Abstract
Inflammatory pain manifests as spontaneous pain and pain hypersensitivity. Spontaneous pain reflects direct activation of specific receptors on nociceptor terminals by inflammatory mediators. Pain hypersensitivity is the consequence of early posttranslational changes, both in the peripheral terminals of the nociceptor and in dorsal horn neurons, as well as later transcription-dependent changes in effector genes, again in primary sensory and dorsal horn neurons. This inflammatory neuroplasticity is the consequence of a combination of activity-dependent changes in the neurons and specific signal molecules initiating particular signal-transduction pathways. These pathways phosphorylate membrane proteins, changing their function, and activate transcription factors, altering gene expression. Two distinct aspects of sensory neuron function are changed as a result of these processes, basal sensitivity, or the capacity of peripheral stimuli to evoke pain, and stimulus-evoked hypersensitivity, the capacity of certain inputs to generate prolonged alterations in the sensitivity of the system. Posttranslational changes largely alter basal sensitivity. Transcriptional changes both potentiate the system and alter neuronal phenotype. Potentiation occurs as a result of the up-regulation in the dorsal root ganglion of centrally acting neuromodulators and simultaneously in the dorsal horn of their receptors. This means that the response to subsequent inputs is augmented, particularly those that induce stimulus-induced hypersensitivity. Alterations in phenotype includes the acquisition by A fibers of neurochemical features typical of C fibers, enabling these fibers to induce stimulus-evoked hypersensitivity, something only C fiber inputs normally can do. Elucidation of the molecular mechanisms responsible provides new opportunities for therapeutic approaches to managing inflammatory pain.
Pain is a state-dependent sensory experience. Normally, it is generated only by activation of a specific subset of high-threshold peripheral sensory neurons, the nociceptors, hence nociception—the detection of noxious or tissue-damaging stimuli. Nociception is important for being aware of and reacting to potentially or actually damaging stimuli in the environment. Absence of this capacity, as in individuals with congenital analgesia, results in ongoing tissue damage. Pain is also, of course, a major clinical problem. After inflammation or nerve injury, dramatic alterations in the somatosensory system occur, amplifying responses and increasing sensitivity to peripheral stimuli so that pain can now be activated by normally innocuous or low-intensity stimuli. Clinical pain is an expression, then, of plasticity in the somatosensory system, operating at multiple sites and due to diverse mechanisms. The purpose of this paper is to highlight key features of the plasticity of primary sensory neurons and of the synaptic contacts they make with dorsal horn neurons, the mechanisms that operate to produce this plasticity, and how this relates to the pathogenesis of clinical pain hypersensitivity. The emphasis will be on posttranslational and transcriptional changes in primary sensory and dorsal horn neurons initiated by electrical activity and peripheral inflammation. We will show that sensory inputs not only produce sensations, painful and nonpainful (basal sensitivity), but also alter the somatosensory system by inducing neural plasticity, and that this changes the responsiveness of the system to subsequent stimuli (stimulus-induced hypersensitivity).
State-Dependent Sensory Processing in the Somatosensory System
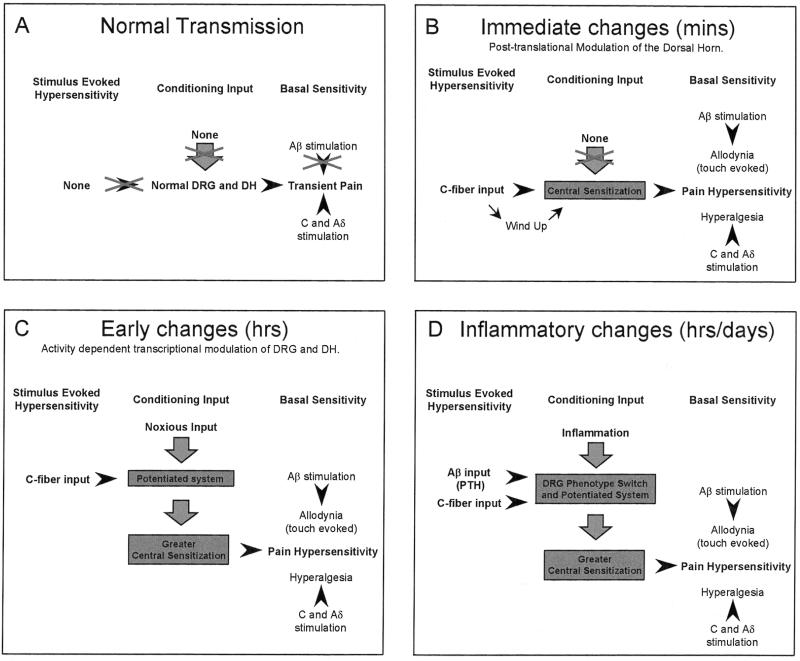
Fig. 1 defines four distinct states of the somatosensory system. The first state is present under normal or physiological circumstances and is responsible for the normal processing of low- and high-intensity peripheral stimuli into innocuous and painful sensations, respectively. The second state follows a noxious C fiber activating input of sufficient intensity or duration to initiate immediate posttranslational changes in membrane-bound receptors of dorsal horn neurons, modifying their excitability and thereby altering sensitivity to subsequent low- and high-intensity peripheral stimuli, the phenomenon of central sensitization. The third state is established several hours after a substantial noxious stimulus, which, by virtue of the activity-dependent changes in the expression of functional genes in sensory and dorsal horn neurons it produces, results in a potentiated system. This transcription-dependent potentiated system now displays an enhanced responsiveness to subsequent central sensitization-inducing inputs. Finally, the last state follows peripheral inflammation, where a combination of activity-dependent and signal molecule-mediated posttranslational and transcriptional changes in the sensory and dorsal horn neurons results in a fundamentally altered system, one in which the transduction sensitivity of the peripheral terminals is reduced (peripheral sensitization) (1), the excitability of dorsal horn neurons is increased (central sensitization) (2), and the phenotype of sensory neurons is altered (3) such that both low-intensity Aβ fiber and C fiber inputs can initiate central sensitization. Here we will discuss each of these states in turn, discussing the molecular mechanisms within the dorsal root ganglion (DRG) and dorsal horn that elicit these changes, from the mediators to the receptors through the intracellular signal-transduction cascades and finally culminating on the posttranslational and transcriptional changes that result.
Figure 1.
State-dependent sensory processing in the somatosensory system. (A) The basal sensitivity of the system is normally such that only a high-intensity C or Aδ stimulus produces pain. (B) C fiber inputs induce immediate posttranslational changes in the dorsal horn, resulting in central sensitization and altering basal sensitivity such that low intensity stimuli result in pain. (C) Conditioning C fiber strength inputs induce activity-dependent transcriptional changes in the DRG and dorsal horn, resulting in a potentiated system augmenting responsiveness to subsequent C fiber inputs. (D) Inflammation results in both a potentiated system and phenotypic switches such that both C fiber and low-intensity Aβ fiber inputs can evoke central sensitization.
State One: Nociception (Normal Transmission).
The activation of high-threshold nociceptive primary afferent neurons, mainly unmyelinated C fibers but Aδ fibers as well, by intense but nondamaging stimuli, results within seconds in a transient, well localized pain that does not require long-term nontranscriptional or transcriptional changes. Primary sensory neurons have three clear functions with respect to their role in nociception: transduction of noxious but not low-intensity peripheral stimuli; conduction of action potentials to the central nervous system; and transmission to central neurons. Distinct transducers, receptors, ion channels, and transmitters mediate these functions. Tables 1 and 2 summarize the key ion channels and receptors expressed in dorsal root ganglion cells, the functions they mediate, and their posttranslational modulation or transcriptional regulation.
Table 1.
Ionotropic receptors and ligand-gated ion channels expressed by nociceptive primary afferent neurons
| Receptor/Channel | Major subtypes | Ligand | Expression (cell size/type) | Notes | Function |
|---|---|---|---|---|---|
| Purine | P2X2 P2X3 | ATP, ADP, Adenosine | P2X3, IB4 positive cells | P2X2 modulated by protons; P2X3 GDNF regulated | IMR, SyMR, AR |
| Acid-sensing proton-gated | DRASIC, ASIC (α, β) | Protons | DRASIC, Small. ASICs α, Small β, Small/large | Amiloride sensitive; Mechanosensitive? | ST, IMR, SyMR |
| Vanilloid | VR1 | Heat, capsaicin | Small | Sensitized by heat; Proton-gated; NGF-regulated | ST, IMR |
| Sodium TTXr | SNS/PN3 SNS2/NaN | N/A | Small | PKA and PKC substrate; NGF regulated | EMC |
| Sodium TTXs | PN1, PN4, rB(I-III) | N/A | All cells | NGF regulated | EMC |
| Calcium voltage-gated channels | T- (low-threshold) L-, N- (highthreshold) | N/A | T, Small/medium L, N, Mainly small | Secondary calcium currents mediated by G protein-coupled receptors, e.g. bradykinin; Enhancement of high threshold current by PKC | SyMC |
| Serotonin | 5HT-3 | Serotonin | Small | IMR, ST | |
| NMDA | NR1 NRgbs | Glutamate/aspartate | Small/large | Controls SP release | SyMR, AR |
| AMPA | iGluR1-3 | Glutamate | R1, Small | SyMR, AR | |
| R2–3, Small/large | |||||
| Kainate | iGluR5 | Glutamate | Small | Controls neurotransmitter release | SyMR, AR |
IMR, inflammatory mediator receptor; SyMR, synaptic modulator receptor; SyMC, synaptic modulator channel; ST, signal transducer; EMC, excitatory modulator channel; AR, autoreceptor; N/A, not applicable.
Table 2.
Metabotropic receptors of nocieptive primary afferent neurons
| G protein-coupled receptor | Subtypes present | Ligand | Expression (cell size/type) | Notes | Function |
|---|---|---|---|---|---|
| Prostanoids | |||||
| PGE | EP1-4 | PGE1-4 | EP1, EP2, EP4, Unspecified. EP3, Mainly small. | PGE2 sensitizes some normally unresponsive intermediate size cells to bradykinin; PGE2–PKA/C mediated modulator of TTXr; Sensitizes heat stimuli. | IMR, AR, SyMR? |
| IP | IP | PGI2 | Mainly small | ||
| Histamine | H1 | Histamine | Some small | Itch mediator. | IMR |
| Serotonin | 5-HT1A 5-HT4 5-HT2A | Serotonin | 5-HT1A/5-HT4, Small. 5-HT2A, Most | 5HT4 increases TTXr small cell currents via PKA/C. | IMR |
| Bradykinin | B1 (induced) | Bradykinin | B1, Small/large | Sensitizes small cells to heat via PKA. | IMR |
| B2 | B2, Small | Increases the number of cells that respond to capsaicin and protons. | |||
| Cannabinoid | CB1-2 | Anandamide | Unspecified | Inhibits peripheral activation. | IMR(in) |
| Tachykinin | NK1 | Sub P, NKA | Small cells | SyMR, AR | |
| Opioid | μ, δ, κ | Enkephalins Dynorphins β-Endorphins | Small cells | Inflammatory cells release endogenous opioids. Inhibits peripheral activation. | SyMR IMR(in) |
Abbreviations are as in Table 1. IMR(in), IMR-inhibitory.
Nociceptive transduction.
Peripheral nociceptive transduction involves the detection of hot and cold (but not warm or cool) thermal stimuli and intense (but not innocuous) mechanical stimuli, as well as a sensitivity to chemical irritants such as the pungent ingredient in chili peppers, capsaicin. This sensitivity is mediated by multiple specialized receptors, including heat-sensitive ion channels like the vanniloid receptor VR1, which is gated by protons and activated by capsaicin (4, 5), channels sensitive to protons and possibly mechanical stimuli, the Na+/degenerin family including ASIC (acid-sensing ionic channel) and DRASIC (dorsal root acid-sensing ionic channel) (6), and receptors sensitive to chemical stimuli alone (the histamine, bradykinin, purine, and serotonin receptors) (7–10). Chemical stimuli are a very prominent component of inflammatory pain, where they are generated as part of the inflammatory response (see below). A chemical component of nociception occurs only on exposure to nondamaging external chemical irritants, such as plant or insect stings. The relative expression of these transduction elements in specific subsets of nociceptor sensory neurons is beginning to reveal specialization of modality sensitivity. The purine receptor P2X3, for example, is expressed only on glial cell line-derived neurotrophic factor-responsive c-Ret-expressing C fibers (11), whereas VR1 is expressed both in these cells and in the TrkA-expressing nerve growth factor (NGF)-responsive neurons (5).
Nociceptive conduction.
Voltage-gated sodium channels, which are responsible for the rising phase of the action potential and play a key role, with potassium channels, in determining the excitability of the sensory neurons, mediate conduction in nociceptors by transferring input from the peripheral nerve terminals to the spinal cord. Neuronal voltage-gated sodium channels can be classified into two types: those sensitive to nanomolar concentrations of the puffer fish toxin (tetrodotoxin-sensitive, TTXs) and those resistant to all but micromolar concentrations of tetrodotoxin (tetrodotoxin- resistant, TTXr) (12). DRG neurons express several distinct kinetic types of sodium current. Small-diameter, high-threshold nociceptor neurons coexpress a rapidly inactivating, fast TTXs current and a slowly activating and inactivating TTXr sodium current (13, 14). Large diameter cells only express a TTXs sodium current (13).
Within the DRG, several voltage-gated sodium channels are known to be responsible for the TTXs current (PN1, rSCP6/PN4, rBI, rBII, and rBIII), and these show a wide distribution across large and small neuronal cells (15–17). Two sensory neuron-specific TTXr sodium channels have been cloned [SNS/PN3 (18, 19) and SNS2/NaN (20, 21)]. SNS2/NaN is found only in small sensory neurons, where it is colocalized with SNS/PN3 (20), with which it may form functional heteromultimers, generating the native TTXr current. SNS/PN3 is also found without SNS2 in a subpopulation of larger neurons (20). The expression of TTXr sodium channels in nociceptive neurons points to a specific role for these channels in contributing to the excitability of nociceptors, although the TTXs current may be sufficient for conduction, and the TTX current plays more of a modulatory role in the peripheral terminals and in generating ectopic inputs.
Nociceptive transmission.
Transfer of synaptic input from nociceptors to specific laminae in the dorsal horn is highly topographically organized and activates particular subsets of second-order projection neurons ultimately leading, following activation of specific brain centers, to the sensation of acute pain as well emotional, cognitive, and autonomic responses. Primary afferent neurons, by virtue of their peripheral transduction specialization, central termination site, or temporal characteristics, encode stimulus modality, intensity, location, and duration (22). This is relayed with great fidelity to second-order neurons. Synaptic transmission for C fibers is mediated by glutamate acting on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors on dorsal horn neurons, generating fast excitatory postsynaptic potentials with a slow trailing edge contributed to by the N-methyl-d-aspartate (NMDA) receptor (23, 24). If the stimulus intensity is high enough, release of neuropeptides from dense core vesicles—particularly substance P—will occur, which will, via an activation of the NK1 receptor, generate a greater postsynaptic response, reflecting the greater input and representing, therefore, a form of intensity coding (25). Antagonism or deletion of the NMDA, NK1, or mGluR receptor does not normally interfere, though, with the nociceptive response to normal non-tissue-damaging noxious stimuli (26); this is largely mediated by glutamate acting on its AMPA receptor and reflects the necessity for a high-safety, fast early warning protective pain system. The NMDA, NK1, or mGluR receptors play a key role in the initiation or maintenance of changes in synaptic transmission that constitute central sensitization (27–32), where the specific link between a noxious stimulus and pain is lost and pain can be produced by normally innocuous inputs (2, 33–35).
Neural plasticity and pain.
Sensory neurons can undergo functional, chemical, and structural changes in response to changes in their environment that modify their transduction, conduction, and transmission and move them from their specific role in mediating normal nociceptive transmission to a new modified condition contributing to an altered state of sensibility. The key issues, then, are what produces these changes, how and where they manifest, and what the sensory consequences are. We will analyze this in terms of two forms of plasticity, activity- and signal molecule-dependent, and two kinds of change, that induced immediately by posttranslational modifications in the neurons and that caused by an alteration in expression of key effector molecules, which takes some time to manifest. The functional significance of this plasticity, both for basal sensibility and stimulus-evoked hypersensitivity, will be discussed.
State Two: Immediate Changes (Posttranslational Modulation of the Dorsal Horn).
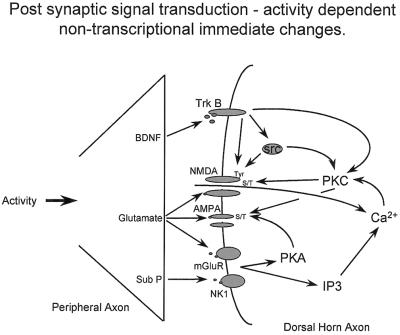
The first example of stimulus-evoked hypersensitivity is that of C fiber-mediated central sensitization (2). Nociceptive pain is characterized by a clear distinction in the sensory responses to a noxious or low-intensity stimulus, pain, and an innocuous sensation, respectively. Activation of C fibers, can, however, result in a situation where basal sensitivity changes such that normal low-threshold Aβ inputs begin to evoke pain and tactile allodynia and noxious inputs provoke a greater pain response, hyperalgesia (refs. 2 and 33–35; Fig. 1). Central sensitization, which in normal noninflamed situations, can be produced only by C fiber input (36), is the direct manifestation of a complex set of activity-dependent posttranslational changes in dorsal horn neurons. Relatively brief C fiber inputs (lasting only tens of seconds) can initiate very rapid changes in membrane excitability, which manifest both as a progressive increase in excitability during the course of the stimulus, windup, and poststimulus changes that can last for several hours (central sensitization) (2, 36). Windup is a manifestation of the removal by successive synaptic depolarization of the voltage-dependent Mg2+ block of the NMDA receptor amplifying the response to each subsequent input. Central sensitization is quite different. Fig. 2 summarizes some of the salient features. Presynaptic transmitter/neuromodulator release [glutamate, substance P, and brain-derived neurotrophic factor (BDNF)] results in changes in signal-transduction pathways in dorsal horn neurons as a result of the activation of ligand-gated ion channels (NMDA-R–glutamate), metabotropic receptors (mGluR–glutamate, NK1–substance P) and tyrosine kinase receptors (TrkB–BDNF). Not shown in Fig. 2 but likely to have major effects on posttranslational changes in the dorsal horn are the prostanoids. These act through prostaglandin E and prostacyclin and IP receptors and may be released pre- and postsynaptically (reviewed in ref. 37).
Figure 2.
Posttranslational changes within dorsal horn neurons after release of transmitters from C fiber central terminals. These transmitters/neuromodulators act on receptors and ion channels in the dorsal horn to activate protein kinases that phosphorylate membrane-bound NMDA and AMPA receptors and alter their functional properties, increasing membrane excitability and thereby eliciting central sensitization.
Activation of these multiple receptors results in an increase in intracellular calcium, both via calcium inflow and release from intracellular stores (38) and a consequent activation of calcium-dependent enzymes (protein kinase C and calcium calmodulin kinase), protein kinase A (via G protein coupled-receptors), and tyrosine kinases (via the TrkB receptor) (see Fig. 2). These pathways converge and diverge in a complex fashion and are linked such that TrkB, which itself is a tyrosine kinase, also activates other tyrosine kinases src and protein kinase C (39, 40). The targets for these different kinases are membrane-bound receptors/ion channels, of which the NMDA and AMPA are best characterized, although others are certainly involved, including activation of neuronal nitric-oxide synthase and prostaglandin production, with generation of retrograde signals to the presynaptic terminal. Phosphorylation of the NMDA receptor at either serine/threonine residues on its NR1 subunit (41) or on tyrosine residues on its NR2 subunit (42, 43) is a major factor. This posttranslational modification results in dramatic changes in NMDA-receptor channel kinetics and a reduction in its voltage-dependent Mg2+ block (42, 44, 45). Both of these changes augment subsequent responsiveness to synaptically released glutamate, increasing synaptic strength and enabling previously subthreshold inputs to drive the output of the cell (46). This effective increase in gain alters receptive field properties (46–48) and pain sensitivity, causing tactile allodynia and pin prick hyperalgesia, far beyond the site of the C fiber-activating stimulus that initiated the central sensitization (49).
State Three: Early Changes in Transcription.
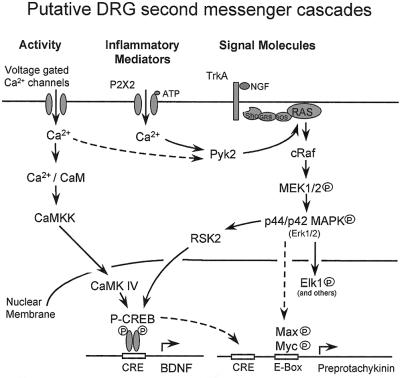
C fiber inputs, in addition to the generation of central sensitization, which occurs within seconds of the appropriate activation of dorsal horn neurons, also generate an activity-dependent change in transcription in dorsal root ganglion and dorsal horn neurons, which has functional consequences that take hours to manifest. BDNF mRNA levels, for example, increase in the DRG 2 hours after C fiber stimuli, whereas such inputs also increase TrkB message in the dorsal horn (unpublished observations). These changes are very likely to result from an increase in calcium influx through voltage-gated calcium channels in the DRG, secondary to increased electrical activity. Such changes in intracellular free calcium will cause changes in the phosphorylation and therefore activation of a whole host of transcription factors, a well characterized example of which in neuronal systems is the cAMP responsive element-binding protein (CREB) (Fig. 3). Seconds to minutes after calcium influx, neuronal CREB is phosphorylated at Ser-133 (50). Activity-dependent CREB Ser-133 phosphorylation has also been demonstrated in mouse embryonic DRG cells in culture as has activity-dependent regulation of mitogen-activated protein (MAP) kinase (51). Multiple signal-transduction cascades converge on CREB Ser-133 phosphorylation (reviewed in ref. 52). In hippocampal neurons, phosphorylation of CREB is mediated by the enzyme CaMKIV (calcium–calmodulin-dependent protein kinase IV). Activation of this enzyme in turn is mediated by CaMK kinase (CamKK). Interestingly, Ji et al. (53) have shown that CamKIV immunostaining is present mostly in small neurons of the DRG. Calcium-dependent BDNF transcription in cortical neurons is mediated through CREB (54, 55). It is likely, then, that at least some of the activity-dependent up-regulation of BDNF within the DRG is also CREB-dependent. Calcium influx into PC12 cells, mediated by P2X2 receptors as opposed to voltage-gated calcium channels, can activate the MAP kinase cascade via Pyk2 (56). Pyk2 provides a link between free cellular calcium and the Map kinase cascade (57).
Figure 3.
Putative DRG activity- and signal molecule-dependent second-messenger cascades leading to transcription. Activity-dependent calcium flux through voltage-gated calcium channels may increase BDNF transcription via CREB phosphorylation on Ser-133. NGF acts via TrkA to activate the MAP kinase cascade through ras. Myc phosphorylation through this pathway can result in E box transactivation via its association with P Max. The preprotachykinin promoter contains multiple E box units and thus maybe regulated by these transcription factors. RSK2 links the MAP kinase cascade to CREB phosphorylation. ATP can stimulate the MAP kinase cascade via Pyk2.
BDNF produced in TrkA-expressing small neurons in the DRG is transported to their central terminals, where BDNF is located in dense core vesicles (58) and contributes not to basal sensitivity, which appears to be the result primarily of glutamate acting on AMPA receptors, but to C fiber-induced central sensitization (unpublished observations) (Fig. 2). An increase in the amount of this presynaptic neuromodulator in C fibers as a result of prior activity in these fibers, combined with an increase in its high-affinity receptor in the dorsal horn, will result in what we have termed a potentiated system. A potentiated system is one where the same stimulus applied a second time, some considerable time after the first, will produce a greater response, because the first stimulus has changed the system after a slow onset, but for a relatively long period (Fig. 1). There are features of this change into a potentiated sensory system that are reminiscent of the activity-dependent transcriptional changes that contribute to the persistence of long-term potentiation in cortical cells and imply that this state constitutes a kind of pain memory. The potentiation of the sensory system needs, though, to be differentiated from windup, where a progressive increase in responsiveness occurs rapidly (within 1 or 2 seconds), during the course of a train of inputs. Potentiation refers to an effect that only manifests several hours after the input and is due to a change in transcription. Although the system is potentiated, this is still only to C fiber inputs, which evoke an augmented hypersensitivity because of changes intrinsic only to these fibers and their receptive dorsal horn neurons. This situation differs from inflammation where A fibers acquire the capacity to induce central hypersensitivity (see below).
State Four: Inflammatory Changes in DRG and Dorsal Horn Neurons.
Inflammation is associated with tissue damage, which results in the leak of intracellular contents into the extracellular fluid, the recruitment of inflammatory cells, and the production and release of a broad range of neuroactive agents by inflammatory and noninflammatory cells, including ions (K+ and H+) (6), amines (5-hydroxytryptamine, histamine) (9), kinins (bradykinin) (8), prostanoids (PGE2) (37), purines (ATP) (7), NO, cytokines (IL-1, TNFα, Il-6) (9, 10) and growth factors (leukemia inhibitory factor, NGF) (10). Some of these inflammatory agents may be sufficient by themselves to activate the peripheral nerve endings of those nociceptors that express the appropriate receptors (Tables 1 and 2), generating inward currents and sensory inflow. Most of these agents, however, act by changing the sensory neuron rather than directly activating it. These changes include early posttranslational changes both in the peripheral terminals of nociceptors, altering transduction sensitivity (peripheral sensitization), and in dorsal horn neurons secondary to activity in C fibers (central sensitization). Both peripheral sensitization and central sensitization alter basal sensitivity to noxious and normally innocuous stimuli. There are, in addition, later and longer-lasting transcription-dependent changes in the DRG and in the dorsal horn that are due to a complex combination of activity and retrograde transport of specific signal molecules produced as a result of the inflammation. These changes result both in a potentiated nociceptive system, as described above, and one in which phenotypic switches alter the central responses elicited by low-threshold Aβ fiber inputs, the phenomenon of progressive tactile hypersensitivity. Both the potentiation of the system and the phenotypic changes manifest as a change in stimulus-evoked rather than basal hypersensitivity.
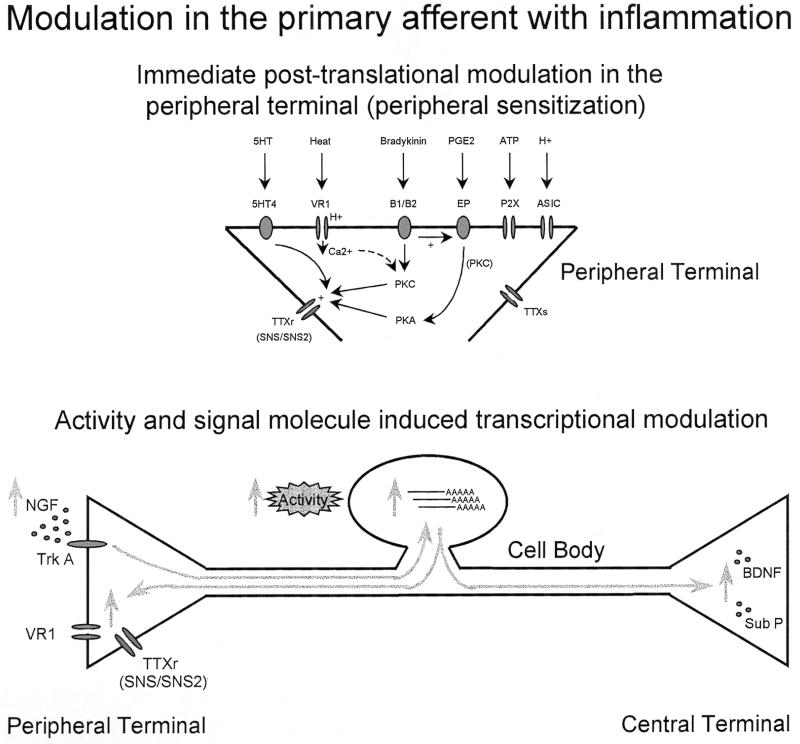
Posttranslational changes.
A defining feature of nociceptors is their normal high threshold for activation. After inflammation, or even after repeated noxious stimuli, however, the peripheral terminal threshold drops such that lower intensity stimuli can now initiate activity in the nociceptors. This peripheral sensitization, which can be detected within a very short period, is the result of changes either in the transducing receptor molecules themselves or in sodium channels in the terminal (Fig. 4). A change in the transducer molecule is best exemplified by VR1, where repeated heat stimuli by themselves result in a progressively augmenting inward current through the ion channel, and exposure to protons does the same thing, by mechanisms currently not known (4, 5). The other contributor to peripheral sensitization is phosphorylation of membrane-bound receptors/ion channels. Many inflammatory mediators activate either protein kinase A or C (see Fig. 4), both of which can phosphorylate the TTXr sodium channel SNS/PN3 and contribute to a greater sodium current in the terminal (59–61). The recently discovered TTXr channel SNS2 has different protein kinase C and protein kinase A consensus regions on its intracellular loops (20), and its contribution to the change in sodium current is undetermined. These sensitizing changes occur locally in the peripheral terminal, independent of transcription in the DRG. However, transcription of the very elements that show posttranslational changes in these terminals is highly regulated in the cell soma. Inflammation up-regulates both VR1 and SNS/SNS2 (5, 20, 62). Inflammation is associated with an increase in peripheral NGF levels (63), and this may be the key signal molecule for many of the transcriptional changes (see below). Although peripheral sensitization does not itself require transcription, an increase in the substrate for such sensitization is very likely to amplify the phenomenon. Because of the delay inherent in the initiation of changes in expression and transport of proteins, such transcription-dependent augmentation will only manifest many hours after the onset of inflammation (Fig. 4).
Figure 4.
Posttranslational changes in transduction mechanisms/ion channels at nociceptor peripheral terminals induced by inflammatory mediators increases sensitivity and reduces threshold (peripheral sensitization), which occurs as a result of changes in the transducer proteins themselves (e.g., VR1) and as a result of a PKC- and PKA-mediated phosphorylation of TTXr sodium channels. Both activity and retrograde transport of signal molecules can also induce transcriptional changes in the DRG, which increase transducer molecules (VR1), ion channels (SNS/SNS2), and synaptic neuromodulators (BDNF/substance P) both altering phenotype and potentiating the system.
Apart from the increased sensitivity of the peripheral terminals of nociceptors on exposure to inflammatory mediators, which will result in an area of increased sensitivity to thermal and mechanical stimuli localized to the site of inflammation, any C fiber input activated at the time of or during inflammation will also initiate central sensitization. This C fiber input will result in an NMDA receptor-sensitive increase in responsiveness to low- and high-intensity stimuli, both when applied to the site of the inflammation and in the contiguous noninflamed area. Tactile allodynia and pin prick hyperalgesia in the zone of secondary hyperalgesia (64), for example, are characteristic NMDA-receptor-mediated (65) features of central sensitization.
Transcriptional changes.
In addition to these posttranslational changes, an alteration in the expression of effector molecules in the DRG (66) and dorsal horn (67–69) is a prominent feature of inflammation and can be initiated in two ways. The first is as a result of an activity-dependent activation of the CREB transcription factor both in DRG and dorsal horn neurons (ref. 70; Fig. 3). As discussed above, this will result, after a delay of several hours for transcription and translation in the DRG and protein transport to central terminals, in a potentiated system in which the C fibers are primed to exert a greater effect on dorsal horn neurons as a result of an increased expression of neuromodulators like BDNF. In addition, the dorsal horn is simultaneously made hyperresponsive to such neuromodulators as a result of an activity-dependent increased expression of the TrkB receptor. The second way that transcriptional changes occur after inflammation is via the production in the inflamed tissue of specific signal molecules that bind to receptors on nociceptor sensory terminals. The ligand–receptor complex is then internalized and retrogradely transported to the cell body, where it activates specific signal-transduction cascades (Fig. 3). NGF is the prototypical example of such a signal molecule whose level increases substantially in inflamed tissue and neutralization of which massively reduces inflammatory hypersensitivity (reviewed in ref. 71).
Signals from extracellular growth factors such as NGF are transduced into intracellular responses through the small G protein Ras and MAP kinase (reviewed in refs. 72–74). The Ras cascade (see Fig. 3) involves the sequential activation of Ras, Raf, MAP kinase kinase, and MAP kinase itself [p44/p42 MAP kinase is also known as ERK 1/2 (extracellular signal regulated kinase)]. Subsequent to phosphorylation, a fragment of activated MAP kinase translocates to the nucleus, where it then regulates gene expression through the phosphorylation, and therefore activation, of various transcription factors including c-Myc, Elk-1, c-Fos, and c-Jun (72, 73). Phosphorylation of these transcription factors results in their association into complexes. These complexes activate transcription of many downstream genes via their association with response elements present in the response gene promoter regions (Fig. 3). The MAP kinase cascade can also phosphorylate and thus activate CREB within neurons via the activation of Rsk2 by MAP kinase itself (75, 76).
One consequence of transcriptional changes in DRG neurons after inflammation is that some low-threshold Aβ neurons acquire the chemical phenotype typical of C fibers. For example, the neuropeptide substance P is normally found almost exclusively in a subset of the TrkA-expressing C fibers, with only a very small number in TrkA expressing small myelinated Aδ fibers (77, 78). After inflammation, there is a NGF-dependent increase in substance P expression in C fibers (63), but also a novel expression of this neuropeptide in some large A fibers as well (3). This new expression, together with the inflammation-induced increase in NK1 receptors in the dorsal horn (79), will result not only in a potentiated system, but one in which the specific type of stimulus that can evoke central sensitization has changed. Stimulus-induced hypersensitivity can thus be mediated by low-intensity Aβ inputs as well as high-intensity C fiber inputs (Fig. 1). This heightened sensibility manifests as progressive tactile hypersensitivity, where low-intensity mechanical stimulation of inflamed skin (light touch) produces a progressively incrementing increase in the excitability of spinal neurons, something they never can elicit in the normal situation, and this can be mimicked by Aβ fiber stimulation (3, 80–83).
CONCLUSION
A clear distinction needs to be made between nociception, the detection only of intense noxious stimuli, and inflammatory pain, which is evoked by normally innocuous stimuli and incorporates an exaggerated response to noxious stimuli. The transition from one state to the other involves multiple changes, some early and others late, some mediated by posttranslational changes and others reliant on altered gene expression, some evoked by activity and others in response to specific inflammatory mediators/signal molecules. Enormous progress has been made in unraveling what changes occur, when they occur, and the molecular mechanisms involved. Although much still remains to be determined, this new insight is translating into novel and more targeted approaches to treating inflammatory pain.
Acknowledgments
The reviewed work was supported by the Medical Research Council G9431792, the Human Frontier Science Program RG73/96, the Wellcome Trust 039614, the European Union BMH4-CT 95 0172, and the National Institute of Neurological Disorders and Stroke R01 NS38253–01.
ABBREVIATIONS
- DRG
dorsal root ganglion
- NGF
nerve growth factor
- TTX
tetrodotoxin
- TTXr
TTX-resistant
- TTXs
TTX-sensitive
- BDNF
brain-derived neurotrophic factor
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CREB
cAMP responsive element-binding protein
- MAP
mitogen-activated protein
- NMDA
N-methyl-d-aspartate
References
- 1.Bessou P, Perl E R. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- 2.Woolf C J. Nature (London) 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 3.Neumann S, Doubell T P, Leslie T A, Woolf C J. Nature (London) 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 4.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilbert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann R, Lazdunski M. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G, Wood J N. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 8.Walker K, Perkins M, Dray A. Neurochem Int. 1995;26:1–17. doi: 10.1016/0197-0186(94)00114-a. [DOI] [PubMed] [Google Scholar]
- 9.Dray A. Br J Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 10.Levine J D, Taiwo Y O. In: Textbook of Pain. Wall P D, Melzack R, editors. New York: Churchill Livingstone; 1994. pp. 45–46. [Google Scholar]
- 11.Bradbury E J, Burnstock G, McMahon S B. Mol Brain Res. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 12.Catterall W A. Physiol Rev. 1992;72:S15–48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- 13.Caffrey J M, Eng D L, Black J A, Waxman S G, Kocsis J D. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- 14.Roy M L, Narahashi T. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waxman S G, Kocsis J D, Black J A. J Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black J A, Waxman S G. Dev Neurosci. 1996;18:139–152. doi: 10.1159/000111403. [DOI] [PubMed] [Google Scholar]
- 17.Black J A, Dib-Hajj S, McNabola K, Jeste S, Rizzo M A, Kocsis J D, Waxman S G. Brain Res Mol Brain Res. 1996;43:117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 18.Akopian A N, Sivilotti L, Wood J N. Nature (London) 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 19.Sangameswaran L, Delgado S G, Fish L M, Koch B D, Jakeman L B, Stewart G R, Sze P, Hunter J C, Eglen R M, Herman R C. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 20.Tate S, Benn S, Hick C, Trezise D, John V, Mannion R J, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf C J. Nat Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- 21.Dib-Hajj S, Tyrrell L, Black J A, Waxman S G. Proc Natl Acad Sci USA. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis W D, Coggeshall R E. Sensory Mechanisms of the Spinal Cord. New York: Plenum; 1991. [Google Scholar]
- 23.Yoshimura M, Jessell T M. J Physiol (London) 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King A E, Thompson S W N, Urban L, Woolf C J. Neurosci Lett. 1988;89:286–292. doi: 10.1016/0304-3940(88)90541-1. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y Q, Mantyh P W, Carlson E J, Gillespie A M, Epstein C J, Bausbaum A I. Nature (London) 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 26.Woolf C J, Mannion R J, Neumann S. Neuron. 1998;20:1063–1066. doi: 10.1016/s0896-6273(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 27.Woolf C J, Thompson S W N. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 28.Ma Q-P, Woolf C J. J Physiol (London) 1995;486(3):769–777. doi: 10.1113/jphysiol.1995.sp020852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubner R. In: Neuronal Plasticity and Pain Following Peripheral Tissue Inflammation or Nerve Injury. Bond M R, Charlton J E, Woolf C J, editors. Amsterdam: Elsevier; 1991. pp. 263–276. [Google Scholar]
- 30.McMahon S B, Lewin G R, Wall P D. Curr Opin Neurobiol. 1993;3:602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- 31.Coderre T J, Melzack R. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren K, Dubner R. Neurosci Lett. 1993;163:22–26. doi: 10.1016/0304-3940(93)90220-f. [DOI] [PubMed] [Google Scholar]
- 33.Woolf C J, Shortland P, Sivilotti L G. Pain. 1994;58:141–155. doi: 10.1016/0304-3959(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 34.Koltzenburg M, Torebjork H E, Wahren L K. Brain. 1994;117:579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- 35.Kilo S, Schmelz M, Koltzenburg M, Handwerker H O. Brain. 1994;117:385–396. doi: 10.1093/brain/117.2.385. [DOI] [PubMed] [Google Scholar]
- 36.Woolf C J, Wall P D. J Neurosci. 1986;6:1433–1443. doi: 10.1523/JNEUROSCI.06-05-01433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bley K R, Hunter J C, Eglen R M, Smith J A. Trends Pharmacol Sci. 1998;19:141–147. doi: 10.1016/s0165-6147(98)01185-7. [DOI] [PubMed] [Google Scholar]
- 38.Heath M J, Womack M D, MacDermott A B. J Neurophysiol. 1994;72:1192–1198. doi: 10.1152/jn.1994.72.3.1192. [DOI] [PubMed] [Google Scholar]
- 39.Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H, Lindholm D. J Neurochem. 1995;65:2241–2250. doi: 10.1046/j.1471-4159.1995.65052241.x. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki Y, Gay B, Wada K, Koizumi S. J Neurochem. 1998;71:106–111. doi: 10.1046/j.1471-4159.1998.71010106.x. [DOI] [PubMed] [Google Scholar]
- 41.Suen P-C, Wu K, Xu J-L, Lin S Y, Levine E S, Black I B. Brain Res Mol Brain Res. 1998;59:215–228. doi: 10.1016/s0169-328x(98)00157-0. [DOI] [PubMed] [Google Scholar]
- 42.Yu X M, Askalan R, Keil G J2, Salter M W. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 43.Lin S Y, Wu K, Levine E S, Mount H T, Suen P-C, Black I B. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- 44.Wang T, Salter M W. Nature (London) 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Huang L-Y M. Nature (London) 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 46.Woolf C J, King A E. J Neurosci. 1990;10:2717–2726. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook A J, Woolf C J, Wall P D, McMahon S B. Nature (London) 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- 48.Simone D A, Baumann T K, Collins J G, LaMotte R H. Brain Res. 1989;486:185–189. doi: 10.1016/0006-8993(89)91293-6. [DOI] [PubMed] [Google Scholar]
- 49.Torebjork H E, Lundberg L E R, LaMotte R H. J Physiol (London) 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginty D D, Kornhauser J M, Thompson M A, Bading H, Mayo K E, Takahashi J S, Greenberg M E. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 51.Fields R D, Eshete F, Stevens B, Itoh K. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bito H, Deisseroth K, Tsien R W. Curr Opin Neurobiol. 1997;7:419–429. doi: 10.1016/s0959-4388(97)80072-4. [DOI] [PubMed] [Google Scholar]
- 53.Ji R R, Shi T-J, Xu Z-Q, Zhang Q, Sakagami H, Tsubochi H, Kondo H, Hokfelt T. Brain Res. 1996;721:167–173. doi: 10.1016/0006-8993(95)01316-4. [DOI] [PubMed] [Google Scholar]
- 54.Tao X, Finkbeiner S, Arnold D B, Shaywitz A J, Greenberg M E. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 55.Shieh P B, Hu S C, Bobb K, Timmusk T, Ghosh A. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 56.Swanson K D, Reigh C, Landreth G E. J Biol Chem. 1998;273:19965–19971. doi: 10.1074/jbc.273.32.19965. [DOI] [PubMed] [Google Scholar]
- 57.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 58.Michael G J, Averill S, Nitkunan A, Rattray M, Bennett D L H, Yan Q, Priestley J V. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gold M S, Levine J D, Correa A M. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.England S, Bevan S, Docherty R J. J Physiol (London) 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gold M S, Reichling D B, Schuster M J, Levine J D. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okuse K, Chaplan S R, McMahon S B, Luo Z D, Calcutt N A, Scott B P, Akopian A N, Wood J N. Mol Cell Biol. 1997;10:196–207. doi: 10.1006/mcne.1997.0657. [DOI] [PubMed] [Google Scholar]
- 63.Woolf C J, Safieh-Garabedian B, Ma Q-P, Crilly P, Winter J. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 64.Koltzenburg M, Lundberg L E R, Torebjork H E. Pain. 1992;51:207–220. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- 65.Stubhaug A, Breivik H, Eide P K, Kreunen M, Foss A. Acta Anaesthesiol Scand. 1997;41:1124–1132. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 66.Leslie T A, Emson P C, Dowd P M, Woolf C J. Neuroscience. 1995;67:753–761. doi: 10.1016/0306-4522(95)00101-n. [DOI] [PubMed] [Google Scholar]
- 67.Noguchi K, Dubner R, Ruda M A. Neuroscience. 1992;46:561–570. doi: 10.1016/0306-4522(92)90144-q. [DOI] [PubMed] [Google Scholar]
- 68.Noguchi K, Kowalski K, Traub R, Solodkin A, Iadarola M J, Ruda M A. Mol Brain Res. 1991;10:227–233. doi: 10.1016/0169-328x(91)90065-6. [DOI] [PubMed] [Google Scholar]
- 69.Ruda M A, Iadarola M J, Cohen L V, Young W S., III Proc Natl Acad Sci USA. 1988;85:622–626. doi: 10.1073/pnas.85.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ji R R, Rupp F. J Neurosci. 1997;17:1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woolf C J. Philos Trans R Soc London B. 1996;351:441–448. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- 72.Davis R J. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 73.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 74.Fukunaga K, Miyamoto E. Mol Neurobiol. 1998;16:79–95. doi: 10.1007/BF02740604. [DOI] [PubMed] [Google Scholar]
- 75.Xing J, Ginty D D, Greenberg M E. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 76.Impey S, Obrietan K, Wong S T, Poser S, Yano S, Wayman G, Deloulme J C, Chan G, Storm D R. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 77.Averill S, McMahon S B. Eur J Neurosci. 1998;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McMahon S B, Armanini M P, Ling L H, Phillips H S. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 79.Krause J E, DiMaggio D A, McCarson K E. Can J Physiol Pharmacol. 1995;73:854–859. doi: 10.1139/y95-117. [DOI] [PubMed] [Google Scholar]
- 80.Ma Q-P, Woolf C J. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- 81.Ma Q-P, Woolf C J. NeuroReport. 1997;8:807–810. doi: 10.1097/00001756-199703030-00001. [DOI] [PubMed] [Google Scholar]
- 82.Ma Q-P, Allchorne A J, Woolf C J. Pain. 1998;77:49–57. doi: 10.1016/S0304-3959(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 83.Ma Q-P, Woolf C J. Eur J Pharmacol. 1997;322:165–171. doi: 10.1016/s0014-2999(97)00014-9. [DOI] [PubMed] [Google Scholar]