Abstract
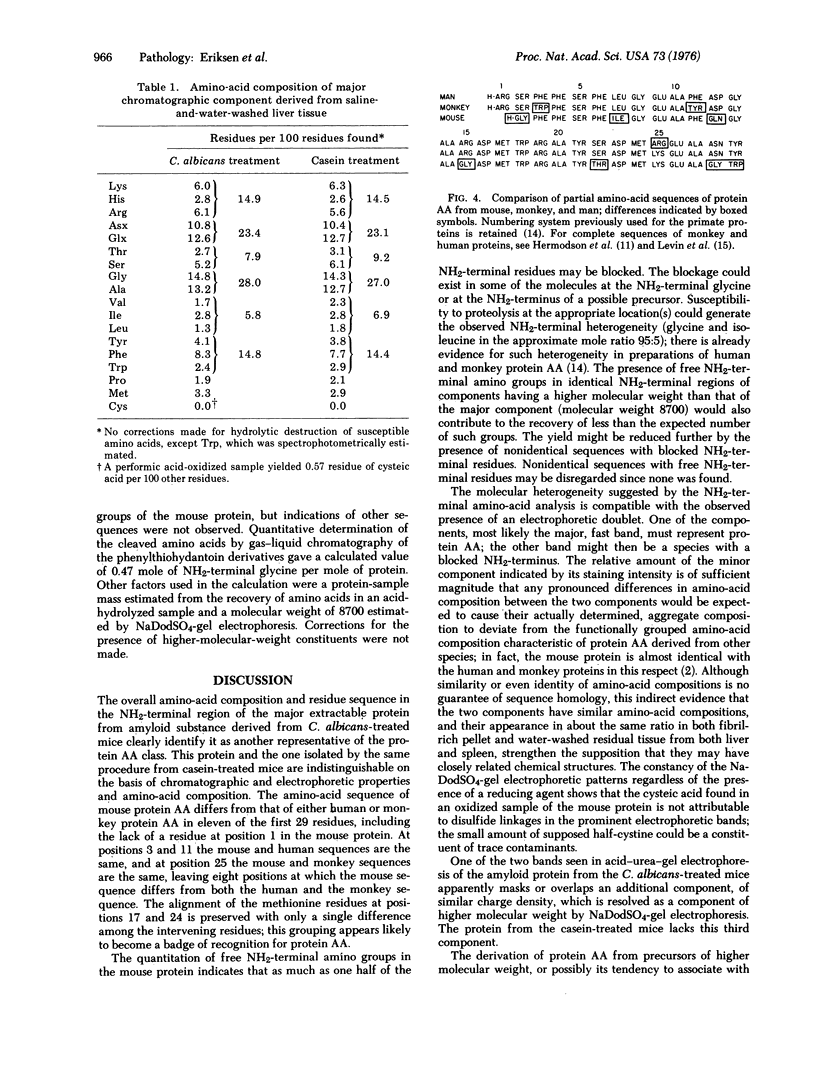
The major protein extracted from anyloid deposits induced in mice by injection of either Candida albicans cells or sodium caseinate was found to have chromatographic and electrophoretic properties and an amino-acid compostiion characteristic of the AA class of amyloid proteins. The homology of the mouse protein with protein AA from man and monkey was established by determination of the sequence of the first 28 amino-acid residues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Natvig J. B., Michaelsen T. E., Husby G. Isolation and characterization of amyloid-related serum protein SAA as a low molecular weight protein. Scand J Immunol. 1975;4(4):397–401. doi: 10.1111/j.1365-3083.1975.tb02642.x. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid. 3. A protein related to the subunit structure of human amyloid fibrils. Proc Natl Acad Sci U S A. 1966 Feb;55(2):308–316. doi: 10.1073/pnas.55.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical classes of amyloid substance. Am J Pathol. 1971 Oct;65(1):231–252. [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical similarity among amyloid substances associated with long standing inflammation. Lab Invest. 1972 Jun;26(6):615–625. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Benson M. D., Skinner M., Lian J., Cohen A. S. "A" protein of amyloidosis. Isolation of a cross-reacting component from serum by affinity chromatography. Arthritis Rheum. 1975 Jul-Aug;18(4):315–322. doi: 10.1002/art.1780180404. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Page D., Isersky C., Harada M., Cuatrecasas P., Eanes E. D., DeLellis R. A., Bladen H. A., Keiser H. R. Murine amyloid fibril protein: isolation, purification and characterization. J Histochem Cytochem. 1971 Jan;19(1):16–28. doi: 10.1177/19.1.16. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W. D., Isersky C. Amyloidosis: its nature and pathogenesis. Semin Hematol. 1973 Jan;10(1):65–86. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R. P., Sipe J. D., Pollock P. S., Ignaczak T. F., Glenner G. G. Isolation of a low-molecular-weight serum component antigenically related to an amyloid fibril protein of unknown origin. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1473–1476. doi: 10.1073/pnas.72.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E. Amino acid sequence of chymotryptic peptides from horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2161–2174. [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M., Cathcart E. S., Cohen A. S., Benson M. D. Isolation and identification by sequence analysis of experimentally induced guinea pig amyloid fibrils. J Exp Med. 1974 Sep 1;140(3):871–876. doi: 10.1084/jem.140.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]




