The prognostic role, in terms of the overall survival outcome, of serum parathyroid hormone evaluated at baseline and after 3 months of zoledronic acid or placebo treatment in hormone-refractory prostate cancer patients with bone metastases enrolled in a randomized clinical trial was assessed.
Keywords: Bone metastasis, Parathyroid hormone, Prostate cancer, Zoledronic acid
Abstract
Background.
Secondary hyperparathyroidism is frequent in prostate cancer patients with bone metastases, and this condition is worsened by the administration of potent bisphosphonates. Serum parathyroid hormone (PTH) elevation can impair the efficacy of these drugs in terms of survival.
Methods.
The prognostic role of elevated serum PTH levels at baseline and after 3 months of zoledronic acid administration was assessed prospectively in 643 bone metastatic prostate cancer patients enrolled in a prospective randomized, placebo-controlled study.
Results.
On multivariate analysis, after adjusting for major prognostic factors and bone turnover markers, elevated baseline serum PTH level was negatively associated with overall survival (hazard ratio [HR], 1.448; 95% confidence interval [CI], 1.045–2.006; p < .03) in zoledronic acid–treated patients but not in placebo-treated patients. In patients with normal baseline PTH levels, there was a trend but insignificant association between zoledronic acid administration and a better survival outcome than with placebo (HR, 0.81; 95% CI, 0.65–1.01; p = .065), whereas a trend in the opposite direction was observed in patients with elevated PTH levels (HR, 1.45; 95% CI, 0.87–2.39; p = .151); interaction test, p = .040. Elevated serum PTH level after 3 months of zoledronic acid treatment was not significantly associated with survival outcome.
Conclusions.
Secondary hyperparathyroidism has a negative prognostic impact in metastatic prostate cancer patients undergoing zoledronic acid administration. Counteracting elevated PTH levels by adequate doses of vitamin D may improve the efficacy of this drug.
Introduction
The skeleton is the most frequent site of metastatic disease [1] resulting from prostate cancer. Metastatic cancer cells in the bone microenvironment release cytokines and growth factors that stimulate bone resorption and formation [1, 2].
The osteoblastic nature of bone lesions from prostate cancer leads to calcium entrapment in bone [3] and secondary hyperparathyroidism in response to the increased calcium demand [4, 5]. Increased serum parathyroid hormone (PTH) levels in prostate cancer patients are further enhanced by vitamin D deficiency, which is frequent in elderly males [6].
PTH elevation can adversely affect the natural history of the disease by several mechanisms: PTH increases the availability of cytokines, such as interleukin-6, tumor necrosis factor-α, and growth factors (transforming growth factor-β) in the bone microenvironment [7–9]. These effects may favor an increase in the bone metastatic burden [10, 11]. PTH has an amino-terminal sequence homology with PTH-related protein (PTHrP) [12, 13], a hormone that stimulates cell growth and inhibits apoptosis in different cell types [14–16]. PTHrP and PTH bind to the same receptor (PTH/PTHrP receptor) that is expressed in cancer cells [13, 17].
Bisphosphonates are inhibitors of osteoclast-mediated bone resorption [18], and zoledronic acid is the most potent bisphosphonate. The efficacy of zoledronic acid against placebo in preventing skeletal-related events (SREs) in bone metastatic prostate cancer patients with hormone-refractory disease was assessed in a randomized, multicenter trial [19].
The potent osteoclast inhibition induced by zoledronic acid reduces the contribution from bone resorption to calcium homeostasis and may enhance the secondary hyperparathyroidism of prostate cancer patients [5]. In the present study, we explored the prognostic role, in terms of the overall survival outcome, of serum PTH evaluated at baseline and after 3 months of zoledronic acid or placebo treatment in hormone-refractory prostate cancer patients with bone metastases enrolled in the randomized clinical trial previously mentioned [19]. A secondary aim of this study was to conduct an exploratory analysis to assess whether zoledronic acid or placebo varied by PTH status.
Materials and Methods
Patients and Study Design
This analysis was based on data from patients enrolled in a randomized, multicenter, double-blind, phase III clinical trial evaluating the safety and efficacy of zoledronic acid in patients with malignant bone disease from prostate cancer (Protocol 039) [19]. The main efficacy endpoint was the occurrence of one or more SREs. Secondary study aims were disease progression in bone and the overall survival outcome.
Patients in the study were randomly assigned to treatment with 4 mg zoledronic acid, 8 mg zoledronic acid, or placebo every 3 or 4 weeks for up to 24 months. During the course of the trial, the 8-mg zoledronic acid dose was reduced to 4 mg to ensure renal safety in all patients.
The current analyses were performed using PTH measurements at baseline and a 3-month assessment; follow-up was up to 24 months.
Patient Evaluation
Urine specimens (from a morning second void) and a venous blood sample were collected to measure biochemical markers of bone metabolism and serum PTH in all patients at baseline, 1 month after enrolment, and then every 3 months. The urinary N-telopeptide/creatinine ratio (NTX) was used to assess bone resorption activity, and serum bone-specific alkaline phosphatase (BAP) was used as an indicator of bone formation. A central laboratory (Mayo Medical Laboratories, Rochester, MN) performed measurements of urine and serum biochemical markers of bone metabolism for all patients.
Serum PTH was considered as a continuous variable or categorized both as normal (≤5.2 pmol/L) or supranormal (>5.2 pmol/L), with cutoffs corresponding to the approximate upper limit of normal (ULN) in age-matched healthy male individuals and according to quartile distribution.
Quality-of-life parameters included a pain score, assessed using the Brief Pain Inventory completed by patients every 6 weeks, Eastern Cooperative Oncology Group (ECOG) performance status score, scores from two quality of life questionnaires (Functional Assessment of Cancer Therapy–General and EuroQol), and analgesic scores recorded by the investigator on the basis of the type of pain medication administered.
Statistical Methods
Logistic regression analysis was used to assess, in multivariate analysis, the independent factors predicting PTH elevation.
Cox regression analysis was used to assess the impact of the PTH value either at baseline or at 3 months on the risk for death among patients receiving zoledronic acid or placebo [20–22]. Both univariate models, which examined one factor at a time, and multivariate models, which examined multiple factors at the same time, were used. A full multivariate model was fit by including all factors simultaneously, and a reduced model was identified by eliminating factors that were not independently associated with survival. The reduced model therefore identified the factors that were independently associated with survival outcome. In the multivariate models, cancer duration, age, ECOG score, hemoglobin level, elevated lactate dehydrogenase (LDH), bone pain index composite pain score, prior SREs, lymph node metastases, visceral disease (lung, liver, brain, pleura, skin, eye, or other metastases versus none), analgesic consumption (minor analgesic, tranquilizers and antidepressants, mild narcotics, or strong narcotics versus none), and baseline serum creatinine, serum albumin, serum calcium, serum prostate-specific antigen (PSA), serum BAP, and NTX were controlled to assess the independent prognostic role of elevated PTH level at baseline. When analyses to assess the independent prognostic role of serum PTH after 3 months were conducted, the following variables were added to the previous ones: 3-month serum PSA level, 3-month urinary NTX, and 3-month serum BAP. In the analyses reported, events were counted from the time of randomization until the end of the scheduled follow-up, censoring, or death, in which case the 3-month PTH value could have been recorded after the clinical event. Note that, in the last analyses, we restricted attention to patients who survived to the 3-month assessment. For both the univariate and multivariate models, relative risks were computed comparing elevated with normal PTH levels for baseline evaluations, whereas the second, third, and fourth quartiles of PTH levels to the first quartile level were considered for the 3-month evaluation. In addition, a global three degree of freedom test was conducted to test the effect of the 3-month PTH level. Homogeneity tests of the null hypothesis of a common treatment effect for groups with different PTH levels were carried out by fitting the interaction between treatment and PTH level.
Results
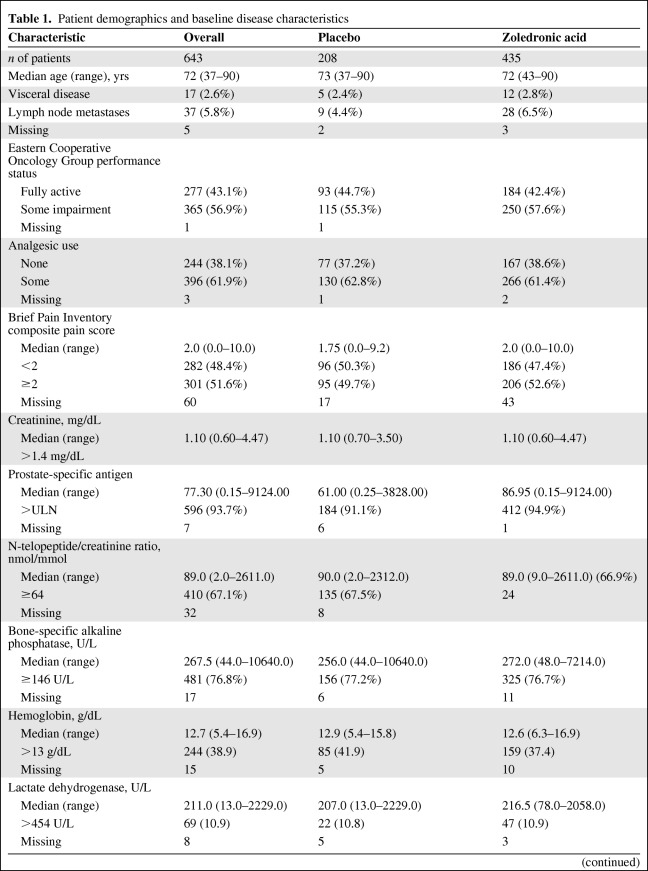
Six hundred forty-three patients were enrolled in this trial. Six hundred twenty-five (97.2%) had serum PTH levels assessed at baseline, 503 (78.2%) had serum PTH levels assessed both at baseline and at 3 months. The distributions of patient demographics, disease characteristics, prognostic markers, bone turnover markers, and serum PTH levels in cases overall and in the placebo and zoledronic acid arms are outlined in Table 1.
Table 1.
Patient demographics and baseline disease characteristics
Table 1a.
(continued)
Abbreviation: ULN, upper limit of normal.
Baseline serum PTH was significantly associated with age. Using tertiles to categorize patient age at baseline, supranormal PTH levels were seen in 24 of 204 (11.8%) patients in the first age tertile (<69 years of age), 29 of 180 (16.1%) patients in the second tertile (69–75 years of age), and 59 of 241 (24.5%) patients in the third tertile (≥75 years of age) (p = .0022).
Prognostic Role of Elevated Serum PTH Level at Baseline
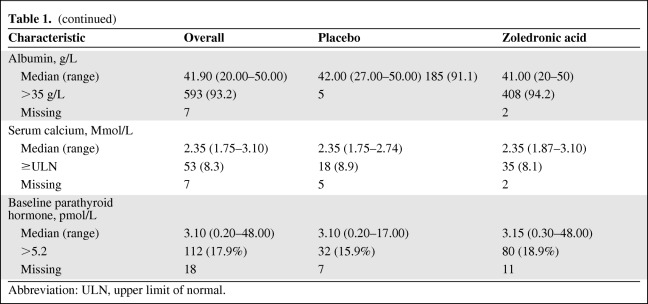
Elevated serum PTH at baseline in patients treated with zoledronic acid was significantly correlated with a higher risk for death in both the univariate analysis (hazard ratio [HR], 1.483; 95% confidence interval [CI], 1.077–2.041; p < .02) and multivariate analysis (HR, 1.448; 95% CI, 1.045–2.006; p < .03) after adjusting for commonly recognized prognostic markers and bone turnover markers (Table 2). The median time to death among zoledronic acid–treated patients with elevated PTH levels was 376 days (95% CI, 332–494 days), and for those with a PTH level ≤5.2 pmol/L, it was 572 days (95% CI, 496–670 days). The respective medians for placebo-treated patients were 501 days (95% CI, 199 to no upper limit) and 493 days (95% CI, 385–637 days), respectively. An elevated serum PTH level at baseline in the placebo arm was not associated with patient outcome in either the univariate (HR, 0.840; 95% CI, 0.494–1.426; p = .52) or multivariate (HR, 0.764; 95% CI, 0.423–1.380; p = .37) analysis (data not shown).
Table 2.
Univariate and multivariate analyses of baseline prognostic factors and supranormal serum parathyroid hormone
Patients had to have a complete baseline PTH assessment and assessment of baseline prognostic factors. Patients entered the risk set at the time of study entry.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PTH, parathyroid hormone; RR, relative risk.
Efficacy of Zoledronic Acid Treatment over Placebo in Patients Stratified According to Baseline PTH Status
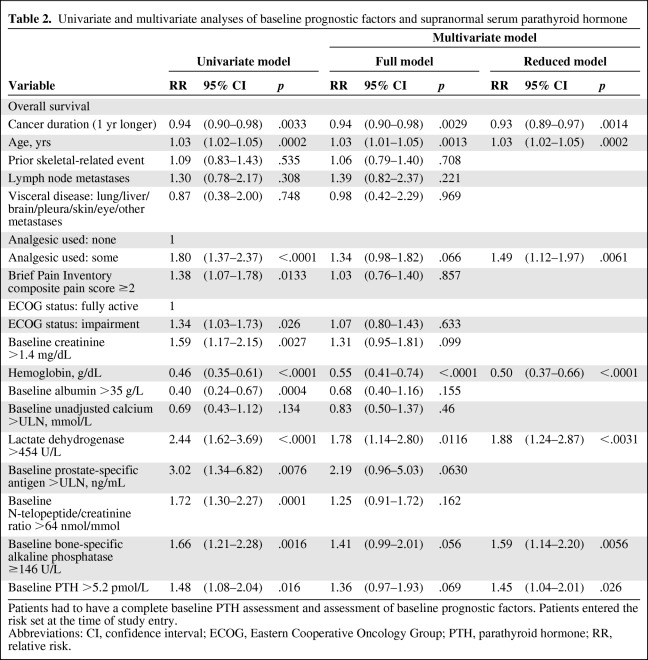
Zoledronic acid administration resulted in a nonsignificant greater survival benefit overall (Fig. 1) [19], but analyses according to patient baseline PTH status revealed a suggestive trend toward a lower risk for death in patients receiving zoledronic acid administration than in those receiving placebo among patients with a normal baseline serum PTH level (HR, 0.81; 95% CI, 0.65–1.01; p = .065) and a nonsignificant higher risk for death in patients with supranormal baseline PTH levels (HR, 1.45; 95% CI, 0.87–2.39; p = .151). These two HRs were significantly different based on a test of homogeneity (p = .040).
Figure 1.
Forrest plot of the prognostic role of zoledronic acid administration versus placebo. A nonsignificant greater survival benefit overall in favor of zoledronic acid was observed. Analyses according to patients' baseline parathyroid hormone (PTH) status revealed opposite trends—a nonsignificant lower risk for death was seen in patients receiving zoledronic acid, compared with placebo, among those with a normal serum PTH level, and a nonsignificant greater risk for death was seen in patients with supranormal baseline PTH levels (test of homogeneity, p = .040).
Factors Predictive of Increased PTH Level after 3 Months of Zoledronic Acid Administration
A multivariate logistic regression analysis was performed to identify categorical baseline factors that were predictive of PTH elevation after 3 months of zoledronic acid administration. Cancer duration (odds ratio [OR], 0.84; 95% CI, 0.75–0.94; p = .0019) and hemoglobin value (OR, 0.33; 95% CI, 0.17–0.63; p = .0008) were the two independent factors negatively associated with 3-month PTH elevation, whereas a baseline serum PSA level above the ULN (OR, 5.13; 95% CI, 1.01–26.07; p = .0489), baseline serum NTX >64 nmol/mmol (OR, 6.39; 95% CI, 3.26–12.52; p < .0001), and baseline serum PTH level >2.8 pmol/L (OR, 4.85; 95% CI, 2.51–9.37; p < .0001) were the independent factors positively associated with 3-month PTH elevation. Other potential independent variables such as age, prior SRE, lymph node metastases, visceral disease, performance status, bone pain, analgesic consumption, serum creatinine, serum albumin, serum calcium, serum LDH, and serum alkaline phosphatase failed to enter the model.
Prognostic Effect of Serum PTH after 3 Months on Zoledronic Acid Efficacy
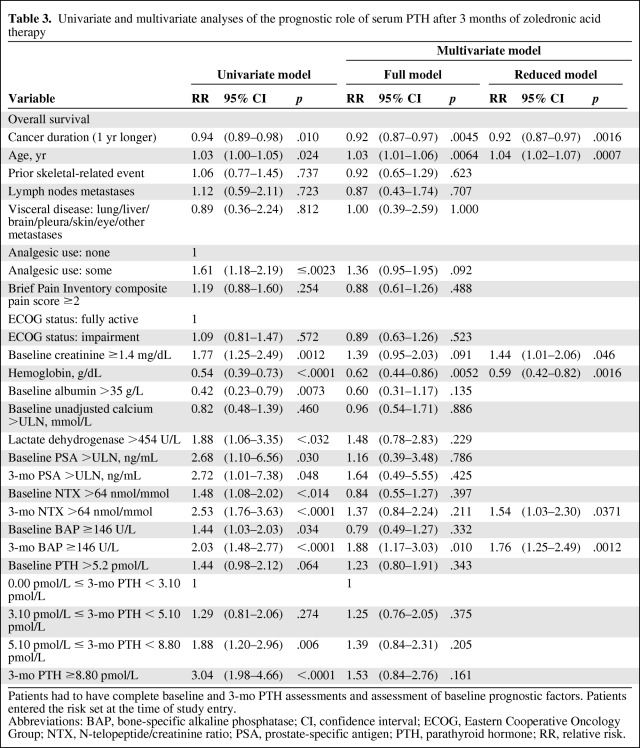
The prognostic effect of serum PTH (categorized according to quartile distribution) after 3 months of zoledronic acid treatment on the overall survival outcome is reported in Table 3. The 3-month PTH level distribution in the third and fourth quartiles had a significant negative prognostic impact versus the first quartile (HR, 1.88; 95% CI, 1.20- 2.96; p = .006 and HR, 3.04; 95% CI, 1.98–4.66; p < .0001, respectively) in the univariate analysis, but it just failed to attain statistical significance in the multivariate analysis (HR, 1.39; 95% CI, 0.84–2.31; p = .204 and HR, 1.53; 95% CI, 0.84–2.76; p = .161, respectively).
Table 3.
Univariate and multivariate analyses of the prognostic role of serum PTH after 3 months of zoledronic acid therapy
Patients had to have complete baseline and 3-mo PTH assessments and assessment of baseline prognostic factors. Patients entered the risk set at the time of study entry.
Abbreviations: BAP, bone-specific alkaline phosphatase; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; NTX, N-telopeptide/creatinine ratio; PSA, prostate-specific antigen; PTH, parathyroid hormone; RR, relative risk.
In placebo-treated patients, the serum PTH level after 3 months failed to correlate with the risk for death in both the univariate and multivariate analyses (data not shown).
Discussion
Elevated serum PTH levels are frequent in bone metastatic prostate cancer patients, and this condition can be stimulated by zoledronic acid administration [4]. Secondary hyperparathyroidism can adversely influence the natural history of the disease, but to our knowledge this hypothesis has never been explored.
In the present study, elevated serum PTH level at baseline had a significant negative impact in zoledronic acid–treated patients in the multivariate analysis adjusting for major prognostic parameters and bone turnover markers, but this was not seen in placebo-treated patients. A possible explanation for this discrepancy is that zoledronic acid treatment consistently enhances serum PTH elevation and the higher PTH levels attained after zoledronic acid can have a negative prognostic role. Vitamin D deficiency could be a key factor responsible for PTH elevation in prostate cancer patients with metastatic bone disease, suggesting that secondary hyperparathyroidism in these patients is a biochemical hallmark for insufficient vitamin D status. Unfortunately, vitamin D was not measured in the patients enrolled in this trial and this represents a limitation. Vitamin D deficiency or insufficiency is a potential confounder in the interpretation of these study results. Vitamin D, in fact, exerts potent antiproliferative effects on normal cells and cancer cells [23], and hypovitaminosis D is associated with a higher risk for death from prostate cancer [24].
Noteworthy is the fact that a divergent effect of zoledronic acid on overall survival was observed when stratifying patients according to baseline PTH level. A trend close to statistical significance of a better survival outcome in favor of zoledronic acid treatment over placebo was observed in patients with normal PTH levels, but an opposite tendency was observed in patients with elevated PTH levels. The two HRs were significantly different using a test for heterogeneity. The antineoplastic effect of zoledronic acid administration has been shown in preclinical and clinical studies [25]. We suggest that, for the present study, the antitumor effect of zoledronic acid in prostate cancer patients could have been masked by a pre-existing condition of an elevated serum PTH level, implying that the correction of secondary hyperparathyroidism using adequate vitamin D supplementation can potentially increase zoledronic acid efficacy.
Serum PTH increased significantly after zoledronic acid treatment despite vitamin D supplementation at 400 IU daily. Forty-one percent of patients with normal PTH values at baseline had elevated PTH levels after 3 months of zoledronic acid treatment, versus 5% of patients receiving placebo. Among the independent factors predictive of a PTH increase in the multivariate logistic analysis, the direct relationship with serum PSA and with urinary NTX and the inverse relationship with blood hemoglobin suggest a contribution of the disease extent in bone.
Data on the relationship between the serum PTH level measured after 3 months and prognosis are less impressive than those of PTH measured at baseline. An elevated PTH level after 3 months of zoledronic acid treatment was significantly correlated with a poor prognosis in the univariate analysis, but it failed to show a relationship with the survival outcome in the multivariate analysis. It should be noted, however, that (a) because of early death and missing values, a lower number of patients were included in the model and (b) the HR for death of the fourth quartile was close to attaining statistical significance.
Conclusion
This study shows, for the first time, that serum PTH status has a negative prognostic role in hormone-refractory prostate cancer patients undergoing zoledronic acid administration. Caution should be adopted in generalizing results emerging from an unplanned analysis, and a potential confounder in this analysis is vitamin D deficiency itself.
These limitations notwithstanding, these data have several implications: (a) vitamin D and PTH status should be assessed before zoledronic acid administration and regularly monitored afterwards, (b) the calcium and vitamin D supplementation performed in this study was inadequate to control PTH elevation under zoledronic acid administration and could be increased on an individual basis, (c) baseline PTH levels should not simply be considered a predictive factor for zoledronic acid efficacy, and (d) elevated PTH may be a target for vitamin D supplementation. The survival improvement obtained with zoledronic acid in the patient subset with normal baseline PTH levels suggests that the efficacy of this drug could be improved if secondary hyperparathyroidism is effectively counteracted. A trial testing this hypothesis would be of great value.
Acknowledgement
Research and technical support were provided by a grant from Novartis Pharmaceuticals Corporation and the Italian Ministry of University and Research (MIUR).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Alfredo Berruti, Richard Cook
Provision of study material or patients: Allan Lipton, Fred Saad, Robert E. Coleman, Matthew R. Smith
Collection and/or assembly of data: Allan Lipton, Fred Saad, Robert E. Coleman, Matthew R. Smith, Ker-Ai Lee
Data analysis and interpretation: Alfredo Berruti, Richard Cook, Fred Saad, Robert E. Coleman, Matthew R. Smith, Ker-Ai Lee
Manuscript writing: Alfredo Berruti, Allan Lipton, Consuelo Buttigliero, Marco Tampellini
Final approval of manuscript: Alfredo Berruti, Richard Cook, Allan Lipton, Fred Saad, Consuelo Buttigliero, Robert E. Coleman, Matthew R. Smith, Marco Tampellini
References
- 1.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 2.Clines GA, Guise TA. Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocr Relat Cancer. 2005;12:549–583. doi: 10.1677/erc.1.00543. [DOI] [PubMed] [Google Scholar]
- 3.Berruti A, Dogliotti L, Tucci M, et al. Hyperparathyroidism due to the so-called bone hunger syndrome in prostate cancer patients. J Clin Endocrinol Metab. 2002;87:1910–1911. doi: 10.1210/jcem.87.4.8423. author reply 1911. [DOI] [PubMed] [Google Scholar]
- 4.Murray RM, Grill V, Crinis N, et al. Hypocalcemic and normocalcemic hyperparathyroidism in patients with advanced prostatic cancer. J Clin Endocrinol Metab. 2001;86:4133–4138. doi: 10.1210/jcem.86.9.7864. [DOI] [PubMed] [Google Scholar]
- 5.Tucci M, Mosca A, Lamanna G, et al. Prognostic significance of disordered calcium metabolism in hormone-refractory prostate cancer patients with metastatic bone disease. Prostate Cancer Prostatic Dis. 2009;12:94–99. doi: 10.1038/pcan.2008.10. [DOI] [PubMed] [Google Scholar]
- 6.Mosekilde L. Vitamin D and the elderly. Clin Endocrinol (Oxf) 2005;62:265–281. doi: 10.1111/j.1365-2265.2005.02226.x. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi S, Hakuta M, Aiba K, et al. Elevation of circulating plasma cytokines in cancer patients with high plasma parathyroid hormone-related protein levels. Endocr Relat Cancer. 2003;10:403–407. doi: 10.1677/erc.0.0100403. [DOI] [PubMed] [Google Scholar]
- 8.Pollock JH, Blaha MJ, Lavish SA, et al. In vivo demonstration that parathyroid hormone and parathyroid hormone-related protein stimulate expression by osteoblasts of interleukin-6 and leukemia inhibitory factor. J Bone Miner Res. 1996;11:754–759. doi: 10.1002/jbmr.5650110606. [DOI] [PubMed] [Google Scholar]
- 9.Salgado R, Junius S, Benoy I, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 10.Eriksen EF, Eghbali-Fatourechi GZ, Khosla S. Remodeling and vascular spaces in bone. J Bone Miner Res. 2007;22:1–6. doi: 10.1359/jbmr.060910. [DOI] [PubMed] [Google Scholar]
- 11.McCarty MF. Parathyroid hormone may be a cancer promoter—an explanation for the decrease in cancer risk associated with ultraviolet light, calcium, and vitamin D. Med Hypotheses. 2000;54:475–482. doi: 10.1054/mehy.1999.0880. [DOI] [PubMed] [Google Scholar]
- 12.Murray TM, Rao LG, Divieti P, et al. Parathyroid hormone secretion and action: Evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr Rev. 2005;26:78–113. doi: 10.1210/er.2003-0024. [DOI] [PubMed] [Google Scholar]
- 13.Gensure RC, Gardella TJ, Jp̈pner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–678. doi: 10.1016/j.bbrc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 14.Hastings RH, Burton DW, Quintana RA, et al. Parathyroid hormone-related protein regulates the growth of orthotopic human lung tumors in athymic mice. Cancer. 2001;92:1402–1410. doi: 10.1002/1097-0142(20010915)92:6<1402::aid-cncr1463>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Birch MA, Carron JA, Scott M, et al. Parathyroid hormone (PTH)/PTH-related protein (PTHrP) receptor expression and mitogenic responses in human breast cancer cell lines. Br J Cancer. 1995;72:90–95. doi: 10.1038/bjc.1995.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamura M, Abrahamsson PA, Foss KA, et al. Parathyroid hormone-related protein: A potential autocrine growth regulator in human prostate cancer cell lines. Urology. 1994;43:675–679. doi: 10.1016/0090-4295(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 17.Carron JA, Fraser WD, Gallagher JA. PTHrP and the PTH/PTHrP receptor are co-expressed in human breast and colon tumours. Br J Cancer. 1997;76:1095–1098. doi: 10.1038/bjc.1997.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep. 2003;1:45–52. doi: 10.1007/s11914-003-0008-5. [DOI] [PubMed] [Google Scholar]
- 19.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 20.Cook RJ, Lawless JF. New York: Springer; 2007. The Statistical Analysis of Recurrent Events. [Google Scholar]
- 21.McCullagh P, Nelder JA. London: Chapman and Hall; 1989. Generalized Linear Models. [Google Scholar]
- 22.Therneau TA, Grambsch PM. New York: Springer; 2000. Extending the Cox Model. [Google Scholar]
- 23.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 24.Tretli S, Hermes E, Berg JP, et al. Association between serum 25(OH)D and death from prostate cancer. Br J Cancer. 2009;100:450–454. doi: 10.1038/sj.bjc.6604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santini D, Virzi V, Fratto ME, et al. Can we consider zoledronic acid a new antitumor agent? Recent evidence in clinical setting. Curr Cancer Drug Targets. 2010;10:46–54. doi: 10.2174/156800910790980223. [DOI] [PubMed] [Google Scholar]