Abstract
Increased trophoblast TNFα production is an important component of placental dysfunction in preeclampsia. However, the mechanism of increased TNFα production in the preeclamptic placenta is largely unknown. ADAM17 is a metallopeptidase that functions as a TNFα converting enzyme. In this study, we examined ADAM17 expression in placentas from normal and preeclamptic pregnancies and found increased ADAM17 expression in preeclamptic placentas compared to those from normal placentas, p<0.05. Since hypoxia/oxidative stress is an underlying pathophysiology in the preeclamptic placenta, we further determined if hypoxia/oxidative stress could modulate ADAM17 expression and subsequently induce TNFα production in placental trophoblasts. Trophoblasts were isolated from normal term placentas and treated with cobalt (II) chloride (CoCl2), a hypoxia mimetic agent, at different concentrations. Our results showed that CoCl2 induced a dose-dependent increase in TNFα production that is associated with enhanced ADAM17 expression. Trophoblast expressions of HO-1 (a sensor of cellular oxidative stress) and caspase-3 (an indicator of apoptosis) in response to CoCl2 stimulation were also examined. We further found that metallopeptidase inhibitor GM6001 and ADAM17 siRNA could block CoCl2 induced TNFα production, demonstrating the role of ADAM17 in TNFα production in placental trophoblasts. These results suggest that oxidative stress-induced increased ADAM17 expression could contribute to the increased TNFα production in preeclamptic placentas.
Keywords: ADAM17, TNFα, oxidative stress, trophoblast, preeclampsia
Introduction
Preeclampsia is a multisystem disorder in human pregnancy that is characterized by maternal hypertension and renal dysfunction. It affects about 5-7% of pregnant women and is one of the leading causes of maternal and fetal morbidity and mortality in human pregnancy. Although the cause of preeclampsia is not clear, the initial pathophysiological event in preeclampsia is thought to be associated with poor trophoblast invasion during early placental development that results in placental hypoxia/ischemia and a reduction of uteroplacental blood perfusion. Increased placental oxidative stress is also associated with increased trophoblast release of inflammatory cytokines (1) and anti-angiogenic factor sFlt-1 (2) and maternal vascular endothelial dysfunction (3, 4). TNFα expression is increased in placentas from women with preeclampsia (5). Increased placental TNFα production in response to local hypoxia is likely to play an important role in placental oxidative stress and endothelial injury associated with preeclampsia (5-8). However, the mechanism of TNFα production by placental cells is largely unknown.
TNFα is a potent cytokine with a wide range of pro-inflammatory activities including stimulation of collagenase and prostaglandin production and activation of neutrophils via activation of NFκB and MAPK signaling pathways (9-11). TNFα, a 17kDa soluble molecule, is converted from proTNF, a 26 kDa transmembrane protein (12). TNFα induces inflammatory activities on target cells, whereas proTNF is involved in cellular immune responses (13, 14). Studies have shown that proteinases such as a disintegrin and metalloproteinases (ADAMs) and matrix metalloproteases (MMPs) are able to cleave proTNF into its soluble form (15-17). Among them, ADAM17 is thought to be a major sheddase for TNFα, therefore named as TNFα converting enzyme or TACE (15, 18). Other than TNFα, several transmembrane proteins have also been identified as substrates for ADAM17, including TNF receptor II (16), IL-15 receptor (19, 20), EGF-R (20), L-selectin (21), etc. Ectodomain shedding has a significant impact on the biological function of these proteins by converting their membrane form into soluble format. Although TNFα is one of the most important substrates for ADAM17, little information is available about ADAM17 expression in preeclamptic placentas. The present study was undertaken to determine if ADAM17 expression was increased in the preeclamptic placenta. Since oxidative stress is an event of underlying pathophysiology in the preeclamptic placenta, we then determined if ADAM17 was responsible for TNFα shedding by placental trophoblasts under oxidative stress challenge. Effects of metallopeptidase ADAM inhibitor GM6001 and ADAM17 siRNA on TNFα production and/or ADAM17 expression were also determined.
Materials and Methods
Materials
GM6001 and GM6001 negative control were from Calbiochem (San Diego, CA). Antibodies for ADAM17 (H-300), HO-1 (C-18), and caspase-3 (E-8) were from Santa Cruz (San Diego, CA). Antibody for ADAM17 (57484) and isotype IgG control (18421) from Abcam Inc., (Cambridge, MA) was also used. ADAM17 siRNA was from Thermo Scientific (Rockford, IL). Trypsin and DNase I were from Washington Biochemical Corp. (Lakewood, NJ). TNFα ELISA kit was from R&D System (Minneapolis, MN). All other chemicals and reagents were from Sigma (St. Louis, MO) unless otherwise noted.
Placenta collection
Placentas delivered by normal and preeclamptic pregnant women were collected immediately after delivery. A total of 43 placentas were used in this study. Among them, tissue sections from 15 placentas were used for the immunohistochemistry experiment, snap frozen tissue pieces from 11 placentas were used for detection of protein and mRNA expression, and 17 placentas from normal term placentas were used for trophoblast isolation and in vitro cell culture studies. Normal pregnancy was defined as pregnancy with normal blood pressure (<140/90mmHg), negative proteinuria, and absence of obstetrical and medical complications. Preeclampsia was defined as follows: sustained systolic blood pressure of ≥ 140 mmHg or a sustained diastolic blood pressure of ≥ 90mmHg on two separate readings; proteinuria measurement of 1+ or more on dipstick, or 24 hrs urine protein with ≥ 300mg in the specimen. No patient had signs of infection. Smokers and patients complicated with HELLP syndrome (hemolysis, elevated liver enzyme and low platelet count), diabetes and/or renal disease were excluded. Placental collection was approved by the Institutional Review Board (IRB) for Human Research at Louisiana State University Health Sciences Center-Shreveport.
Immunohistochemistry
Expression for ADAM17 was examined by immunohistochemistry in paraffin embedded placental tissue sections. A standard immunohistochemistry staining procedure was performed as previously described (22, 23). Briefly, a series of deparaffinization was done with xylene and ethanol alcohol. Antigen retrieval was performed by boiling tissue slides in 0.01M citric buffer. Hydrogen peroxide was used to quench the endogenous peroxidase activity. After blocking, tissue sections were incubated with primary monoclone antibody specific against human ADAM17 (Abcam). Corresponding secondary antibody and DAB chromogen ABC staining system (Santa Cruz, San Diego, CA) were used. Slides stained with isotype IgG were used as the negative control. The nuclei were counterstained by haematoxylin. Stained slides were examined by an Olympus microscope (Olympus IX 71). Images were captured by a digital camera with PictureFrame computer software (Uptronics Inc., Sunnyvale, CA) and recorded to a microscope linked PC computer.
The intensity of ADAM17 expression was evaluated semiquantitatively as previously described (23) by two laboratory personnel independently using the following categories: 0 (no staining); 1+ (detectable but weak staining); 2+ (moderate or distinct staining), and 3+ (intensive staining). In each slide, five different areas with 15-20 villi per area were evaluated microscopically with a 20x objective magnification. For each specimen, a H-score value was derived by calculating the sum of the percentage of villi that stained in each intensity category and multiplying that value by the weighted intensity of the staining, using the formula H-score = ∑Pi (i + l), where i represents the intensity score and Pi is the corresponding percentage of villi.
Trophoblast isolation and culture conditions
Normal term placentas were collected. Trophoblasts were isolated by trypsin digestion (0.125 % trypsin solution containing 0.1mg/ml DNase I and 5mM MgCl2) in Dulbecco’s Modified Eagle Medium (DMEM) at 37°C for 90 min. Isolated trophoblasts were further purified by Percoll gradient centrifugation and contaminated red blood cells were eliminated by incubation of isolated trophoblast cells with red blood cell lysis buffer (2). Isolated trophoblasts (5×106cells/well) were then incubated with DMEM containing 5% fetal bovine serum (FBS) and antibiotics in 6-well plates. After overnight culture, fresh medium was replaced and trophoblasts were then treated with cobalt (II) chloride (CoCl2, a hypoxic mimetic agent) at different concentrations of 0, 100, 250, and 500μM for 48 hrs to induce oxidative stress. Culture medium was collected at the end of the experiments and medium concentrations for TNFα were measured by enzyme-linked immunoassay (ELISA). Cellular protein was obtained by lysis of trophoblasts with ice-cold protein lysis buffer and used for protein expression by Western blot. In separate experiments, trophoblasts were treated with CoCl2 at different concentrations of 0, 100, 250, and 500μM in the presence or absence of GM6001, a metallopeptidase inhibitor. GM6001 at a concentration of 50μM was used. Culture medium was collected at the end of the experiment and TNFα concentrations were measured by ELISA.
ADAM17 siRNA transfection assay
To determine if ADAM17 specifically regulates TNFα production by placental trophoblasts, ADAM17 siRNA transfection assay was performed. Briefly, 30nM of ADAM17 siRNA or control siRNA were transfected into primary isolated trophoblasts (5 ×106 cells/well) 24 hrs after seeding using Lipofectamine™ RNAiMAX transfection agent (Invitrogen, Carlsbad, CA). Total cellular protein was collected after 48 hrs of transfection and protein expression for ADAM17 was determined by Western blot. Medium was collected and TNFα concentrations were measured by ELISA.
Measurement of TNFα production
Trophoblast production of TNFα was measured by ELISA. The assay was performed following the manufacturer’s instructions. The range of standard curve for TNFα was 0.98 to 125 pg/ml. An aliquot of 100μl of trophoblast culture medium was assayed in duplicate. Within- and between-assay variations were < 8% for all assays. For data calculation, TNFα concentration (pg/5×106 cells/ml) in control cells was presented as 1 and fold changes were then calculated for the treated cells.
Protein expression by Western blot
Protein expression of ADAM17 was examined by Western blot in snap frozen placental tissues and in isolated trophoblasts after culture. For placental tissue expression, total tissue protein was extracted from snap frozen tissue using protein lysis buffer. For trophoblast expression, cellular protein was extracted after cells were treated with CoCl2 as stated above. An aliquot of total protein (10μg of each sample) was subject for electrophoresis (Bio-Rad, Hercules, CA) and then transferred to Hybond-protein transfer membrane (Amersham Corp, Arlington Heights, IL). The membrane was blocked with 5% milk in phosphate buffered saline (PBS) and then probed with ADAM17 antibody (Santa Cruz) at 4°C overnight. The bound antibody was visualized with an enhanced chemiluminescent (ECL) detection Kit (Amersham Corp). β-actin expression was determined and used as the loading control for each sample. The density was scanned and analyzed by NIH Image 1.16. Relative density for ADAM17 expression was normalized by β-actin expression for each sample. Trophoblast expressions for HO-1 and caspase-3 in response to CoCl2 stimulation were also determined by Western blot.
mRNA expression
Total RNA was isolated from snap frozen tissue and an aliquot of total RNA (1μg) per sample was used for reverse transcription with AffinityScript QPCR cDNA synthesis kit (Stratagene, La Jolla, CA). First-strand cDNA (2μl) was then used as a template for PCR by GoTaq PCR Core System I (Promaga, Madison, MI). The ADAM17 primer was designed based on accession # NM_003183. The forward sequence is 5′-ATTGGTGGTAGCAGATCATCG-3′ and reverse sequence is 5′-TGGGAGAGCCAACATA AGCTA-3′, which produces a 389bp fragment. mRNA expression for glyceraldehyd 3-phosphate dehydrogenase (GAPDH) was also determined as a house keeping gene. The forward primer sequence is 5′- CAAAAGGGTCATCATCTCTGC -3′ and reverse sequence is 5′- AGTTGTCATGGATGACCTTGG-3′. Primers were synthesized by Integrated DNA Technologies, Inc. (IDT, Coralville, CA). PCR products were separated by 1% agarose electrophoresis. The gel density was captured by VersaDoc Imaging System (Bio-Rad) and analyzed by NIH Image 1.16. Relative density of ADAM17 mRNA expression was normalized by GAPDH expression for each sample.
Statistical analysis
Data is presented as mean±SE. The immunostaining data for ADAM17 expression was analyzed by nonparametric Mann Whitney test, and paired and unpaired t-test. The ELISA data for TNFα production and Western blot data for ADAM17, HO-1, and caspase-3 expressions were analyzed by analysis of variance (ANOVA). Fisher’s PLSD test or Student-Newman-Keuls test was used as post hoc tests. The computer software program StatView was used. A probability level less than 0.05 was set as statistically significant.
Results
ADAM17 expression in normal and preeclamptic placentas
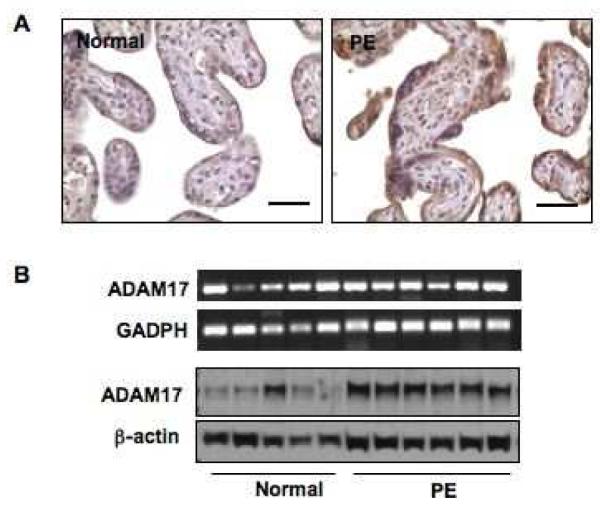
ADAM17 protein expression was determined by immunohistochemistry and Western blot, and mRNA expression by reverse transcription polymerase chain reaction (RT-PCR). For immunohistochemistry, tissue sections from 15 placentas were examined, 7 from normal pregnant women and 8 from women with preeclampsia. For total protein and mRNA expressions, snap frozen tissue pieces from 11 placentas, 5 from normal pregnant women and 6 from women with preeclampsia, were used. The patient demographic information was summarized in Table 1. Figure 1A shows representative ADAM17 immunostaining of normal and preeclamptic tissue sections. Positive ADAM17 staining was mainly localized in the syncytiotrophoblast layer in placental tissue sections. ADAM17 immunostaining was intensively expressed in trophoblasts of preeclamptic placentas compared to those of normal placentas. Slides stained with isotype IgG antibody showed negative staining (data not shown). The H-score for ADAM17 was 2.984±0.764 for preeclamptic placentas, which was significantly higher than 1.629±0.482 for normal placentas, p<0.05.
Table 1.
Demographic characteristics for normal and preeclamptic pregnant women
| Normal (n=12) | Preeclampsia (n=14) | p value | |
|---|---|---|---|
| Maternal Age (years) | 26 ± 8 | 24 ± 4 | 0.98 |
| Racial Status | |||
| White | 3 | 4 | NC |
| Black | 8 | 10 | NC |
| Other | 1 | ||
| Gestational Age (weeks) | 39 ± 2 | 32 ± 4 | <0.05 |
| Blood Pressure (mmHg) | |||
| Systolic | 119 ± 11 | 164 ± 14 | <0.01 |
| Diastolic | 62 ± 15 | 98 ± 11 | <0.01 |
| Gravidity | |||
| Pimigravid | 5 | 7 | NC |
| Multigravid | 7 | 7 | NC |
| Mode of Delivery | |||
| Vaginal | 4 | 9 | NC |
| Caesarean Section | 8 | 5 | NC |
| Placenta Weight (gram) | 563 ± 100 | 319 ± 132 | <0.01 |
Data presented as mean ± SD. NC: not calculated
Figure 1.
ADAM17 expression in normal and preeclamptic placentas. A: Representative ADAM17 immunostaining in normal and preeclamptic (PE) placentas. ADAM17 immunostaining is mainly localized in the syncytiotrophoblasts in placental villous tissue. Intensive ADAM17 immunostaining is shown in placentas from women with PE compared to those from normal pregnant controls. Bar = 50 micron. B: mRNA and protein expressions for ADAM17 in normal and preeclamptic placentas. Protein expression, but not mRNA, of ADAM17 is increased in preeclamptic placentas.
Figure 1B shows mRNA and total protein expressions of ADAM17 in normal and preeclamptic placentas. There was no significant difference for ADAM17 mRNA expression between normal and preeclamptic placentas (0.948±0.160 vs. 0.904 ± 0.049, p=0.465). However, total protein expression of ADAM17 was significantly increased in preeclamptic placentas compared to normal placentas, 1.014±0.049 vs. 0.594± 0.12, p<0.05. The increased protein, but not mRNA, expression in the preeclamptic placental tissue suggests that ADAM17 expression/activity is mediated through post-transcriptional/translational regulatory mechanisms.
ADAM17 is responsible for TNFα production in trophoblasts
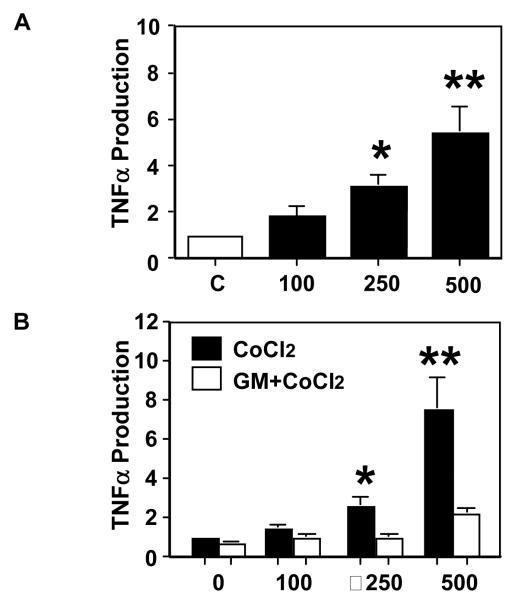
As shown in Figure 1A, ADAM17 is mainly expressed in syncytiotrophoblasts. To study if ADAM17 is responsible for TNFα production/release, a cellular hypoxia/oxidative stress model was used in which primary isolated trophoblasts from normal term placentas were treated with CoCl2. CoCl2 is a hypoxia mimetic agent which has been used in various in vitro cell culture studies including trophoblasts (24-27). This cell culture model is used because increased oxidative stress is an underlying pathophysiology in the preeclamptic placenta. Our results showed that trophoblast release of TNFα was significantly increased in a dose-dependent manner in response to CoCl2 stimulation, Figure 2A.
Figure 2.
Hypoxia promotes TNFα production and metallopeptidase ADAM inhibitor blocks TNFα production by placental trophoblasts in culture. A: TNFα production in trophoblasts treated with CoCl2 at concentration of 100, 250, and 500μM for 48hrs. CoCl2 induced TNFα production is in a dose-dependent manner. Data are mean ± SE from 4 independent primary trophoblast culture experiments. B: TNFα production by trophoblasts treated with CoCl2 at different concentrations (0, 100, 250, and 500μM) in the presence or absence of a metallopeptidase inhibitor GM6001. GM6001 at 50μM concentration was used. Trophoblast production of TNFα induced by CoCl2 was significantly inhibited by GM6001. TNFα production by untreated cells is presented as 1 for each experiment. The data is presented as mean ± SE of fold changes for TNFα productions in CoCl2 treated cells vs. untreated controls Data are from 6 independent primary trophoblast culture experiments.
We next investigated if ADAM17 was responsible for TNFα release in placental trophoblasts treated with CoCl2. Cultured trophoblasts were treated with different concentrations of CoCl2 with or without pretreatment of the cells with GM6001. As shown in Figure 2B, we found that trophoblasts treated with GM6001+ CoCl2 produced significantly less TNFα than those treated with CoCl2 alone, whereas GM6001 negative control had no effect (data not shown). Although GM6001 is not a specific inhibitor for ADAM17, GM6001 has been used to inhibit ADAM17 activity by many investigators (28, 29). These results indicate that metalloproteinase/ADAM plays, at least in part, a role in regulating TNFα production by placental trophoblasts.
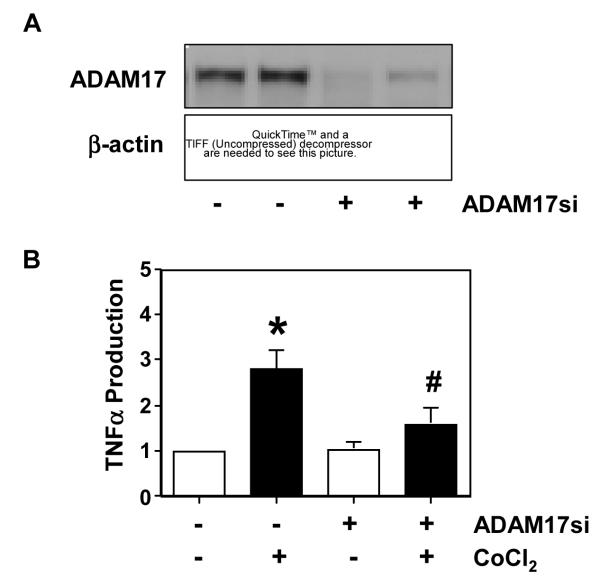
The specificity of ADAM17 in regulating TNFα production was further studied by transfection of trophoblasts with ADAM17 siRNA. Figure 3A shows downregulation of ADAM17 expression in trophoblasts transfected with ADAM17 siRNA. Figure 3B shows TNFα production by trophoblasts with or without ADAM17 siRNA transfection in the presence or absence of CoCl2 at a concentration of 250μM. It is clear that cells transfected with ADAM17 siRNA produced significantly less TNFα than those without siRNA transfection in the presence of CoCl2. These results further support ADAM17 being responsible for TNFα production/shedding by trophoblasts.
Figure 3.
Effects of ADAM17 siRNA on ADAM17 expression and TNFα production in primary isolated placental trophoblasts in culture. A: ADAM17 expression in cells with or without transfection of ADAM17 siRNA. β-actin expression was used as control. B: TNFα production by trophoblasts transfected ADAM17 siRNA in the presence or absence of CoCl2 at a concentration of 250μM in culture. Untransfected cells were used as control. Our results showed that reduced TNFα production is correlated with downregulation of ADAM17 expression in cells transfected with ADAM17 siRNA. TNFα production by untreated cells is presented as 1 for each experiment. The data is presented as mean ± SE of fold changes for TNFα productions from 4 independent primary trophoblast culture experiments, *p<0.05: CoCl2 treated vs. untreated control; and # p<0.05: siRNA transfected cells vs. untransfected cells treated with. CoCl2.
Effects of oxidative stress on ADAM17, HO-1, and caspase-3 expressions in trophoblasts
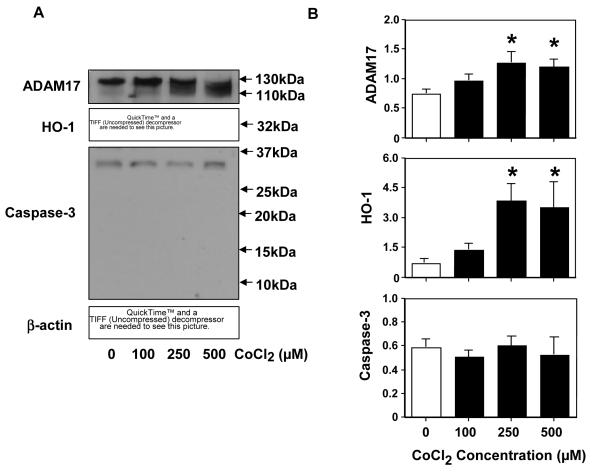
ADAM17 expression in placental trophoblasts in response to CoCl2 stimulation was determined by Western blot. As shown in Figure 4, ADAM17 expression was upregulated in trophoblasts treated with CoCl2 at concentrations of 100, 250, and 500μM compared to untreated control cells. Upon treatment with CoCl2, not only was there an increase in ADAM17 expression but also a shift of the ADAM17 band to a lower molecular weight on the Western blot. Previous study has shown that two molecular weight bands at about 130 and 100kDa can be detected by Western blot, which correspond to the precursor and mature (active) forms of ADAM17, respectively (30). Although it cannot be judged from such a small change on the gel, it appears that oxidative stress could lead to cleavage of the pro-form of ADAM17 to the mature form. In addition, we also examined HO-1 and caspase-3 expressions in trophoblasts treated with CoCl2. HO-1 is a sensor of cellular oxidative stress and caspase-3 is an apoptosis indicator. Our results showed that HO-1 expression was significantly upreguated in trophoblasts treated with CoCl2. The increased HO-1 expression is in a dose-dependent manner, which indicates that trophoblasts experienced oxidative stress when exposed to CoCl2. For caspase-3 expression, a band about 32kDa, a mature form for procaspase-3, was detected (Figure 4A). Since caspase-3 activation generates 17 and 12kDa catalytic subunit proteins, our results suggest that cultured trophoblasts did not undergo apoptosis in response to CoCl2. The bar graphs show relative protein expression for ADAM17, HO-1, and caspase-3 after being normalized with β-actin expression.
Figure 4.
Expression of ADAM17, HO-1, and caspase-3 in placental trophoblasts treated with different concentrations of CoCl2. A: ρεπρεσεντατιψε Ωεστερν βλοτ οφ AΔAM17, HO–1, ανδ ξασπασε–3 εχπρεσσιονσ. Mολεξνλαρ ωειγητ ισ γιψεν ον τηε ριγητ. B: ρελατιψε δενσιτυ οφ AΔAM17, Ho–1, ανδ ξασπασε–3 εχπρεσσιονσ βυ Ωεστερν βλοτ αφτερ νορμαλιζεδ βυ β-actin expression, *p<0.05: treated vs. control, respectively. β-actin expression was determined as an indicator of equal loading of protein samples in electrophoresis. The data is presented as mean ± SE from 3 independent primary trophoblast culture experiments.
Discussion
Placental expression of TNFα is increased in preeclamptic placentas (5, 31) and oxidative stress promotes placental cell release of TNFα (32). ADAM17 is a TNFα converting enzyme. In the present study, we investigated if ADAM17 is responsible for trophoblast production/shedding of TNFα in the human placenta. We found that the ADAM17 protein, but not the mRNA, expression was significantly increased in preeclamptic placentas compared to that in normal placentas. This was demonstrated by both immunostaining studies and Western blot analysis. Through immunostaining, we noticed that ADAM17 expression was mainly localized in syncytiotrophoblasts. Although the precise mechanism of increased protein expression for ADAM17 in preeclamptic placentas is not known, ADAM17 localization in syncytiotrophoblasts suggests that these cells could be a major source of TNFα produced by the placenta during pregnancy. The syncytiotrophoblasts are a continuous, specialized layer of epithelial cells covering the entire surface of villous trees and are in direct contact with maternal blood. They are the interface between the maternal and fetal compartment, therefore it is expected that TNFα released by syncytiotrophoblasts could directly enter the maternal circulation and contribute to the maternal TNFα levels during pregnancy (33, 34).
Oxidative stress is an underlying pathophysiology in the preeclamptic placenta. The findings of increased superoxide generation, increased lipid peroxide production, and reduced expressions of antioxidant enzyme CuZn-SOD and glutathione peroxidase in preeclamptic placentas support this notion (35-37). In preeclampsia, increased oxidative stress and increased inflammatory response are tightly connected to each other (32, 38). ADAM17 is expressed in placental trophoblasts throughout pregnancy and hypoxia-induced ADAM17 expression has been found at both mRNA and protein levels in the placenta (39). To study the mechanism of increased TNFα production in the preeclamptic placenta, we specifically investigated if ADAM17 was accountable for increased TNFα production/shedding in placental trophoblasts under oxidative stress. Primary isolated placental trophoblasts from normotensive term placentas were treated with CoCl2. CoCl2 is a hypoxia mimetic agent that has been widely used as a hypoxia/oxidative stress inducer in numerous in vitro cell culture studies including adipocytes, astrocytes, retinal ganglion cells, and trophoblasts (24, 25, 27, 40). Using CoCl2 to induce trophoblast oxidative stress, we found that TNFα production was significantly increased when cells were treated with CoCl2. The increased TNFα production was in a dose-dependent manner, and the CoCl2 induced TNFα production could be blocked by a metalloproteinase inhibitor GM6001. GM6001 is a broad range metalloproteinase inhibitor. Although GM6001 is not a specific inhibitor for ADAM17, it has been used by many investigators to inhibit ADAM17 activity and TNFα shedding (28, 29). Nonetheless, our data provide evidence that metalloproteinase/ADAM contributes to TNFα production by placentas trophoblasts.
The specificity of ADAM17 mediated TNFα production in trophoblasts was further confirmed by ADAM17 siRNA transfection experiments. We found that inhibition of ADAM17 expression is correlated with the reduced TNFα production in trophoblasts that was transfected with ADAM17 siRNA. These data provide convincing evidence that ADAM17 is responsible for TNFα production by placental trophoblasts. Although ADAM17 is considered a major metallopeptidase that induces TNFα shedding, other metallopeptidases have also been reported to be able to mediate TNFα production. For example, a study by Mezyk-Kopeć et al has shown that ADAM10 is a major TNF sheddase in ADAM17-deficient fibroblasts (41). Matrix metalloproteinase (MMP)-7 was also found to be associated with TNFα production in cancer cells (17). It is very likely that TNFα shedding/production is dependent on the difference of the cell type and the presence of ADAM17. We previously reported increased ADAM10 expression in preeclamptic placentas (23). Since ADAM10 is also able to regulate TNFα shedding, it is possible that both ADAM17 and ADAM10 may contribute to TNFα shedding/production in the preeclamptic placenta. However, which one plays the dominant role in controlling TNFα shedding/production warrants further investigation.
Trophoblast ADAM17 expression was increased when cells were treated with CoCl2, indicating hypoxia/oxidative stress could promote ADAM17 expression or mature form formation. This result is consistent with the work published by Hung et al. in which ADAM17 expression was increased in placental villous tissues when cultured under a low oxygen condition (39). HO-1 is a sensor of cellular oxidative stress. It is now recognized that HO-1 is not only a rate-limiting enzyme for heme catabolism but also exerts cytoprotective activity against endoplasmic reticulum stress and plays a role in anti-inflammation and anti-proliferation (42). The observation of increased ADAM17 expression correlating with upregulation of HO-1 expression in trophoblasts treated with CoCl2 further supports the concept that hypoxia/oxidative stress regulates ADAM17 expression that is correlated with cellular response to stress challenge in placental trophoblasts. In addition, upregulation of ADAM17 and HO-1 expressions in response to CoCl2-induced oxidative stress in placental trophoblasts does not appear to be related to apoptosis since no active caspase-3 catalytic subunit was detected. Therefore, other mechanisms may be involved in the increased apoptotic process in placental trophoblasts in preeclampsia (43).
In conclusion, we have made several important findings in this study. First, we have demonstrated that ADAM17 expression, a major TNFα converting enzyme, is increased in syncytiotrophoblasts in preeclamptic placentas. The increased ADAM17 expression occurs at the protein level, but not at mRNA level, which suggests that ADAM17 expression/activity is probably mediated through post-transcriptional regulatory mechanisms. Second, using hypoxic mimetic agent CoCl2, we have found that hypoxia promotes TNFα production, which is associated with increased ADAM17 expression and HO-1 expression in primary isolated placental trophoblasts. Last but not least, results from metalloproteinase inhibitor experiments and ADAM17 siRNA studies confirm that ADAM17 plays a role in TNFα release/shedding by placental trophoblasts. These results suggest that oxidative stress could be a causative factor in upregulating ADAM17 expression and/or increasing ADAM17 activity in placental trophoblasts. Our results also suggest that increased ADAM17 expression may play a major role in increased TNFα production by placental trophoblasts in preeclampsia.
Acknowledgement
This study was supported in part by grants from National Institute of Health grants RO1 NICHD (HD36822) and RO1 NHLBI (HL65997) to Y.W. and presented at the 58th Annual Meeting for the Society of Gynecologic Investigation (SGI), Miami Beach, Florida, USA, March 16-19, 2011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures The authors have no financial conflicts of interest.
References
- [1].Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- [2].Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;25:210–217. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- [3].Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354:788–789. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- [4].Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30:S32–37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang Y, Walsh SW. TNFα concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol. 1996;32:157–169. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- [6].Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metabol. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- [7].Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- [8].Malek A, Sager R, Schneider H. Effect of hypoxia, oxidative stress and lipopolysaccharides on the release of prostaglandins and cytokines from human term placental explants. Placenta. 2001;22(Suppl A):S45–50. doi: 10.1053/plac.2001.0635. [DOI] [PubMed] [Google Scholar]
- [9].Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985;162:2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wright HL, Chikura B, Bucknall RC, Moots RJ, Edwards SW. Changes in expression of membrane TNF, NF-{kappa}B activation and neutrophil apoptosis during active and resolved inflammation. Ann Rheum Dis. 2011;70:537–543. doi: 10.1136/ard.2010.138065. [DOI] [PubMed] [Google Scholar]
- [11].Li W, Li H, Bocking AD, Challis JR. Tumor necrosis factor stimulates matrix metalloproteinase 9 secretion from cultured human chorionic trophoblast cells through TNF receptor 1 signaling to IKBKB-NFKB and MAPK1/3 pathway. Biol Reprod. 2010;83:481–487. doi: 10.1095/biolreprod.109.082578. [DOI] [PubMed] [Google Scholar]
- [12].Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS, Torrance DS, Otten-Evans C, Greenstreet T, Weerawarna K, KronheiM SR, Petersen M, Gerhart M, Kozlosky CJ, March CJ, Black RA. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- [13].Waetzig GH, Rosenstiel P, Arlt A, Till A, Bräutigam K, Schäfer H, Rose-John S, Seegert D, Schreiber S. Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis via reverse TNF signaling and autocrine transforming growth factor-beta1. FASEB J. 2005;19:91–93. doi: 10.1096/fj.04-2073fje. [DOI] [PubMed] [Google Scholar]
- [14].Mueller C, Corazza N, Trachsel-Løseth S, Eugster HP, Bühler-Jungo M, Brunner T, Imboden MA. Noncleavable transmembrane mouse tumor necrosis factor-alpha (TNFalpha) mediates effects distinct from those of wild-type TNFalpha in vitro and in vivo. J Biol Chem. 1999;274:38112–38118. doi: 10.1074/jbc.274.53.38112. [DOI] [PubMed] [Google Scholar]
- [15].Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- [16].Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem. 2000;275:14608–14614. doi: 10.1074/jbc.275.19.14608. [DOI] [PubMed] [Google Scholar]
- [17].Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- [18].Reddy AB, Ramana KV, Srivastava S, Bhatnagar A, Srivastava SK. Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-alpha via protein kinase C-delta and TNF-alpha converting enzyme in vascular smooth muscle cells. Endocrinology. 2009;150:63–74. doi: 10.1210/en.2008-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Budagian V, Bulanova E, Orinska Z, Ludwig A, Rose-John S, Saftig P, Borden EC, Bulfone-Paus S. Natural soluble interleukin-15Ralpha is generated by cleavage that involves the tumor necrosis factor-alpha-converting enzyme (TACE/ADAM17) J Biol Chem. 2004;279:40368–40375. doi: 10.1074/jbc.M404125200. [DOI] [PubMed] [Google Scholar]
- [20].Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Lee DC. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem. 2002;277:12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- [21].Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93:260–266. doi: 10.1210/jc.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y. Proteases and sFlt-1 release in the human placenta. Placenta. 2010;31:512–518. doi: 10.1016/j.placenta.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kamiya T, Hara H, Inagaki N, Adachi T. The effect of hypoxia mimetic cobalt chloride on the expression of EC-SOD in 3T3-L1 adipocytes. Redox Rep. 2010;15:131–134. doi: 10.1179/174329210X12650506623483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183:145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- [26].Goldberg MA, Schneider TJ. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994;269:4355–4359. [PubMed] [Google Scholar]
- [27].Chen JK, Zhan YJ, Yang CS, Tzeng SF. Oxidative stress-induced attenuation of thrombospondin-1 expression in primary rat astrocytes. J Cell Biochem. 2011;112:59–70. doi: 10.1002/jcb.22732. [DOI] [PubMed] [Google Scholar]
- [28].Rabie T, Strehl A, Ludwig A, Nieswandt B. Evidence for a role of ADAM17 (TACE) in the regulation of platelet glycoprotein V. J Biol Chem. 2005;280:14462–14468. doi: 10.1074/jbc.M500041200. [DOI] [PubMed] [Google Scholar]
- [29].Mol MA, van den Berg RM, Benschop HP. Involvement of caspases and transmembrane metalloproteases in sulphur mustard-induced microvesication in adult human skin in organ culture: directions for therapy. Toxicology. 2009;258:39–46. doi: 10.1016/j.tox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- [30].Boutet P, Agüera-González S, Atkinson S, Pennington CJ, Edwards DR, Murphy G, Reyburn HT, Valás-Gámez M. Cutting edge: the metalloproteinase ADAM17/TNF-alpha-converting enzyme regulates proteolytic shedding of the MHC class I-related chain B protein. J Immunol. 2009;182:49–53. doi: 10.4049/jimmunol.182.1.49. [DOI] [PubMed] [Google Scholar]
- [31].Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Jr, Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181:915–920. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- [32].Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164:1049–1061. doi: 10.1016/s0002-9440(10)63192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-α is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–1759. [PubMed] [Google Scholar]
- [34].Lewis DF, Canzoneri BJ, Wang Y. Maternal circulating TNFα levels are highly correlated with IL-10 levels, but not IL-6 and IL-8 levels, in women with preeclampsia. Am J Reprod Immunol. 2006;62:269–274. doi: 10.1111/j.1600-0897.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in preeclampsia. Placenta. 2001;22:206–212. doi: 10.1053/plac.2000.0608. [DOI] [PubMed] [Google Scholar]
- [36].Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Invest. 1996;3:179–184. [PubMed] [Google Scholar]
- [37].Wang Y, Walsh SW, Kay HH. Placental lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. Am J Obstet Gynecol. 1992;167:946–949. doi: 10.1016/s0002-9378(12)80017-2. [DOI] [PubMed] [Google Scholar]
- [38].Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- [39].Hung TH, Chen SF, Hsieh CC, Hsu JJ, Li MJ, Yeh YL, Hsieh TT. Tumor necrosis factor-alpha converting enzyme in the human placenta throughout gestation. Reprod Sci. 2008;15:195–209. doi: 10.1177/1933719107310709. [DOI] [PubMed] [Google Scholar]
- [40].Tulsawani R, Kelly LS, Fatma N, Chhunchha B, Kubo E, Kumar A, Singh DP. Neuroprotective effect of peroxiredoxin 6 against hypoxia-induced retinal ganglion cell damage. BMC Neurosci. 2010;11:125. doi: 10.1186/1471-2202-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mezyk-Kopeć R, Bzowska M, Stalińska K, Chełmicki T, Podkalicki M, Jucha J, Kowalczyk K, Mak P, Bereta J. Identification of ADAM10 as a major TNF sheddase in ADAM17-deficient fibroblasts. Cytokine. 2009;46:309–315. doi: 10.1016/j.cyto.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [42].Kim HP, Pae HO, Back SH, Chung SW, Woo JM, Son Y, Chung HT. Heme oxygenase-1 comes back to endoplasmic reticulum. Biochem Biophys Res Commun. 2011;404:1–5. doi: 10.1016/j.bbrc.2010.11.067. [DOI] [PubMed] [Google Scholar]
- [43].Cobellis L, De Falco M, Torella M, Trabucco E, Caprio F, Federico E, Manente L, Coppola G, Laforgia V, Cassandro R, Colacurci N, De Luca A. Modulation of Bax expression in physiological and pathological human placentas throughout pregnancy. In Vivo. 2007;21:7777–7783. [PubMed] [Google Scholar]