Abstract
The PI3K (phosphatidylinositol-3-kinase)/mTOR (mammalian target of rapamycin) pathway is frequently activated in endometrial cancer through various PI3K/AKT-activating genetic alterations. We examined the antitumor effect of NVP-BEZ235—a dual PI3K/mTOR inhibitor—and RAD001—an mTOR inhibitor—in 13 endometrial cancer cell lines, all of which possess one or more alterations in PTEN, PIK3CA, and K-Ras. We also combined these compounds with a MAPK pathway inhibitor (PD98059 or UO126) in cell lines with K-Ras alterations (mutations or amplification). PTEN mutant cell lines without K-Ras alterations (n = 9) were more sensitive to both RAD001 and NVP-BEZ235 than were cell lines with K-Ras alterations (n = 4). Dose-dependent growth suppression was more drastically induced by NVP-BEZ235 than by RAD001 in the sensitive cell lines. G1 arrest was induced by NVP-BEZ235 in a dose-dependent manner. We observed in vivo antitumor activity of both RAD001 and NVP-BEZ235 in nude mice. The presence of a MEK inhibitor, PD98059 or UO126, sensitized the K-Ras mutant cells to NVP-BEZ235. Robust growth suppression by NVP-BEZ235 suggests that a dual PI3K/mTOR inhibitor is a promising therapeutic for endometrial carcinomas. Our data suggest that mutational statuses of PTEN and K-Ras might be useful predictors of sensitivity to NVP-BEZ235 in certain endometrial carcinomas.
Introduction
Constitutive activation of the PI3K (phosphatidylinositol 3-kinase) pathway results from various types of alterations, including changes to RTKs (receptor tyrosine kinases), Ras, PIK3CA (the p110alpha catalytic subunit of PI3K), and PTEN [1]. Endometrial cancer is the fourth most frequent cancer in women [2]. There are two pathogenetic types of endometrial carcinomas: estrogen-dependent type I (endometrioid adenocarcinomas) and estrogen-independent type II (high-grade carcinomas). Approximately 80% of endometrial carcinomas are classified as type I [3], [4]. Mutations of K-Ras (10–20%), PTEN (34–56%), and PIK3CA (25–36%) are frequently observed in endometrial cancer [5]–[8]. In addition, we previously revealed that chromosomal imbalances in the Ras-PI3K pathway genes (NF1, PTEN, K-Ras, and PIK3CA) are also common in endometrial cancer [9], indicating that the Ras-PI3K pathway is activated in the majority of endometrial cancers.
Novel therapeutics targeting the PI3K/mTOR (mTORC1/2) pathway are being intensively developed. The first clinically approved inhibitors are rapamycin analogs (rapalogs), such as everolimus (RAD001) and temsirolimus, targeting the mTORC1 complex for use with advanced renal cell carcinomas [10]–[13]. However, clinical trials with single-agent rapalog therapies have shown limited response rates in other cancer types [14]. Several potent and selective PI3K inhibitors have recently entered early-phase clinical trials for treatment of various malignant tumors [15]. The limitation of the rapalogs might be explained by the activity of the mTORC1-independent substrates of Akt, including GSK3beta and FOXO1/3a. Rapalogs do not prevent mTORC2-dependent phosphorylation of Akt on Ser-473 or PDK1-dependent phosphorylation of Akt on Thr-308 [16], [17]. In addition, rapalogs may cause feedback activation of the PI3K-Akt pathway mediated by insulin-like growth factor-1 receptor (IGF-1R) signaling [18]. Therefore, a dual PI3K/mTOR inhibition might be a more rational therapeutic option than mTOR inhibition alone in tumors with PI3K-activating mutations.
Developing predictive biomarkers of the PI3K/mTOR inhibitors is important; however, the existence of alterations in the PI3K pathway (or elevated AKT phosphorylation) alone is not necessarily a good biomarker for these compounds. Indeed, tumors with alterations in Ras and RTK do not respond sufficiently to simple PI3K pathway inhibition [19]–[22]. Moreover, multiple genetic alterations in the RTK-Ras-PI3K pathway are reported in many cancers [1]. It remains to be determined which types of alterations are useful as predictive biomarkers.
In this study, we firstly evaluated the antitumor effect of a dual PI3K/mTOR inhibitor, NVP-BEZ235, and an mTOR inhibitor, RAD001 (everolimus), in a panel of endometrial cancer cell lines. Second, we analyzed the antitumor effect of NVP-BEZ235 and RAD001 in vivo. Third, we focused on the predictive biomarkers to the PI3K/mTOR inhibitors, using the mutational status of K-Ras, PTEN, and PIK3CA. Finally, we addressed the antitumor effect of the combined inhibition of the PI3K/mTOR and MAPK pathways in cells with K-Ras alterations.
Materials and Methods
Cell lines and reagents
Culture conditions of 13 endometrial cancer cell lines (endometrioid adenocarcinomas) were described previously [8]. NVP-BEZ235 and RAD001 (everolimus) were kindly provided by Novartis Pharma AG (Basel, Switzerland). MAPK pathway (MEK) inhibitors PD98059 and UO126 were purchased from Cell Signaling Technology (Beverly, MA).
PCR and sequencing
The mutational status of 13 cell lines was analyzed by PCR and direct sequencing. The PCR conditions and primers for PTEN (exons 1–9), K-Ras (exon 1 and 2), and AKT1 (exon 4) were described previously [8], [23], [24]. The mutational status of PIK3CA was analyzed by RT-PCR with LA-Taq according to the manufacturer's protocol (Takara BIO, Madison, WI) to cover entire coding region. The PCR primers were the following: forward, 5′-CCCGAGCGTTTCTGCTTTGGGACAACC-3′; reverse, 5′-AGCGTTTCTGCTTTGGGACAACCATACATC-3′.
Immunoblotting
Cells were treated with each drug for the indicated time and concentrations and then lysed as described previously [7]. Antibodies to total Akt, phospho-Akt (Ser473), phospho-Akt (Thr308), phospho-GSK3beta (Ser9), total S6, phospho-S6 (Ser235/236, Ser 240/244), p-4EBP1 (Thr37/46), total FoxO1, phospho-FoxO1 (Thr24), phospho-FoxO3a (Thr32), phospho-ERK (ERK1/2-Thr202/Tyr204), total ERK (Cell Signaling Technology, Beverly, MA), and beta-actin (Sigma-Aldrich, St. Louis, MO) were used for immunoblotting, as recommended by the manufacturer, and were detected by an ECL western blot detection kit (Amersham Biosciences, Piscataway, NJ) or Immobilon western detection reagents (Millipore Biosciences, Temecula, CA).
Proliferation assays
Cell viability assays were performed with the Cell Counting Kit-8 (cell viability colorimetric assay), using the tetrazolium salt WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) (Dojindo, Tokyo, Japan), as an MTT (Methyl thiazolyl tetrazorium) assay. In 96-well plates, 1×105 cells were seeded with 10% fetal bovine serum (FBS) and treated with increasing doses of NVP-BEZ235 or RAD001 for 72 h, starting 24 h after seeding. The WST-8 colorimetric assay was quantified at 415 nm and normalized to the value of cells treated with DMSO alone. All experiments were repeated twice.
Cell cycle analysis
Cells (5×105) were seeded in 60-mm dishes (with 10% FBS) and treated with reagents (such as NVP-BEZ235, RAD001, PD98059, or UO126) for 48 h. Floating and adherent cells were collected by trypsinization and washed twice with PBS. Cells were resuspended in buffer containing ethanol and PBS at a ratio of 7∶3 at −20°C overnight. After being washed twice with PBS, cells were incubated in ribonuclease solution (0.25 mg/mL) (Sigma) for 30 min at 37°C, followed by staining with propidium iodide (50 µg/mL) (Dojindo, Japan) on ice for 30 min in the dark. Cells were then incubated in 70% ethanol at −20°C overnight, treated with 20 µg/mL RNase A, stained with 0.5 µg/mL propidium iodide, and evaluated by flow cytometry (BD FACS Calibur HG, Franklin Lakes, NJ). Cell cycle distribution was analyzed with CELL Quest pro ver. 3.1. (Beckman Coulter Epics XL, Brea, CA). The experiments were repeated 3 times.
Ethics statement for animal experiments and clinical data
Ethics statement for animal experiments: This study was approved by Animal Care and Use Committee, The University of Tokyo. The approval number is Med-P09-051. Athymic BALB/c mice (CLEA JAPAN, Tokyo, Japan) were maintained in an SPF (Specific Pathogen Free) facility according to our institutional guidelines, and experiments were conducted under an approved animal protocol.
This manuscript includes clinical data, which were previously published elsewhere [7]–[9], [25]. The authors declare that all these participants provided written informed consent, and the study design was approved by the Institutional Review Board of the University of Tokyo Hospital. The approval number is 683.
Tumor xenografts in nude mice
Subcutaneous xenograft tumors in BALB/c mice were established by the injection of a 500-µL cell suspension of 10×106 AN3CA and HEC-59 endometrial carcinoma cells in PBS. Tumors were removed after exponential growth, cut into 3-mm pieces, and transplanted subcutaneously into other mice. One week after tumor transplantation, mice were assigned randomly to one of three treatment regimens: (1) vehicle (control), (2) NVP-BEZ235, and (3) RAD001. Each treatment group consisted of 6 mice. NVP-BEZ235 and RAD001 were injected orally (p.o.) at daily doses of 40 mg/kg and 2.5 mg/kg, respectively. Tumor volumes (in mm3) were calculated by the formula: ([major axis]*[minor axis]2/2). After the treatment, the tumors were removed and analyzed by Western blot analysis. Tumor weight (wet weight) was measured, and the average weight was calculated for each group.
Single nucleotide polymorphism typing array and array comparative genomic hybridization
A single nucleotide polymorphism (SNP) array was performed in the HEC-6, 50B, 59, 88, 108, 116, 151, and HHUA cell lines. Experimental procedures for GeneChip were performed according to the GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA, USA) with the use of a human mapping 250K Nsp array [9]. Array comparative genomic hybridization (CGH) was performed using arrays of 2464 BAC clones (HumArray2.0) in the remaining 5 cell lines (AN3CA, HEC-1B, Ishikawa, KLE, and RL95-2) as described previously [7].
Statistical analysis
Statistical comparisons of mean final tumor volumes in the xenograft studies were made using a one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
Results
Classification of endometrial cancer cells according to the mutational status of PIK3CA, PTEN, and K-Ras
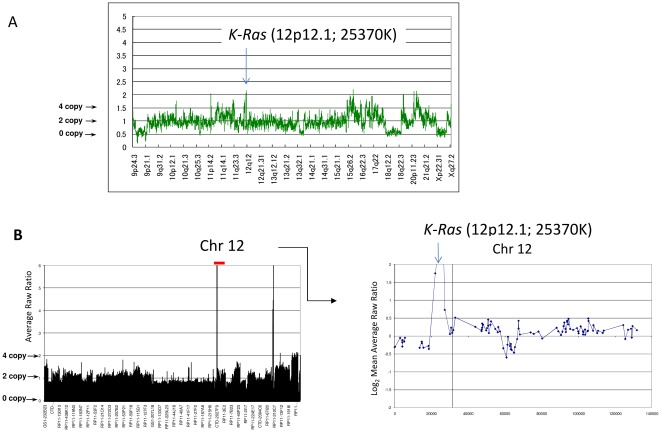
We previously reported copy number losses for PTEN (26%) and gains for PIK3CA (19%) and K-Ras (13%) in our 31 clinical samples, in addition to mutations of K-Ras, PTEN, PIK3CA, and AKT1 [8], [9], [25]. We confirmed that all 13 endometrial cancer cell lines possess one or more alterations (mutations and/or copy number alterations) in the PIK3CA, PTEN, and K-Ras genes (Table 1, Figure 1A and 1B). AKT1 mutations were not detected in these 13 cell lines. We classified 13 endometrial cancer cell lines into 4 groups according to the mutational status of PIK3CA, PTEN, and K-Ras (Table 1): group A (n = 4), with coexistent mutations of PIK3CA and PTEN; group B (n = 5), with PTEN mutation alone; group C (n = 2), with coexistent mutations of K-Ras and PIK3CA; and group D (n = 2), with copy number gain of K-Ras (without any mutations in these 3 genes). We previously reported that PTEN expression was not detected in PTEN mutant endometrial cancer cell lines [8]. We have found no endometrial cell lines without any alterations in the Ras-PI3K pathway, suggesting that this pathway is essentially activated in the majority of endometrial cancer cell lines.
Table 1. Classification of endometrial cancer cell lines by mutational status and IC50 values to NVP-BEZ235 and RAD001.
| Group | Cell line | Mutational status | Copy number alterations | IC50 (nM) | |||||
| PIK3CA | PTEN (mutated exons) | K-Ras | PIK3CA | PTEN | K-Ras | BEZ235 | RAD001 | ||
| A | HEC-116 | Mut (R88Q) | Mut 6(M), 7(N) | wild type | nl | nl | nl | 19 | 6 |
| HEC-6 | Mut (R108H) | Mut 4(F), 8(F) | wild type | nl | nl | nl | 19 | 400 | |
| HEC-59 | Mut (R38C) | Mut 2(M), 7(N), 7(M), 7(F) | wild type | nl | nl | nl | 24 | 220 | |
| HEC-88 | Mut (E365K) | Mut 5(M), 6(M), 8(M), 8(M) | wild type | nl | nl | nl | 44 | 440 | |
| B | AN3CA | wild type | Mut 5(N) | wild type | nl | nl | nl | 20 | 14 |
| Ishikawa | wild type | Mut 8(F), 8(F) | wild type | Gain | nl | nl | 30 | 50 | |
| HEC-151 | wild type | Mut 2(M), 4(F) | wild type | nl | nl | nl | 51 | 130 | |
| HEC-108 | wild type | Mut 1(F), 8(F) | wild type | nl | nl | nl | 55 | 730 | |
| RL95 | wild type | Mut 8(F), 8(F) | wild type | nl | nl | nl | 90 | >1000 | |
| C | HEC-1B | Mut (G1049R) | wild type | Mut (G12D) | Gain | nl | nl | 220 | 200 |
| HHUA | Mut (R88Q) | Mut 5(F), 8(F) | Mut (G12V) | nl | nl | nl | 250 | >1000 | |
| D | KLE | wild type | wild type | wild type | nl | nl | Gain | 110 | >1000 |
| HEC-50B | wild type | wild type | wild type | Gain | nl | Gain | 100 | >1000 | |
(M) Missense mutation, (N) Non-sense mutation, (F) Frameshift mutation.
Figure 1. Copy number gain at the locus of.
K-Ras (12p12.1) in the two group D cell lines. (A) SNP array ‘karyograms’ (250K) of HEC-50B cells. The graph shows the total copy number through chromosome 9p-X. The locus of K-Ras is amplified as indicated. (B) Array CGH of KLE cells. The graphs show total copy number throughout the entire genome (Left) and chromosome 12 (Right). The locus of K-Ras is amplified as indicated.
Mutations in PIK3CA and/or PTEN, in the absence of mutations in K-Ras, define the antiproliferative response to NVP-BEZ235 and RAD001 in endometrial cancer cell lines
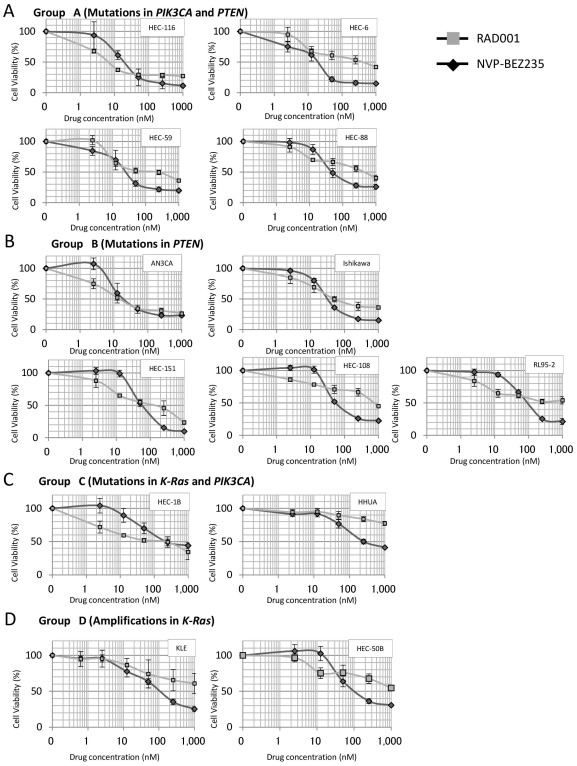
We performed MTT (Methyl thiazolyl tetrazorium) assay by NVP-BEZ235 and RAD001 in the 13 endometrial cell lines. RAD001 (100 nM) showed a growth inhibitory effect against 10 of the 13 cell lines (including all 9 group A and B cell lines), with a 30–70% reduction in cells (Figure 2A–2D). NVP-BEZ235 (100 nM) inhibited cell growth, with a 30–90% reduction in all 13 cell lines. The IC50 values for cell proliferation by RAD001 were greater than 100 nM (non-sensitive) in 6 out of the 9 cell lines in groups A and B, whereas the IC50 values for NVP-BEZ235 were less than 100 nM (sensitive) in all 9 cell lines in groups A and B (Table 1). Dose-dependent growth suppression was more clearly induced by NVP-BEZ235 than by RAD001 in 8 of the 9 cell lines in groups A and B (Figure 2A–2D). The IC50 values for all 4 cell lines in groups C and D were greater than 100 nM for NVP-BEZ235 (Table 1). Taken together, these data show that the existence of PTEN and/or PIK3CA mutations without K-Ras mutations is associated with sensitivity to NVP-BEZ235. In addition, high-dose NVP-BEZ235 might be more broadly effective than RAD001 for treatment of endometrial carcinomas. Growth curves of all cell lines in 1 graph were available for both NVP-BEZ235 and RAD001, respectively (Figures S1 and S2).
Figure 2. Inhibition of cell proliferation by NVP-BEZ235 and RAD001.
(A)–(D) WST-8 assay showing the sensitivity of endometrial cancer cells to NVP-BEZ235 and RAD001 at increasing concentrations of drug (nmol/L) for 72 h. The data was normalized to the value of control cells. (A) Four group A cell lines with double mutations of PIK3CA and PTEN, (B) Five group B cell lines with PTEN mutations, (C) Two group C cell lines with coexistent mutations of K-Ras and PIK3CA, and (D) Two group D cell lines with chromosomal copy number amplification at the locus of K-Ras. All experiments were repeated 3 times, and each value is shown as the mean of 3 experiments ± S.D.
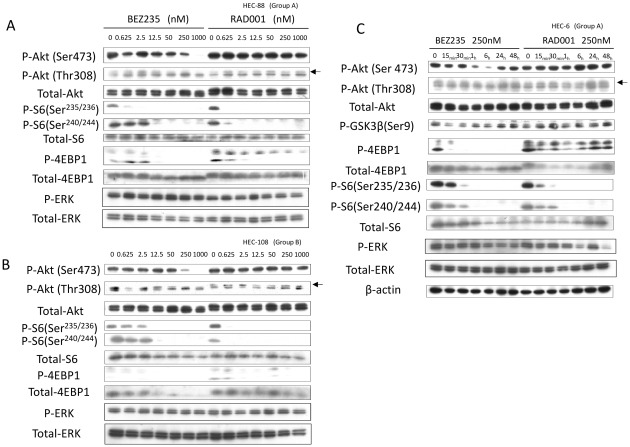
NVP-BEZ235 suppresses phosphorylation of Akt, GSK3beta, S6, and 4EBP1, whereas RAD001 suppresses phosphorylation of S6 and 4EBP1
We performed immunoblotting with lysates prepared from cells treated with NVP-BEZ235 or RAD001. The phosphorylation (p-) levels of 4E-BP1 and S6 were clearly suppressed by both inhibitors at low concentrations (0.625–2.5 nM). NVP-BEZ235 also suppressed the level of p-Akt (Ser473 and Thr308) (50–1000 nM) in these cells (Figure 3A and 3B). RAD001 did not suppress the phosphorylation level of Akt at any dose (Figure 3A and 3B). The dose dependency of the phosphorylation levels of mTORC1-dependent proteins (4E-BP1 and S6) and Akt suggests that NVP-BEZ mainly works as an mTOR (mTORC1) inhibitor at lower concentrations and functions as a dual PI3K/mTOR inhibitor at higher concentrations.
Figure 3. Inhibition of PI3K/mTOR signaling by NVP-BEZ235 and inhibition of mTOR signaling by RAD001 in endometrial cancer cell lines.
(A)–(B) Western blot of total lysates of HEC-88 (group A) and HEC-108 (group B) cells, treated with NVP-BEZ235 or RAD001 at concentrations ranging from 0 to 1,000 nmol/L with 10% fetal bovine serum (FBS). phospho-Akt (Ser473, Thr308), phospho-S6 (Ser235/236, Ser240/244), and phospho-4EBP1 (Thr 37/46) levels are shown with total Akt level for a loading control. (C) Western blot of total lysates of HEC-6 (group A) cells treated with NVP-BEZ235 or RAD001 at a dose of 100 nM for up to 48 hours with 10% FBS. Phosphorylation levels of the PI3K/mTOR signaling are shown with loading controls.
Next, we performed time-course experiments with NVP-BEZ235 and RAD001. Long-term exposure to NVP-BEZ235 (250 nM) resulted in sustained inhibition of p-S6 and p-4E-BP1. However, the phosphorylation levels of Akt and GSK3beta (an mTORC1-independent protein) recovered nearly to the baseline levels within 24 h (Figure 3C). Exposure to RAD001 resulted in a drastic reduction in the level of p-4EBP1 in 15 min, but the level was recovered within 6 hours; the level of p-S6 was continuously suppressed over the time course (Figure 3C). We confirmed that the phosphorylation level of ERK was not affected by both RAD001 and NVP-BEZ235 (Figure 3A–3C).
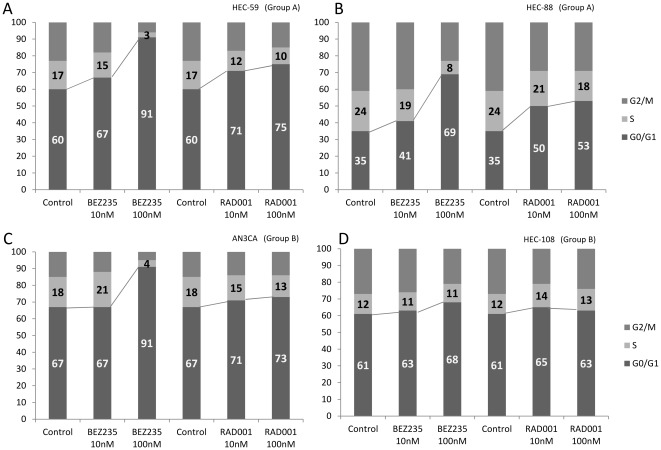
NVP-BEZ235 robustly induces dose-dependent G1 arrest in “sensitive” cells
We conducted fluorescence-activated cell sorting (FACS)-based cell cycle analyses before and after NVP-BEZ235 or RAD001 treatment in a subset of the cell lines. At a low concentration (10 nM), G1 arrest was slightly induced by both RAD001 and NVP-BEZ235 (<15%) in group A and group B cell lines (Figure 4A–4D). At a higher concentration (100 nM), G1 arrest was much more effectively induced by NVP-BEZ235 than by RAD001 in three of the four cell lines (Figure 4A–4D). Dose-dependent G1 arrest by NVP-BEZ235 was confirmed in all the other group A and B cell lines (data not shown). G1 arrest was also observed to be induced by either RAD001 or NVP-BEZ235 in the 4 cell lines of groups C and D; however, the dose-dependent effect of NVP-BEZ235 was not significant, except for HEC-1B cells (Figure S3A–S3D). The sub-G1 population was not significantly induced by either inhibitor in all cell lines examined, suggesting that the antitumor effect of these inhibitors is predominantly cytostatic, not cytotoxic.
Figure 4. Flowcytometric analysis of cell cycle in cancer cells treated with either NVP-BEZ235 or RAD001.
(A–D) Cells (5×105) were seeded in the presence of 10% serum and treated with NVP-BEZ235 or RAD001 for 48 h at a dose of 10 nM or 100 nM, respectively. A higher dose of NVP-BEZ235 (100 nM) significantly augmented the percentage of cells in the G0-G1phase of the cell cycle, compared with that of RAD001 (100 nM). (A)–(B); The data from two group A cells. (C)–(D); the data from two group B cells.
In vivo antitumor effect of NVP-BEZ235 and RAD001 in a mouse xenograft model
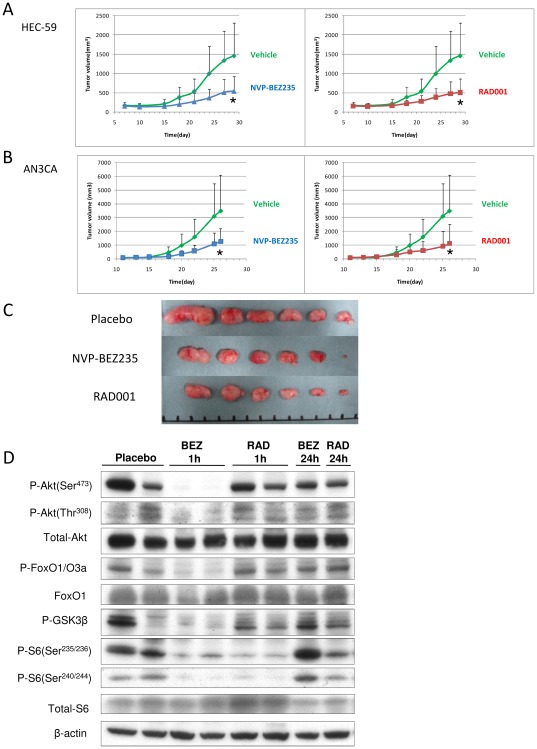
We examined in vivo antitumor activity of both NVP-BEZ235 and RAD001 in mice inoculated with either group A (HEC-59) or group B (AN3CA) cells. Both NVP-BEZ235 and RAD001 significantly suppressed the tumor growth of the xenografts, compared with the control (vehicle). No significant adverse effects, including a body weight loss of more than 10%, were observed in the examined mice (data not shown). Inconsistent with the in vitro data, the effects of NVP-BEZ235 and RAD001 were comparable (Figure 5A–5C).
Figure 5. in vivo anti-tumor effect of NVP-BEZ235 and RAD001 in nude mice.
Subcutaneous xenograft tumors in athymic BALB/c mice were established in the injection of endometrial carcinoma cells. Mice were treated with a daily dose of 40 mg/kg (NVP-BEZ235) or with a daily dose of 2.5 mg/kg RAD001 or vehicle alone (control). Each treatment group contained 6 mice; (A) HEC-59 and (B) AN3CA. Tumor volumes were calculated by the formula {(major axis)*(minor axis)2/2}mm3 twice a week. Groups were compared at the end of treatment. Points, mean; bars, SD, *;p<0.05. (C) Appearance of subcutaneous tumors in HEC-59 xenografts. (D) Western blot of total lysates from the HEC-59 xenografts. p-Akt, p-FOXO1/3a, p-GSK3beta, p-S6 were assessed 1 and 24 h after the last drug administration. Total Akt and beta-actin were shown as loading controls.
We then evaluated the phosphorylation levels of the targeted molecules as pharmacodynamic markers. We extracted proteins from the second, third, and fourth largest tumors of each group. Although there were variations in the phosphorylation levels in the control group, NVP-BEZ235 suppressed the phosphorylation levels of Akt, FOXO1/3a, and S6 at 1 h. However, the phosphorylation levels of these proteins recovered to the baseline levels within 24 h (Figure 5D). RAD001 had clearly suppressed the p-S6 level at 1 h, and the effect partly remained at 24 h after the treatment (Figure 5D). Taken together with the in vitro experiments, these results indicate that the antitumor activity of NVP-BEZ235 might not be sufficiently maintained during treatment.
Inhibition of the MAPK pathway synergistically (or additively) suppresses cell proliferation and induces G1 arrest in K-Ras mutant endometrial cell lines
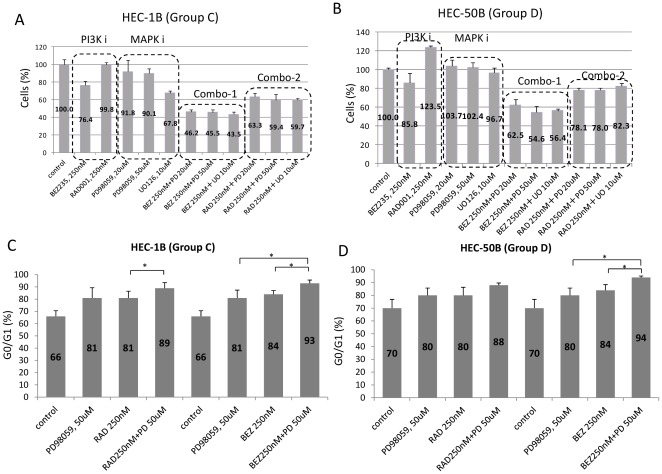
Because the 4 cell lines with K-Ras alterations (HEC-1B, HHUA, KLE, and HEC-50B) were less sensitive to both NVP-BEZ235 and RAD001, compared with K-Ras wild-type cells with PTEN mutations (groups A and B), we combined NVP-BEZ235 (or RAD001) with a MEK inhibitor (PD98059 or UO126) for use in these 4 cell lines (groups C and D). MTT assay revealed that PD98059 (50 µM) or UO126 (10 µM) alone showed a limited growth inhibitory effect with a 10–30% reduction in the 4 cell lines (Figure 6A and 6B, Figure S4A and S4B). However, after treatment with NVP-BEZ235 (250 nM) combined with PD98059 (20–50 µM) or UO126 (10 µM), cell proliferation was suppressed synergistically or additively with a 45–70% reduction (Figure 6A and 6B, Figure S4A and S4B). FACS analysis showed that G1 arrest was markedly induced by a combination of PD98059 (or UO126) and NVP-BEZ235 (Figure 6C and 6D, Figure S4C and S4D). In HEC-1B and HEC-50B cells, the G0/G1 ratio was significantly higher for the combination of PD98059 and NVP-BEZ235 than for either compound alone (Figure 6C and 6D). A similar synergistic effect was also observed with the combination of PD98059 (or UO126) and RAD001 (250 nM), although the effect of RAD001 was weaker than that of NVP-BEZ235 (Figure 6C and 6D, Figure S4C and S4D). The sub-G1 population was not significantly increased in groups C and D cells when using the combination of NVP-BEZ235 and a MEK inhibitor, PD98059 (<5%), as compared to that when using the control or NVP-BEZ235 alone.
Figure 6. Inhibition of cell proliferation and augmentation of G1 arrest by combination of a MEK inhibitor and NVP-BEZ235 (or RAD001) in cells with alterations in.
K-Ras (mutation or amplification). (A)–(B) WST-8 assay was performed in HEC-1B (group C) and HEC-50B (group D) cell lines. All the experiments were repeated three times and each value is shown as the mean of three experiments +/− S.D. The combination of a MEK inhibitor (PD98059 or UO126) and NVP-BEZ235 (or RAD001) significantly augmented anti-proliferative effect in these cell lines, compared with either inhibition alone (p<0.05 by Student's t-test). (C)–(D) Flowcytometric analysis of cell cycle in HEC-1B and HEC-50B cells. All experiments were repeated 3 times, and each value is shown as the mean of 3 experiments ± S.D. Combination of a MEK inhibitor (PD98059 or UO126) and NVP-BEZ235 significantly augmented the percentage of cells in the G0-G1phase of the cell cycle in these cells. *: p<0.05 by Student's t-test.
Phosphorylation levels of Akt, S6, and ERK in cells with K-Ras alterations treated with RAD001, NVP-BEZ235, and PD98059
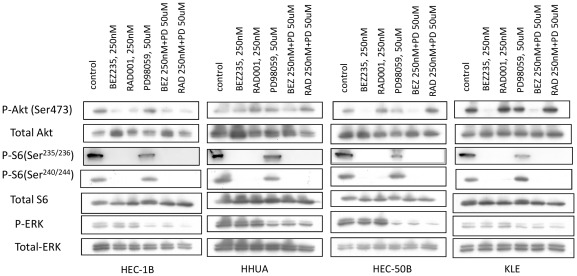
We analyzed the PI3K/mTOR and MAPK signaling pathways in cells of groups C and D by western blotting. NVP-BEZ235 at 250 nM suppressed the phosphorylation levels of AKT and S6 in HEC-1B, HEC-50B, and KLE cells. In HHUA cells, the total AKT level was slightly elevated, although the p-AKT level was not significantly changed by NVP-BEZ235 alone (Figure 7). RAD001 at 250 nM suppressed the p-S6 level in all cells of groups C and D (Figure 7). Both RAD001 and NVP-BEZ235 did not suppress the p-ERK level, whereas a MEK inhibitor, PD98059, decreased the p-ERK level in these cells.
Figure 7. Inhibition of PI3K and MAPK signaling pathway by NVP-BEZ235, RAD001, and a MEK inhibitor (PD98059) in endometrial cancer cell lines with K-Ras alterations.
Western blot analysis of total lysates of HEC-1B and HHUA (group C) and HEC-50B and KLE (group D) cells treated with 1 of the PI3K (or MAPK) signal inhibitors or their combination with 10% FBS. Phospho-Akt, phospho-S6, and phospho-ERK levels are shown with total Akt, total S6, and total ERK levels.
Discussion
We examined activity of the PI3K/mTOR pathway inhibitors in endometrial cancer cell lines with a particular focus on (i) the antitumor effect of an mTOR inhibitor (RAD001) and a dual PI3K/mTOR inhibitor (NVP-BEZ235), (ii) predictive biomarkers of the mutational status of the PI3K pathway genes, and (iii) combined inhibition of the MAPK pathway and the PI3K/mTOR pathway in K-Ras mutant cells. MTT assay and FACS analysis in a panel of endometrial cancer cell lines revealed a clear dose-dependent effect of NVP-BEZ235 on cell proliferation. NVP-BEZ235 induces G1 arrest much more efficiently at a higher concentration (100 nM) than at a lower concentration (10 nM). In contrast, RAD001 does not show evidence of such dose dependency. Previous reports also suggested that NVP-BEZ235 was more effective than rapalogs at higher concentrations [26], [27]. PI3K activity might not be sufficiently suppressed by 100 nM NVP-BEZ235, as indicated by the observation that decreased phosphorylation of Akt (Thr308) is not observed at 50 nM but is observed at 250 nM or higher. In addition, IC50 values were under 100 nM in cells from groups A and B. These data are in agreement with previous reports on other cancers that indicate a discrepancy between the basal activity of the PI3K/Akt pathway and the biochemical activity of NVP-BEZ235 [26]–[29]. Nevertheless, the dose-dependent antiproliferative activity at concentrations ≥250 nM suggests that the effect of NVP-BEZ235 was, at least in part, caused by inhibition of the PI3K/Akt pathway. Our data suggest that a dual inhibitor of PI3K/mTOR might be a more promising therapeutic strategy than a single mTOR inhibitor in endometrial cancer.
Our in vivo studies in 2 cell lines of xenograft mice support the in vitro findings that inhibition of the PI3K/mTOR axis has an antitumor effect in endometrial cancers. We did not see any superior efficacy of NVP-BEZ235 in the in vivo study. The concentrations we used were 40 mg/kg for NVP-BEZ235 and 5 mg/kg for RAD001, which are equivalent with the previous in-vivo experiments [26], [28], [30]–[32]. In a pharmacodynamic analysis, the levels of p-Akt, p-GSK3beta, p-FOXO1/3a, and p-S6 in tumors returned to the baseline levels within 24 h after administration of NVP-BEZ235, suggesting that inhibition of PI3K signaling by NVP-BEZ235 might not be sufficiently maintained over time. This is compatible with previous data showing that inhibition of p-Akt (Ser473) was maintained for 16 h, with recovery to baseline levels at 24 h [33]. It remains to be determined which oral dosing schedule is optimal in treatment of endometrial cancer. As well, the mechanisms of in-vivo anti-tumor effect by these drugs should be more clarified, as inhibition of mTOR might result in anti-angiogenic effect by suppressing HIF1-VEGF pathway [34].
Developing predictive biomarkers in therapeutics targeting the PI3K/mTOR pathway is crucial, as alterations in several molecules are involved in the activation of this pathway. PIK3CA mutation and HER2 amplification have been recommended as useful biomarkers in breast cancer [26], [35], [36]. Mutant oncogenic Ras has been suggested as a dominant determinant of resistance in several solid tumor cells [19], [37]. PTEN deficiency is controversial as a predictive biomarker [26], [36], [38]. The mechanism of resistance in PTEN-deficient tumors might be explained by the predominant activation of p110beta in PTEN mutant tumors [39], [40], as NVP-BEZ235 and most of the other PI3K inhibitors suppress p110beta less preferentially than the other p110 isoforms. However, p110beta is not a predominant isoform in endometrial carcinomas with PTEN mutations [8]. The significance of p110alpha in PTEN mutant endometrial cancer would be helpful to identify patients susceptible to NVP-BEZ235. Thus, the existence of PTEN mutations might be a predictive biomarker for the PI3K/mTOR inhibitors in endometrial carcinomas. Further in vivo analysis, including the anti-tumor effect of NVP-BEA235, RAD001, or a combination of these compounds with a MEK inhibitor on groups C and D tumors would be necessary to evaluate the utility of these factors as biomarkers.
Feasibility of mutational analysis of the predictor genes should be also considered in clinical trials. K-Ras mutational analysis would be reasonably achievable, as “hot spot” mutations are located only in 2 exons (codons 12, 13, and 61). However, mutations of PIK3CA and PTEN are widespread in the entire coding region. Others and we have reported that PTEN expression is sufficiently evaluated by immunohistochemistry and is correlated with mutational status [25], [41]. A combination of screening K-Ras mutations and immunohistochemistry analysis of PTEN might be a useful and feasible strategy in clinical trials of endometrial cancer. We previously reported that PIK3CA mutations frequently coexist with K-Ras muations in endometrial cancer [8]. The two group C cells (HEC-1B and HHUA) with double mutations of PIK3CA and K-Ras were less sensitive to NVP-BEZ235, compared with group A/B cells. Thus, PIK3CA mutation alone might not be a good biomarker in endometrial cancer. Over 5 clinical studies of the rapalogs have been developed in advanced endometrial cancer. Of them, Oza et al reported phase II study of temsirolimus in endometrial cancer, showing clinical benefit rate (partial response and stable disease) of 52% in chemoetherapy-treated patients [42]. They suggested that PTEN loss (immunohistochemistry and mutational analysis) and molecular markers of PI3K/Akt/mTOR pathway did not correlate with the clinical outcome. It would be clarified whether information of K-Ras alterations might be helpful in the clinical settings. As well, further exploration of the other PI3K-activating alterations (such as mutations of FGFR2 and PIK3R1) and other measurable characteristics (such as standardized uptake value by PET imaging) would be also necessary to establish the most useful clinical biomarkers.
She et al reported that various cell lines with double mutations of K-Ras/BRAF and PIK3CA were resistant to AKT inhibitors, and suggested that a MEK inhibitor sensitizes these double mutant cells to AKT or PI3K inhibitors [43]. Our data in the two group C cells support that a combination of a MEK inhibitor and a PI3K (AKT) inhibitor might be effective to various types of cancers with double mutations of K-Ras and PIK3CA. In addition to mutations, copy number gain of oncogenes is also important for “oncogene addiction”. We previously reported that extensive chromosomal instability (with 5 or more copy number alterations) is a poor independent prognostic factor in endometrial carcinomas [10]. Although extensive chromosomal instability is more common in type II endometrial carcinomas [44], the percentage of extensive chromosomal instability was 31% in our clinical endometrioid adenocarcinoma samples [10]. We found that both group D cell lines (KLE and HEC-50B) harbor extensive CNAs (copy number alterations), with copy number gain at the locus of K-Ras, although they do not possess any mutations in K-Ras, PTEN and PIK3CA. The antiproliferative effect of combined inhibition of MAPK pathway and PI3K/mTOR pathway in group D cells suggests that this combination therapy might be an option to treat tumors with CNA in K-Ras. The dual inhibition of the PI3K and MAPK pathways might overcome the resistance to PI3K/mTOR inhibition alone in certain endometrial tumors with K-Ras alterations through its enhanced cytostatic effect (at least in part). Cheung et al reported that endometrial cell lines with wild-type PI3K pathway members were resistant to an mTOR inhibitor, rapamycin, suggesting that other unexamined factors, including CNA in K-Ras, might be involved in the anti-tumor effect of rapalogs [45]. Phosphorylation of 4E-BP1 is not only regulated by mTORC1, but also by ERK signaling [43], [46], suggesting the crosstalk between PI3K/mTOR pathway and MAPK pathway. It would be necessary to evaluate the in vivo effect of the combined therapy in tumors with K-Ras alterations to address the activity of the MAPK pathway in endometrial cancer.
Supporting Information
Inhibition of cell proliferation by NVP-BEZ235 in 13 endometrial cancer cells. The growth curves of all the 13 cells in response to NVP-BEZ235 in the WST-8 assay (in Figure 2) are shown in one graph.
(PPT)
Inhibition of cell proliferation by RAD001 in 13 endometrial cancer cells. The growth curves of all the 13 cells in response to RAD001 in the WST-8 assay (in Figure 2) are shown in one graph.
(PPT)
Flowcytometric analysis of cell cycle in cancer cells treated with either NVP-BEZ235 or RAD001. (A–D) Cells (5×105) were seeded and treated with NVP-BEZ235 or RAD001 for 48 h at a dose of 10 nM or 100 nM, respectively, as described in Figure 4. (A)–(B); The data from the two group C cells (HEC-1B and HHUA). (C)–(D); the data from the two group D cells (KLE and HEC-50B).
(PPT)
Inhibition of cell proliferation and augmentation of G1 arrest by combination of a MEK inhibitor and NVP-BEZ235 (or RAD001) in cells with alterations in K-Ras (mutation or amplification). (A)–(B) WST-8 assay was performed in HHUA (group C) and KLE (group D) cell lines. (C)–(D) Flowcytometric analysis of cell cycle in HHUA (group C) and KLE (group D) cells. All experiments were repeated 3 times, and each value is shown as the mean of 3 experiments ± S.D.
(PPT)
Acknowledgments
We thank Masato Nishida for providing the Ishikawa3-H-12 cell line.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a Grant-in-aid for Scientific Research (C), 19599005 and 23592437, a Grant-in-Aid for Young Scientists (B), grant number 21791544 (to KO), grant number: Scientific Research (S) 20221009 (to HA), and Next-Generation Cancer Research Project, grant number: 117100001060 to TY) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The funders had no rule in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AJ, Susil B, Jobling TW, Oehler MK. Endometrial cancer. Cell Tissue Res. 2005;322:53–61. doi: 10.1007/s00441-005-1109-5. [DOI] [PubMed] [Google Scholar]
- 4.Doll A, Abal M, Rigau M, Monge M, Gonzalez M, et al. Novel molecular profiles of endometrial cancer-new light through old windows. J Steroid Biochem Mol Biol. 2008;108:221–229. doi: 10.1016/j.jsbmb.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Enomoto T, Inoue M, Perantoni AO, Buzard GS, Miki H, et al. K-ras activation in premalignant and malignant epithelial lesions of the human uterus. Cancer Res. 1991;51:5308–5314. [PubMed] [Google Scholar]
- 6.Kong D, Suzuki A, Zou TT, Sakurada A, Kemp LW, et al. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat Genet. 1997;17:143–144. doi: 10.1038/ng1097-143. [DOI] [PubMed] [Google Scholar]
- 7.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 8.Oda K, Okada J, Timmerman L, Rodriguez Viciana P, Stokoe D, et al. PIK3CA cooperates with other phosphatidylinositol 3′-kinase pathway mutations to effect oncogenic transformation. Cancer Res. 2008;68:8127–8136. doi: 10.1158/0008-5472.CAN-08-0755. [DOI] [PubMed] [Google Scholar]
- 9.Murayama Hosokawa S, Oda K, Nakagawa S, Ishikawa S, Yamamoto S, et al. Genome-wide single-nucleotide polymorphism arrays in endometrial carcinomas associate extensive chromosomal instability with poor prognosis and unveil frequent chromosomal imbalances involved in the PI3-kinase pathway. Oncogene. 2010;29:1897–1908. doi: 10.1038/onc.2009.474. [DOI] [PubMed] [Google Scholar]
- 10.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 13.Houghton PJ. Everolimus. Clin Cancer Res. 2010;16:1368–1372. doi: 10.1158/1078-0432.CCR-09-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meric Bernstam F, Gonzalez Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 16.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 17.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 19.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sos ML, Fischer S, Ullrich R, Peifer M, Heuckmann JM, et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci U S A. 2009;106:18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faber AC, Li D, Song Y, Liang MC, Yeap BY, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mordant P, Loriot Y, Leteur C, Calderaro J, Bourhis J, et al. Dependence on phosphoinositide 3-kinase and RAS-RAF pathways drive the activity of RAF265, a novel RAF/VEGFR2 inhibitor, and RAD001 (Everolimus) in combination. Mol Cancer Ther. 2010;9:358–368. doi: 10.1158/1535-7163.MCT-09-1014. [DOI] [PubMed] [Google Scholar]
- 23.Minaguchi T, Yoshikawa H, Oda K, Ishino T, Yasugi T, et al. PTEN mutation located only outside exons 5, 6, and 7 is an independent predictor of favorable survival in endometrial carcinomas. Clin Cancer Res. 2001;7:2636–2642. [PubMed] [Google Scholar]
- 24.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 25.Shoji K, Oda K, Nakagawa S, Hosokawa S, Nagae G, et al. The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br J Cancer. 2009;101:145–148. doi: 10.1038/sj.bjc.6605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 27.Cho DC, Cohen MB, Panka DJ, Collins M, Ghebremichael M, et al. The efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 compared with rapamycin in renal cell carcinoma. Clin Cancer Res. 2010;16:3628–3638. doi: 10.1158/1078-0432.CCR-09-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMillin DW, Ooi M, Delmore J, Negri J, Hayden P, et al. Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Cancer Res. 2009;69:5835–5842. doi: 10.1158/0008-5472.CAN-08-4285. [DOI] [PubMed] [Google Scholar]
- 30.Konstantinidou G, Bey EA, Rabellino A, Schuster K, Maira MS, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–7652. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao P, Maira SM, Garcia Echeverria C, Hedley DW. Activity of a novel, dual PI3-kinase/mTor inhibitor NVP-BEZ235 against primary human pancreatic cancers grown as orthotopic xenografts. Br J Cancer. 2009;100:1267–1276. doi: 10.1038/sj.bjc.6604995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 34.Mabuchi S, Altomare DA, Connolly DC, Klein Szanto A, Litwin S, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67:2408–2413. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 35.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res. 2010;16:3670–3683. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- 37.Ihle NT, Lemos R, Jr, Wipf P, Yacoub A, Mitchell C, et al. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009;69:143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dan S, Okamura M, Seki M, Yamazaki K, Sugita H, et al. Correlating phosphatidylinositol 3-kinase inhibitor efficacy with signaling pathway status: in silico and biological evaluations. Cancer Res. 2010;70:4982–4994. doi: 10.1158/0008-5472.CAN-09-4172. [DOI] [PubMed] [Google Scholar]
- 39.Jia S, Liu Z, Zhang S, Liu P, Zhang L, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wee S, Wiederschain D, Maira SM, Loo A, Miller C, et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci U S A. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Ren Y, Wang L, Li B, Chen Y, et al. PTEN mutation spectrum in breast cancers and breast hyperplasia. J Cancer Res Clin Oncol. 2010;136:1303–1311. doi: 10.1007/s00432-010-0781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oza AM, Elit L, Tsao MS, Kamel Reid S, Biagi J, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A. 2009;106:4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung LW, Hennessy BT, Li J, Yu S, Myers AP, et al. High Frequency of PIK3R1 and PIK3R2 Mutations in Endometrial Cancer Elucidates a Novel Mechanism for Regulation of PTEN Protein Stability. Cancer Discov. 2011;1:170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of cell proliferation by NVP-BEZ235 in 13 endometrial cancer cells. The growth curves of all the 13 cells in response to NVP-BEZ235 in the WST-8 assay (in Figure 2) are shown in one graph.
(PPT)
Inhibition of cell proliferation by RAD001 in 13 endometrial cancer cells. The growth curves of all the 13 cells in response to RAD001 in the WST-8 assay (in Figure 2) are shown in one graph.
(PPT)
Flowcytometric analysis of cell cycle in cancer cells treated with either NVP-BEZ235 or RAD001. (A–D) Cells (5×105) were seeded and treated with NVP-BEZ235 or RAD001 for 48 h at a dose of 10 nM or 100 nM, respectively, as described in Figure 4. (A)–(B); The data from the two group C cells (HEC-1B and HHUA). (C)–(D); the data from the two group D cells (KLE and HEC-50B).
(PPT)
Inhibition of cell proliferation and augmentation of G1 arrest by combination of a MEK inhibitor and NVP-BEZ235 (or RAD001) in cells with alterations in K-Ras (mutation or amplification). (A)–(B) WST-8 assay was performed in HHUA (group C) and KLE (group D) cell lines. (C)–(D) Flowcytometric analysis of cell cycle in HHUA (group C) and KLE (group D) cells. All experiments were repeated 3 times, and each value is shown as the mean of 3 experiments ± S.D.
(PPT)