Abstract
The guanine insertion enzyme from Escherichia coli catalyzes exchange of guanine located at the first position of the anticodon of tRNA with radioactive guanine (N. Okada and S. Nishimura, unpublished data). tRNA isolated from various tumors, including slowly growing Morris hepatoma 7794A, incorporated considerable guanine with E. coli guanine insertion enzyme, whereas tRNA isolated from all normal tissues so far tested, except regenerating rat liver, incorporated scarcely any. In the rat ascites hepatoma AH7974, the guanine was mostly incorporated into minor isoaccepting species of tRNAAsp that contained the guanine residue instead of Q base in the first position of the anticodon. This is a sensitive and easy method for identifying unique tRNA species in tumor tissues.
Full text
PDF

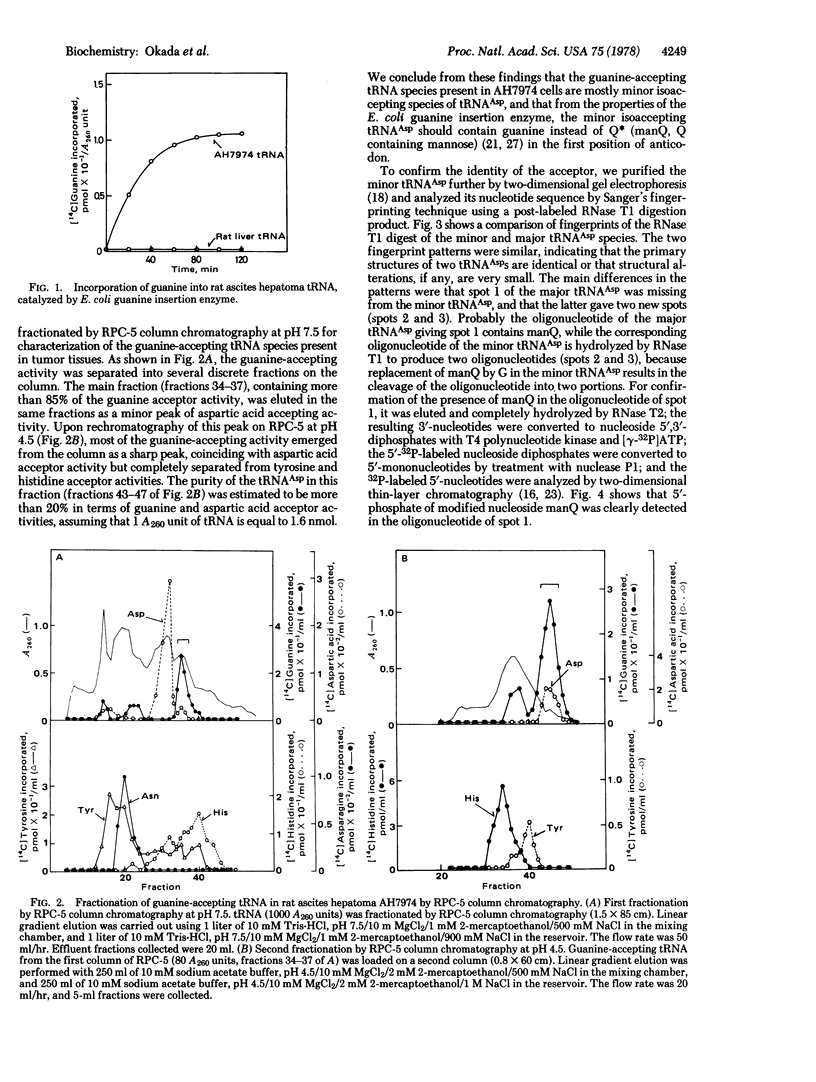
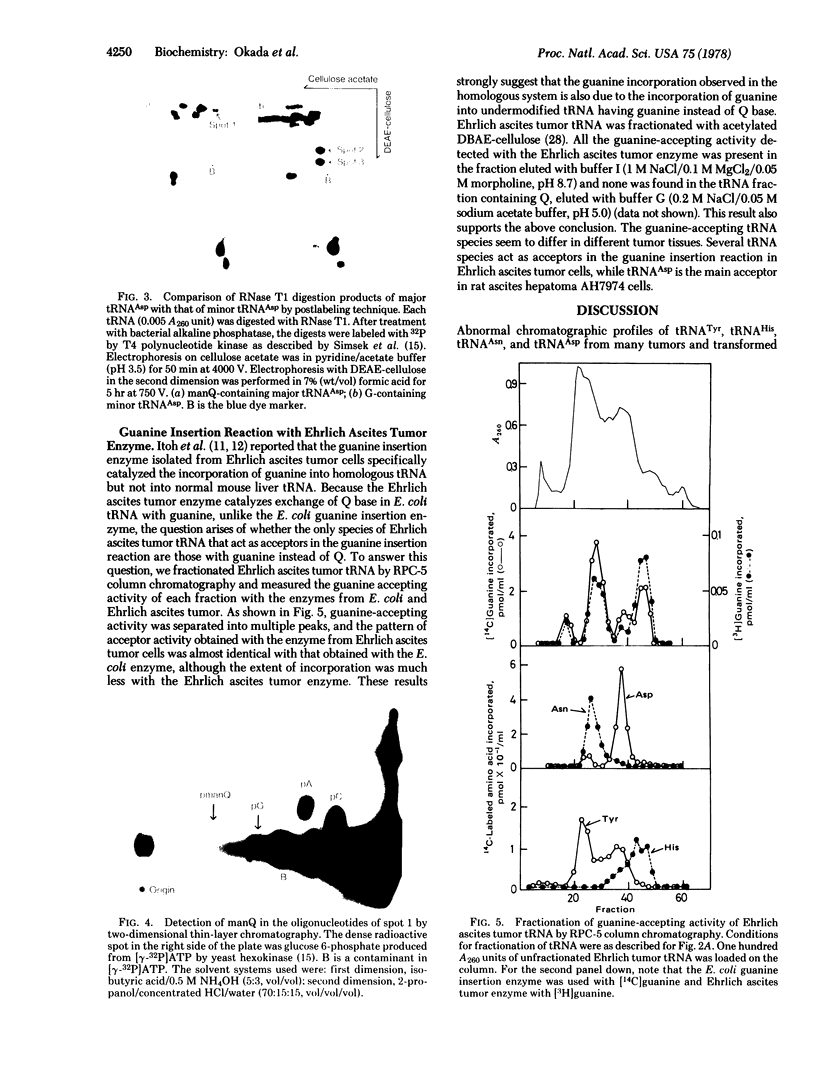

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baliga B. S., Borek E., Weinstein I. B., Srinivasan P. R. Differences in the transfer RNA's of normal liver and Novikoff hepatoma. Proc Natl Acad Sci U S A. 1969 Mar;62(3):899–905. doi: 10.1073/pnas.62.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek E., Kerr S. J. Atypical transfer RNA's and their origin in neoplastic cells. Adv Cancer Res. 1972;15:163–190. doi: 10.1016/s0065-230x(08)60374-7. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Griffin A. C., McBride C., Bowen J. M. The distribution and properties of aspartyl transfer RNA in human and animal tumors. Cancer Res. 1975 Sep;35(9):2586–2593. [PubMed] [Google Scholar]
- Craddock V. M. Transfer RNA methylases and cancer. Nature. 1970 Dec 26;228(5278):1264–1268. doi: 10.1038/2281264a0. [DOI] [PubMed] [Google Scholar]
- Farkas W. R., Chernoff D. Identification of the minor guanylated tRNA of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2521–2528. doi: 10.1093/nar/3.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas W. R., Singh R. D. Guanylation of transfer ribonucleic acid by a cell-free lysate of rabbit reticulocytes. J Biol Chem. 1973 Nov 25;248(22):7780–7785. [PubMed] [Google Scholar]
- Garnett M. E., Dyson R. D., Dost F. N. Pyruvate kinase isozyme changes in parenchymal cells of regenerating rat liver. J Biol Chem. 1974 Aug 25;249(16):5222–5226. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberger D., Weinstein I. B., Mushinski J. F. Deficiency of the Y base in a hepatoma phenylalanine tRNA. Nature. 1975 Jan 3;253(5486):66–67. doi: 10.1038/253066a0. [DOI] [PubMed] [Google Scholar]
- Harada F., Nishimura S. Possible anticodon sequences of tRNA His , tRNA Asm , and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry. 1972 Jan 18;11(2):301–308. doi: 10.1021/bi00752a024. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Irie R. F. Antigenic cross-reactivity between primary spontaneous mouse mammary tumors and their transplantable ascites tumors. Cancer Res. 1971 Nov;31(11):1682–1689. [PubMed] [Google Scholar]
- Ito T., Haruna I., Watanabe I. Incorporation of GMP into specific tRNA molecules by extracts of Ehrlich ascites tumour cells. Nature. 1975 Sep 25;257(5524):327–329. doi: 10.1038/257327a0. [DOI] [PubMed] [Google Scholar]
- Itoh Y. H., Itoh T., Haruna I., Watanabe I. Substitution of guanine for a specific base in tRNA by extracts of Ehrlich ascites tumour cells. Nature. 1977 Jun 2;267(5610):467–467. doi: 10.1038/267467a0. [DOI] [PubMed] [Google Scholar]
- Kasai H., Kuchino Y., Nihei K., Nishimura S. Distribution of the modified nucleoside Q and its derivatives in animal and plant transfer RNA's. Nucleic Acids Res. 1975 Oct;2(10):1931–1939. doi: 10.1093/nar/2.10.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nakanishi K., Macfarlane R. D., Torgerson D. F., Ohashi Z., McCloskey J. A., Gross H. J., Nishimura S. Letter: The structure of Q* nucleoside isolated from rabbit liver transfer ribonucleic acid. J Am Chem Soc. 1976 Aug 4;98(16):5044–5046. doi: 10.1021/ja00432a071. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Alterations in SVT2 cell transfer RNAs in response to cell density and serum type. Biochim Biophys Acta. 1975 Mar 10;383(2):131–139. doi: 10.1016/0005-2787(75)90254-3. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Relation of cell type and cell density to the degree of post-transcriptional modification of tRNALys and tRNAPhe. Biochim Biophys Acta. 1975 Nov 4;407(4):392–398. doi: 10.1016/0005-2787(75)90291-9. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Borek E. Tumour-specific phenylalanine tRNA contains two supernumerary methylated bases. Nature. 1978 Jan 12;271(5641):126–129. doi: 10.1038/271126a0. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA K. A new transplant strain of leukemic ascites tumor of mice. Gan. 1956 Dec;47(3-4):561–565. [PubMed] [Google Scholar]
- Nishimura S., Weinstein I. B. Fractionation of rat liver transfer ribonucleic acid. Isolation of tyrosine, valine, serine, and phenylalanine transfer ribonucleic acids and their coding properties. Biochemistry. 1969 Mar;8(3):832–842. doi: 10.1021/bi00831a011. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada F., Nishimura S. Specific replacement of Q base in the anticodon of tRNA by guanine catalyzed by a cell-free extract of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2593–2603. doi: 10.1093/nar/3.10.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Shindo-Okada N., Nishimura S. Isolation of mammalian tRNAAsp and tRNATyr by lectin-Sepharose affinity column chromatography. Nucleic Acids Res. 1977 Feb;4(2):415–423. doi: 10.1093/nar/4.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Randerath E., Chia L. L., Morris H. P., Randerath K. Transfer RNA base composition studies in Morris hepatomas and rat liver. Cancer Res. 1974 Mar;34(3):643–653. [PubMed] [Google Scholar]
- Sekiya T., Oda K. I. The altered patterns of transfer RNA in SV40-infected and transformed cells. Virology. 1972 Jan;47(1):168–180. doi: 10.1016/0042-6822(72)90250-4. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Transformation of green monkey kidney cells by SV40 genome: the establishment of transformed cell lines and the replication of human adenoviruses and SV40 in transformed cells. Virology. 1971 Jul;45(1):163–171. doi: 10.1016/0042-6822(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Kano-Sueoka T. Transfer RNA and cell differentiation. Prog Nucleic Acid Res Mol Biol. 1970;10:23–55. doi: 10.1016/s0079-6603(08)60560-7. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J Mol Biol. 1973 Mar 15;74(4):635–651. doi: 10.1016/0022-2836(73)90054-5. [DOI] [PubMed] [Google Scholar]