 6.3 (3.4) | 5.1 (3.2) | .047 (.35) § | 2.57 (.115) |
| Other Recent Drug Use | ||||
| Cigarette use (# per day) | 17.9 (7.2) | 13.2 (8.9) | .073 (.50) | 4.03 (.050) |
| Alcohol use (# past 30 days) | 1.8 (3.6) | 2.1 (3.8) | .001 (.06) | 0.06 (.805) |
| Cocaine use | ||||
| # past 30 days | 3.0 (6.8) | 5.7 (7.9) | .031 (.24) | 1.64 (.206) |
| Positive urinalysis (%) | 28 | 68 | 7.53 (.006) | |
| Marijuana use | ||||
| # past 30 days | 0.2 (0.5) | 1.5 (4.4) | .032 (.25) | 1.71 (.197) |
| Positive urinalysis (%) | 11 | 32 | 2.83 (.092) | |
| Past-Month Income/Expenses | ||||
| Total income ($) | 2368 (1204) | 1829 (1287) | .064 (.46) § | 3.57 (.064) |
| Proportion income spent on: | ||||
| Heroin | 76.1 (14.6) | 76.5 (19.5) | .000 (.05) | 0.01 (.942) |
| Cigarettes | 4.8 (3.2) | 2.7 (2.9) | .109 (.70) | 6.36 (.015) |
| Food | 5.3 (4.8) | 5.7 (6.3) | .001 (.06) | 0.05 (.820) |
| Shelter/utilities | 4.2 (6.8) | 5.1 (8.2) | .003 (.07) | 0.16 (.693) |
| Lifetime DSM-IV Diagnoses (%)¶ | ||||
| Antisocial personality disorder | 50 (16) | 20 (25) | ||
| Anxiety disorder (any type) | 13 (16) | 12 (25) | ||
| Major depressive disorder | 31 (16) | 12 (25) | ||
| Alcohol use disorder | 69 (16) | 64 (25) | ||
| Cocaine use disorder | 63 (16) | 68 (25) | ||
| Cannabis use disorder | 64 (14) | 36 (24) | ||
Effect sizes and power are for log10-transformed variables. See text.
Psychiatric diagnostic data (based on SCID) were available for fewer participants than in the overall sample. Sample sizes are shown in parentheses adjacent to each percentage for each diagnosis. Substance use disorders refer to meeting criteria for lifetime abuse or dependence. Antisocial personality disorder refers to cases that also met criteria for childhood conduct disorder. Due to the smaller group sizes for DSM-IV diagnoses, statistical differences were not evaluated.
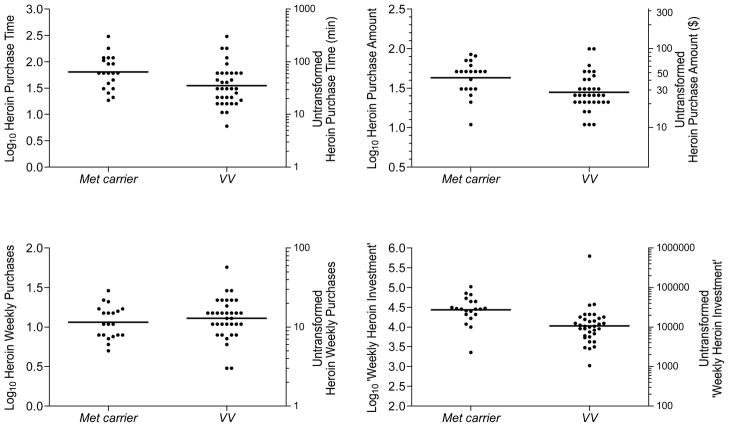
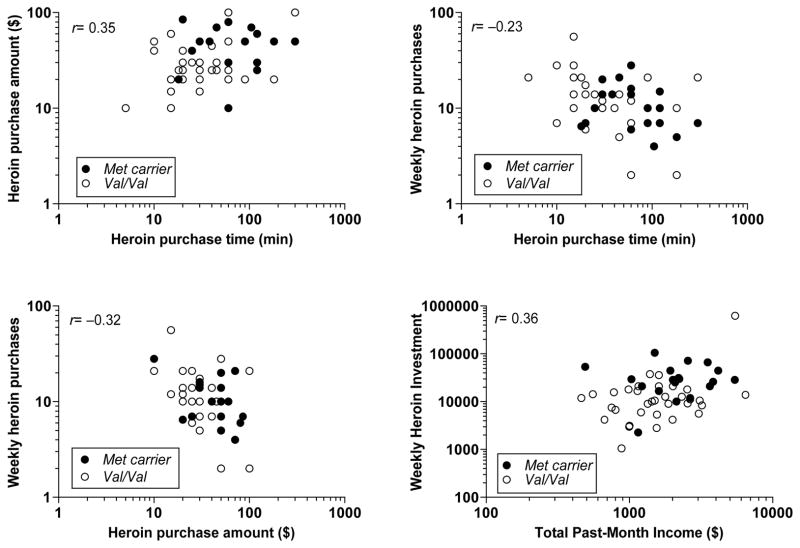
Variables that were not normally distributed (see below) were log10-transformed for correlation and regression analyses. See Figure 1 for distributions of heroin purchasing measures. We conducted tests of BDNF genotype effect for key measures of drug seeking/use behavior, and for control variables that – while not hypothesized as related to the genotype – might need to be included as covariates in regression analyses; Table 2 lists these variables. Pearson correlations were computed among behavioral economic measures (Figure 2), including drug supply factors (log10 past-month income, number of heroin suppliers, and unit cost), heroin purchasing pattern (log10 time, log10 amount, and log10 frequency; percent of income spent on heroin), heroin consumption (log10 total daily bags used), and non-heroin expenses (percent of income spent on food, shelter/utilities, cigarettes). This was also done to identify control variables for regression analyses in which we predicted different heroin seeking phenotypes (Table 3). We derived a novel heroin-purchasing summary score, “weekly heroin investment” [log10 purchase time × purchase amount × number of weekly purchases], expressed in dollar-minutes weekly.
Figure 1.
Response distributions for European American participants (N=54) with means (horizontal bars) by BDNF rs6265 genotype (Met carriers [n=20] vs. Val homozygotes [n=34]) for heroin-seeking phenotypes: Purchase Time (upper left panel), Purchase Amount (upper right panel), Weekly Purchases (lower left panel), and the empirically derived index “Weekly Heroin Investment” (lower right panel), which is the product score of purchase time × purchase amount × weekly purchases (measured in dollar-minutes weekly). For each measure, the log10-transformed scores are shown on the left ordinate, and the corresponding untransformed scores are illustrated on the right ordinate. For all measures except weekly heroin purchases, Met carriers significantly differed from Val homozygotes.
Figure 2.
Relationships between drug-seeking phenotypes in European-American participants (total N=54). Each panel illustrates a significant (p< .05) overall correlation between two heroin-seeking phenotypes (upper left: purchase time × purchase amount; upper right: purchase time × weekly purchases; lower left: purchase amount × weekly purchases), as well as differences in the response distributions between BDNF Met allele carriers (n=20; closed circles) and Val/Val genotype (n=34; open circles). Lower right panel: Combined prediction of ‘weekly heroin investment’ by BDNF genotype and past-month income (see Table 3, regression analysis).
Table 3.
Summary of Stepwise Multiple Regressions, European Americans (N=54)
| Log10 Heroin Purchase Time | |||||
|---|---|---|---|---|---|
| Predictors | ΔR2 | Cum. Adjust. R2 | β | T value | P value |
| BDNF rs6265 Met carrier | .111 | .094 | .334 | 2.554 | .014 |
| Log10 Heroin Unit Purchase Amount | |||||
| Predictor | ΔR2 | Cum. Adjust. R2 | β | T value | P value |
|
| |||||
| Total Past-Month Income | .158 | .142 | .325 | 2.569 | .013 |
| BDNF rs6265 Met carrier | .076 | .204 | .285 | 2.249 | .029 |
| Log10 Number of Weekly Heroin Purchases | |||||
| Predictor | ΔR2 | Cum. Adjust. R2 | β | T value | P value |
|
| |||||
| Number of suppliers | .108 | .090 | .355 | 2.784 | .008 |
| Age | .071 | .146 | −.267 | −2.092 | .041 |
| Log10 ‘Weekly Heroin Investment’ (Purchase Time × Amount × Frequency) | |||||
| Predictor | ΔR2 | Cum. Adjust. R2 | β | T value | P value |
|
| |||||
| BDNF rs6265 Met carrier | .184 | .169 | .361 | 2.882 | .006 |
| Total Past-Month Income | .068 | .223 | .270 | 2.159 | .036 |
| Log10 Daily Bags of Heroin Consumed | |||||
| Predictor | ΔR2 | Cum. Adjust. R2 | β | T value | P value |
|
| |||||
| Total Past-Month Income | .483 | .473 | .655 | 6.637 | .0001 |
| Age | .040 | .503 | −.203 | −2.058 | .045 |
ΔR2 refers to unadjusted change in variance accounted for by the individual predictor variable. Cum. Adjust. R2 refers to the cumulative (stepwise) adjusted variance accounted for. β refers to the standardized beta coefficient. T-test residual degrees of freedom in steps 1 and 2 of all models above are 52 and 51, respectively.
RESULTS
Participant Characteristics
Table 1 presents BDNF genotype and allelic frequencies for rs6265 (Met carrier vs. Val/Val) for African Americans (AA; N=74), European Americans (EA; N=54), and overall sample (N=128). Genotype frequencies for rs6265 did not deviate significantly from Hardy–Weinberg equilibrium in the European- or African-descent groups or the overall sample. Allele frequencies significantly differed between races, with the Met allele extremely rare in AA. Separate analyses were performed for EAs and AAs. For the present purposes, we primarily report results for EAs and include data for AAs in supplementary materials.
BDNF Val66Met Effect on Drug Seeking/Use
Univariate analyses
Some continuous measures of heroin seeking and income were not normally distributed. Figure 1 shows the distributions of responding by BDNF rs6265 genotype (Met carriers vs. Val homozygotes) for three primary heroin-seeking phenotypes: typical purchase time (min), average purchase amount (dollars); number of weekly purchases; and an empirically derived index referred to as “weekly heroin investment” that is the product of these three measures (purchase time × purchase amount × weekly purchases). Supplementary Figure 1 compares the distributions of these four phenotypes in EAs and AAs. Figure 2 illustrates the covariance among these phenotypes in EAs, while demonstrating BDNF Val66Met genotype differences in these response distributions.
One-way ANOVAs found significant BDNF rs6265 genotype differences such that Met carriers had longer purchase times and higher unit purchase amounts than Val homozygotes (see Table 2). Similar non-significant tendencies (Ps < .15) were observed for Met carriers to report longer duration of heroin use, more likely to inject heroin, and more daily bags consumed. Met carriers also reported marginally higher total past-month income. Supplementary Table 1 provides comparable data for AA subjects.
Multivariate analyses
Multivariate ANOVA was used to ascertain whether results remained significant for the four primary outcomes (purchase time, unit purchase amount, weekly purchases, and daily bags consumed) when adjusting for multi-collinearity among these measures (Figure 2); the “heroin investment” measure was excluded because it was derived directly from the first three measures. The MANOVA confirmed that the BDNF genotype effect remained significant, Hotelling F(4,49) = 3.17, p = .022.
Stepwise multiple regression analyses were used to determine whether Val66Met genotype and control variables predicted measures of heroin seeking in the natural environment. Initial analyses were stratified by ancestral race (EA vs. AA), after we determined this factor explained significant variance on some measures. In AAs, the infrequent Met allele was not related to any heroin seeking measure but due to its rarity, there was little power in the AA sample to detect such an effect. Thus, final regression analyses focused on European-Americans. Covariates in all these analyses were age, number of heroin suppliers, current injection heroin use, and total past-month income.
Table 3 shows that, in EAs (N = 54), BDNF Met allele carriers (n = 20) had significantly longer heroin purchasing times and higher purchase amounts than Val homozygotes (n = 34), which accounted for 11.1% and 7.6% of variance in these outcomes, respectively. BDNF Met carriers had significantly higher ‘weekly heroin investment’ scores than Val homozygotes (see Figure 1), which accounted for 18.4% of variance in this measure. Higher total income was the primary significant predictor of greater heroin unit purchase amounts and daily bags consumed (explaining 15.8% and 48.3% of variance in these two measures, respectively); whereas, income was a secondary predictor of the ‘weekly heroin investment’ score (6.8%, in contrast to 18.4% explained by BDNF genotype; see lower right panel of Figure 2 and Table 3).
In parallel regression analyses, BDNF genotype was not significantly related to number of weekly heroin purchases or number of daily bags consumed. Rather, number of weekly purchases was higher for subjects with more heroin suppliers and those who were younger (explaining 10.8% and 7.1% of the incremental variance, respectively).
BDNF Val66Met genotype and cigarette/nicotine use
Prevalence of smoking is very high among heroin-dependent individuals, including this sample, providing the opportunity to examine whether BDNF genotype impacts this other stable form of (legal) drug use. The influence of BDNF genotype on cigarette purchasing and use was examined in EAs (N = 54). Eighty-seven percent reported daily cigarette use. Two stepwise multiple regressions (which included non-smoking participants) were used to predict the proportion of past-month income spent on cigarettes and the number of cigarettes smoked daily (BDNF genotype groups significantly differed in univariate analyses; see Table 2), controlling for age, route of heroin use, total past-month income, and daily bags of heroin consumed. Relative to Val homozygotes, Met carriers reported spending a significantly higher proportion of income on cigarettes (standardized β = 0.33, t = 2.52, adjusted r2 = .092) and smoking significantly more cigarettes daily (standardized β = 0.27, t = 2.01, adjusted r2 = .073). No other predictors were significant.
DISCUSSION
The BDNF 66Met allele has been repeatedly associated with neurobehavioral deficits, including hippocampal and frontal-cortical volume loss, and impaired learning/memory. The Met allele leads to less BDNF secretion and reduced neurotrophic influence, which may decrease organismic behavioral flexibility or adaptive fitness. In this study, we theorized that the 66Met allele may confer resistance to environmentally- or pharmacologically-induced changes in drug seeking, or reduced behavioral flexibility, once addictive behavior has progressed to a chronic stage. To test this hypothesis we assessed several related phenotypes using a validated semi-structured interview method to assess past-month drug seeking/use.
Consistent with previous large population-based data, Met allele frequency was much greater among our European-ancestral than African-ancestral subjects (21 vs. 3%). Important to recognize is that prior associations between BDNF Val66Met genotype and neurobehavioral deficits were observed exclusively among European/Caucasian samples, where the Met allele is more informative.
Among EAs in this study, the 66Met allele was significantly associated with increases in several drug-seeking behaviors. Bivariate relationships between Val66Met genotype and drug seeking/use were initially observed for heroin purchase time, purchase amount, and an empirically derived ‘weekly heroin investment’ score (purchase time × amount × weekly frequency); effect sizes were moderate. In stepwise multiple regression analyses that controlled for other factors that showed zero-order correlations with the BDNF genotype (younger age, higher income, more heroin suppliers, and injection route of heroin use), these bivariate relationships remained significant. A significant genotype effect was not observed for number of weekly purchases or daily bags of heroin consumed. Val66Met genotype accounted for unique variance (change in r2 value) of 7.6%, 11.1%, and 18.4% in heroin unit purchase amount, purchase time, and weekly heroin investment, respectively. Thus, BDNF Val66Met genotype was more closely related to measures of drug seeking than consumption. This is critical for selecting an appropriate phenotype: Heroin and other illegal drug users (including many in our sample) often obtain some drug free (e.g. shared by others at no cost), or through bartering (e.g. providing sex or transportation for drugs). These behavior patterns were assessed in our interview, because we noted in our validation studies the potential for dissociation between drug seeking and drug consumption.
Chen et al. (2006) generated a transgenic mouse model of the BDNF Val66Met polymorphism. Met-homozygous animals – who exhibit 50% lower BDNF levels and ≈30% less activity-dependent BDNFMet release from neurons – demonstrate greater anxiety- and depression-like behaviors (without alterations in locomotion), and loss of hippocampal volume and less dendritic complexity in dentate gyrus neurons. Thus, it may be useful to test associations with addictive behavior in this model. We predict that in mice trained to self-administer heroin-like opioids, Met/Met (versus Val/Val) mice would exhibit higher breakpoints, be less responsive to medication and environmental-incentive induced disruptions of this behavior, and reinstate (following extinction) opioid self-administration more readily.
A limitation of this study is that the sample size was rather small, so hypotheses related to epistatic effects could not be tested with adequate statistical power. The preliminary results of this study are thus hypothesis generating, given the large number of tests performed on key phenotypes (which exhibited multi-collinearity with one another) and control variables, and – despite surviving multivariate adjustment – need to be confirmed. Nevertheless, we believe that several of these results are likely correct, due to BDNF’s biological plausibility as a potential mediator or moderator of addictive behavioral processes. Specifically, there is growing evidence that BDNF is involved in behavioral sensitization following repeated exposure to abused drugs including opioids. For instance, BDNF interacts closely with the dopamine D3 autoreceptor (DRD3; Le Foll et al, 2005), which controls phasic dopamine activity (Sokoloff et al, 2006) and is implicated in conditioned drug seeking behavior (Everitt and Robbins, 2000). Striatal BDNF/DRD3 interactions could be one candidate mechanism by which drug seeking is sensitized and instantiated as habitual behavior (Vanderschuren and Kalivas, 2000; Gerdeman et al, 2003). This suggests important avenues for research, including whether dopamine polymorphisms could act in epistasis with BDNF to modulate drug seeking.
Given the potentially widespread neurotrophic influence of BDNF, an important question concerns the generality of its effects (behavioral specificity). In this study, we observed in EAs that BDNF Val66Met genotype also accounted for 9.2% of variance in purchasing (percent of income spent) and 7.3% of variance in use (daily number) of cigarettes. These findings in our predominantly male subject sample are consistent with a study of nicotine-dependent smokers (Beuten et al, 2005) and a recent large-scale genome-wide association study (The Tobacco and Genetics Consortium, 2010). Although an association of Val66Met with smoking severity was not found in the Beuten et al. study, a haplotype showed a significant relationship in male EA smokers, but not female EAs or AA smokers. Other evidence points to a role of BDNF, D3 and D1 receptor polymorphisms in nicotine addiction, specifically, with quantity of tobacco smoked (Novak et al, 2010). The BDNF Val66Met genotype thus seems to produce an effect on drug seeking/use in EA populations that is broader than for one particular class of abused substances, and may therefore be of general importance to addiction.
On the other hand, urinalysis and self-report data were collected at screening to ascertain recent use of drugs besides opioids. Results indicated that, relative to BDNF Val homozygotes, significantly fewer Met allele carriers tested positive for cocaine. A similar non-significant trend was observed for cannabis use. This finding does not support the idea that the BDNF Met allele is related to generally greater substance use. Rather, these data are consistent with the hypothesis that the Met allele is a risk factor for promoting the engrained use of preferred drugs – in this case, heroin and cigarettes.
Given the present preliminary findings and published data, our working hypothesis is that the 66Met allele, which leads to decreased BDNF secretion and less neuroadaptation due to lower rates of cell proliferation, impairs behavioral flexibility once drug self-administration becomes habitual. This neurotrophic environment may promote a range of neurobehavioral deficits. With specific regard to addiction, reduced BDNF function and its sequelae may strengthen reinforcing efficacy of preferred drugs relative to non-drug alternatives or despite pharmacotherapy. In short, selected forms of drug-seeking behavior may become entrenched and more resistant to change in BDNF Met carriers. We found reliable 66Met genotype differences across opioid seeking phenotypes (i.e. increased purchase time and increased purchase amount), and across drug classes (greater opioid and nicotine use). Taken together with prior findings, the robust effects of this genotype may implicate its broader importance for understanding and treating addictive behavior and underlying processes such as impairments in learning/memory. If results of future work confirm the influence of this neurotrophic genotype, 66Met allele carriers might require higher levels of intervention (e.g. cognitive behavior therapy or pharmacotherapy) to overcome their chronic drug use pattern. Although we observed behavioral effects of the Val66Met genotype in a restricted sample (small in size and primarily for EAs), this converging pattern of association increases confidence that the results are meaningful. Further research could be theoretically and clinically useful by determining whether this hypothesis applies to the habitual use of other substances.
Supplementary Material
Acknowledgments
NIH grant R01 DA015462 from the National Institute on Drug Abuse and a research grant (Joe Young, Sr. Funds) from the State of Michigan supported this research. Data for this study were obtained under registered NIH clinical trials NCT00218309, NCT00218361, and NCT00608504.
The authors thank Ken Bates for recruiting participants; Debra Kish, Joi Moore and Lisa Sulkowski for data collection and management; staff members of the Psychiatric and Addiction Research Center at Wayne State University for clinical data collection and safety monitoring; and Srijan Sen for statistical advice.
Footnotes
AUTHORS CONTRIBUTION
MKG was responsible for the study concept, design, analysis, and drafting the manuscript. CLS contributed to study implementation, interviewing, data coordination and management. ES conducted the genotyping analyses. LHL contributed to psychiatric screening and edited the manuscript. MB was responsible for the genotyping approach, overseeing analysis, and editing the manuscript. All authors have reviewed content and approved the final version for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest with respect to the conduct or content of this work.
References
- 1.Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DW, Lindsay RM, Nestler EJ. Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–79. doi: 10.1016/0306-4522(95)00207-y. [DOI] [PubMed] [Google Scholar]
- 2.Beuten J, Ma JZ, Payne TJ, Dupont RT, Quezada P, Huang W, Crews KM, Li MD. Significant association of BDNF haplotypes in European-American male smokers but not in European-American female or African-American smokers. Am J Med Genet B Neuropsychiatr Genet. 2005;139:73–80. doi: 10.1002/ajmg.b.30231. [DOI] [PubMed] [Google Scholar]
- 3.Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 4.Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta J-K. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153 (Suppl 1):S310–324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng C-Y, Hong C-J, Yu YW-Y, Chen T-J, Wu H-C, Tsai S-J. Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. Mol Brain Res. 2005;140:86–90. doi: 10.1016/j.molbrainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo C, Di Lorenzo G, Sances G, Ghiotto N, Guaschino E, Grieco GS, Santorelli FM, Casali C, Troisi A, Siracusano A, Pierelli F. Drug consumption in medication overuse headache is influenced by brain-derived neurotrophic factor Val66Met polymorphism. J Headache Pain. 2008;10:349–355. doi: 10.1007/s10194-009-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 12.Enoch MA, Hodgkinson CA, Yuan Q, Albaugh B, Virkkunen M, Goldman D. GABRA1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology. 2009;34:1245–1254. doi: 10.1038/npp.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- 14.Frodl T, Schüle C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Möller HJ, Meisenzahl EM. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. 2007;64:410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- 15.Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 16.Goggi J, Pullar IA, Carney SL, Bradford HF. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro. Brain Res. 2002;941:34–42. doi: 10.1016/s0006-8993(02)02505-2. [DOI] [PubMed] [Google Scholar]
- 17.Greenwald MK. Effects of experimental Unemployment, Employment and Punishment analogs on opioid seeking and consumption in heroin-dependent volunteers. Drug Alcohol Depend. 2010;111:64–73. doi: 10.1016/j.drugalcdep.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwald MK, Hursh SR. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: Effects of unit price and pre-session drug supply. Drug Alcohol Depend. 2006;85:35–48. doi: 10.1016/j.drugalcdep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald MK, Steinmiller CL. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: Effects of alternative reinforcer magnitude and post-session drug supply. Drug Alcohol Depend. 2009;104:84–93. doi: 10.1016/j.drugalcdep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 21.Hariri A, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor Val66Met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatami H, Oryan S, Semnanian S, Kazemi B, Bandepour M, Admadiani A. Alterations of BDNF and NT-3 genes expression in the nucleus paragigantocellularis during morphine dependency and withdrawal. Neuropeptides. 2007;41:321–328. doi: 10.1016/j.npep.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Hodgkinson CA, Yuan Q, Xu K, Shen P-H, Heinz E, Lobos EA, et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khokhar JY, Ferguson CS, Zhu AZX, Tyndale RF. Pharmacogenetics of drug dependence: role of gene variations in susceptibility and treatment. Ann Rev Pharmacol Toxicol. 2010;50:39–61. doi: 10.1146/annurev.pharmtox.010909.105826. [DOI] [PubMed] [Google Scholar]
- 25.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 26.Lang UE, Sander T, Lohoff FW, Hellweg R, Bajbouj M, Winterer G, Gallinat J. Association of the met66 allele of brain–derived neurotrophic factor (BDNF) with smoking. Psychopharmacology. 2007;190:433–439. doi: 10.1007/s00213-006-0647-1. [DOI] [PubMed] [Google Scholar]
- 27.Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- 28.Lerman C, Perkins KA, Gould TJ. Nicotine-dependence endophenotypes in chronic smokers. In: Swan GE, Baker TB, Chassin L, Conti DV, Lerman C, Perkins KA, editors. National Cancer Institute. Phenotypes and endophenotypes: foundations for genetic studies of nicotine use and dependence. Tobacco Control Monograph No. 20. Bethesda, MD: US Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. pp. 403–484. NIH Publication No. 09–6366. [Google Scholar]
- 29.Li MD, Burmeister M. New insights into the genetics of addiction. Nat Rev Genet. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- 31.Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- 32.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maisonpierre PC, Le Beau MM, Espinosa R, III, Ip NY, Belluscio L, de la Monte SM, Squinto S, Furth ME, Yancopoulos GD. Human and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics. 1991;10:558–568. doi: 10.1016/0888-7543(91)90436-i. [DOI] [PubMed] [Google Scholar]
- 34.Matsushita S, Kimura M, Miyakawa T, Yoshino A, Murayama M, Masaki T, Higuchi S. Association study of brain-derived neurotrophic factor gene polymorphism and alcoholism. Alcohol Clin Exp Res. 2004;28:1609–1612. doi: 10.1097/01.alc.0000145697.81741.d2. [DOI] [PubMed] [Google Scholar]
- 35.Novak G, LeBlanc M, Zai C, Shaikh S, Renou J, DeLuca V, Bulgin N, Kennedy JL, Le Foll B. Association of polymorphisms in the BDNF, DRD1 and DRD3 genes with tobacco smoking in schizophrenia. Ann Hum Genet. 2010;74:291–298. doi: 10.1111/j.1469-1809.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- 36.Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–10708. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, Waggoner SG, Tahl AR, Sklar P. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 2010;15:810–815. doi: 10.1038/mp.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roddy JK, Greenwald MK. An economic analysis of income and expenditures in heroin-using research volunteers. Substance Use Misuse. 2009;44:1503–1518. doi: 10.1080/10826080802487309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roddy JK, Steinmiller CL, Greenwald MK. Heroin purchasing is income- and price-sensitive. Psychol Addict Behav. 2011;25:358–364. doi: 10.1037/a0022631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56:73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sklair-Tavron L, Shi W-X, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 45.Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, Goldman D, Malhotra AK. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 46.The Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 48.Vargas-Perez H, Kee RT-A, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naïve rats. Science. 2009;324:1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, Sliwerska E, Burmeister M. Association between Val66Met brain-derived neurotrophic factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcohol: Clin Exp Res. 2009;33:1–10. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong CCY, Schumann G. Genetics of addictions: strategies for addressing heterogeneity and polygenicity of substance use disorders. Phil Trans R Soc B. 2008;363:3213–3222. doi: 10.1098/rstb.2008.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann N Y Acad Sci. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.