Abstract
We report equilibrium, relaxation kinetic, and transient electric dichroism studies on the complex of the diamino steroid irehdiamine A with DNA. The results are consistent with a β-kinked structure for the complex at saturation, with a kink in the DNA structure induced by a bound steroid every second base pair. The results that favor this hypothesis include an apparent length decrease of rod-like bacterial DNA molecules when only a small amount of drug is bound, followed by an apparent length increase at saturation. The limiting dichroism amplitude implies a substantial increase in the tilt of the bases relative to the orientation axis; at saturation the base UV transition moments are tilted about 31° from the plane perpendicular to the orientation axis. Because of the direction of polarization of the 260-nm transition moments, the results indicate that the tilt of the bases must be predominantly in the short rather than the long axis of the base pair. The large hyperchroism of the complex is consistent with loss of base stacking, as required by a kinked structure. The kinetic results imply a bimolecular reaction mechanism, with a temperature-dependent association rate constant of roughly 108 M-1 sec-1, and a dissociation rate constant of about 5 × 103 sec-1, nearly independent of temperature. The association activation energy and apparent reaction enthalpy vary from 12 to 22 kcal mol-1; heat is absorbed on complex formation as expected for loss of base-stacking interactions. An anomalous result of the experiments is the larger apparent length increase (13%) exhibited by two eukaryotic DNAs, compared to 6% for three prokaryotic DNAs. Differences were also observed in the kinetic properties of the complexes.
Keywords: electric dichroism, relaxation kinetics, conformational change
Full text
PDF
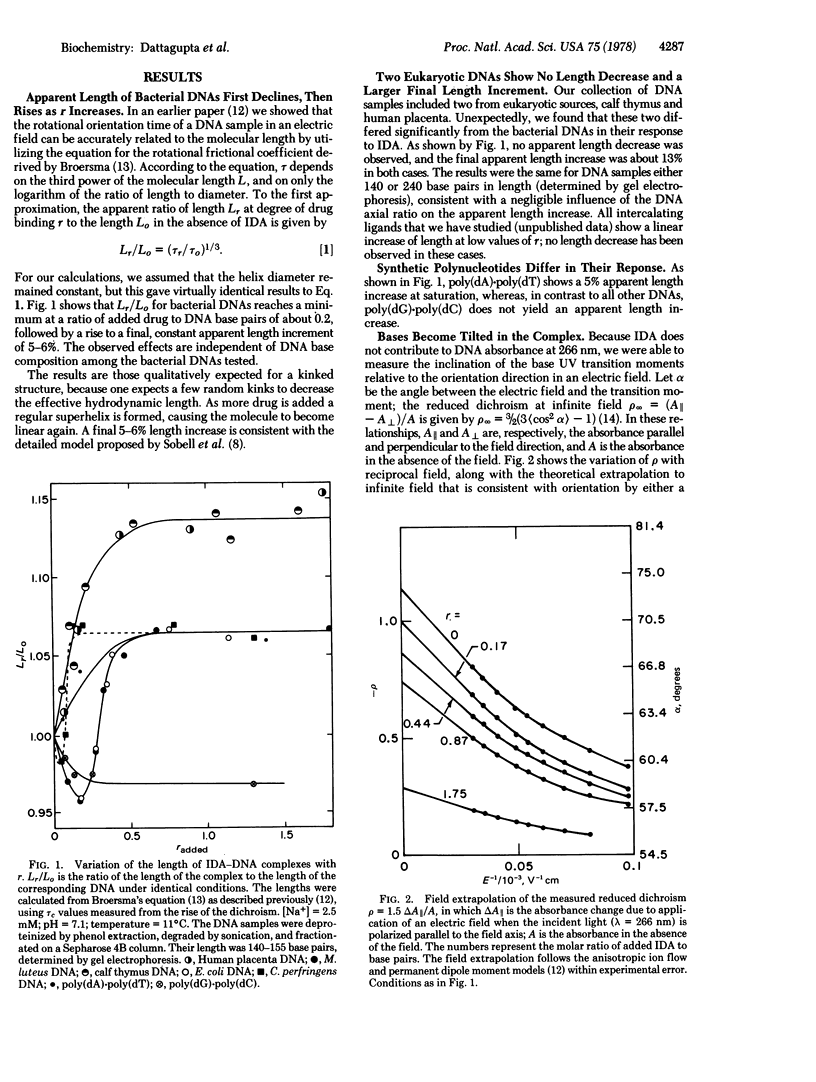
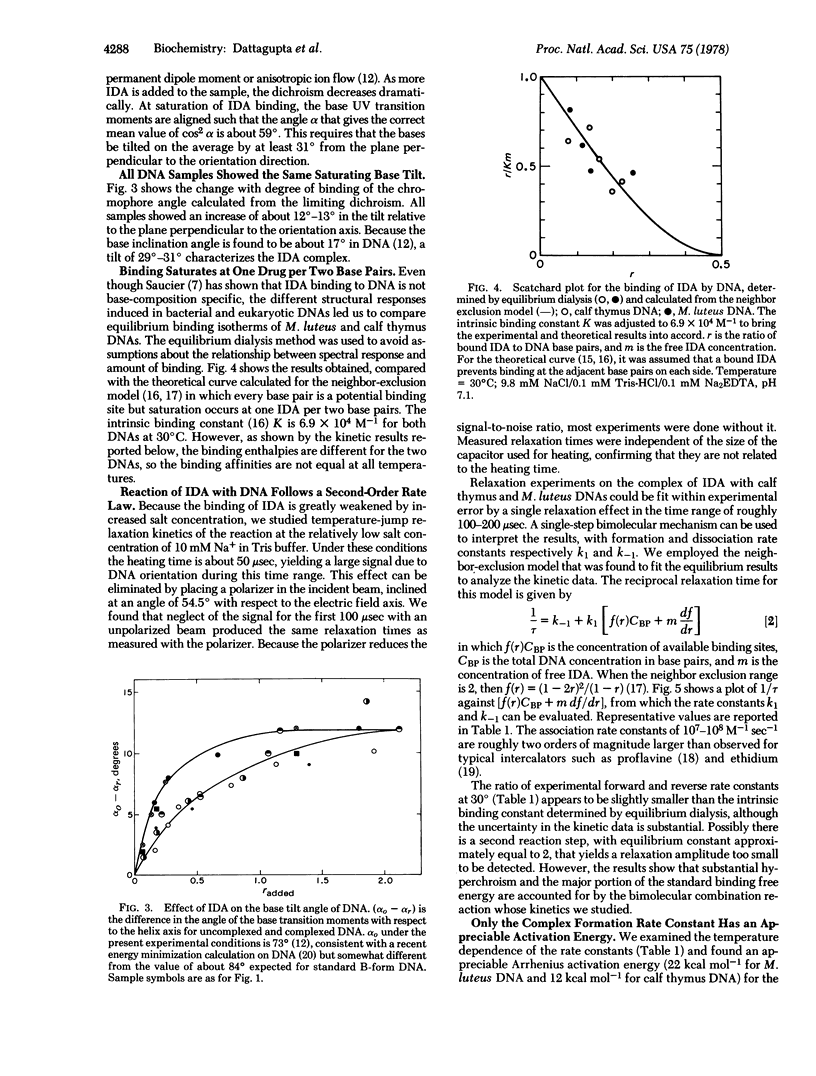
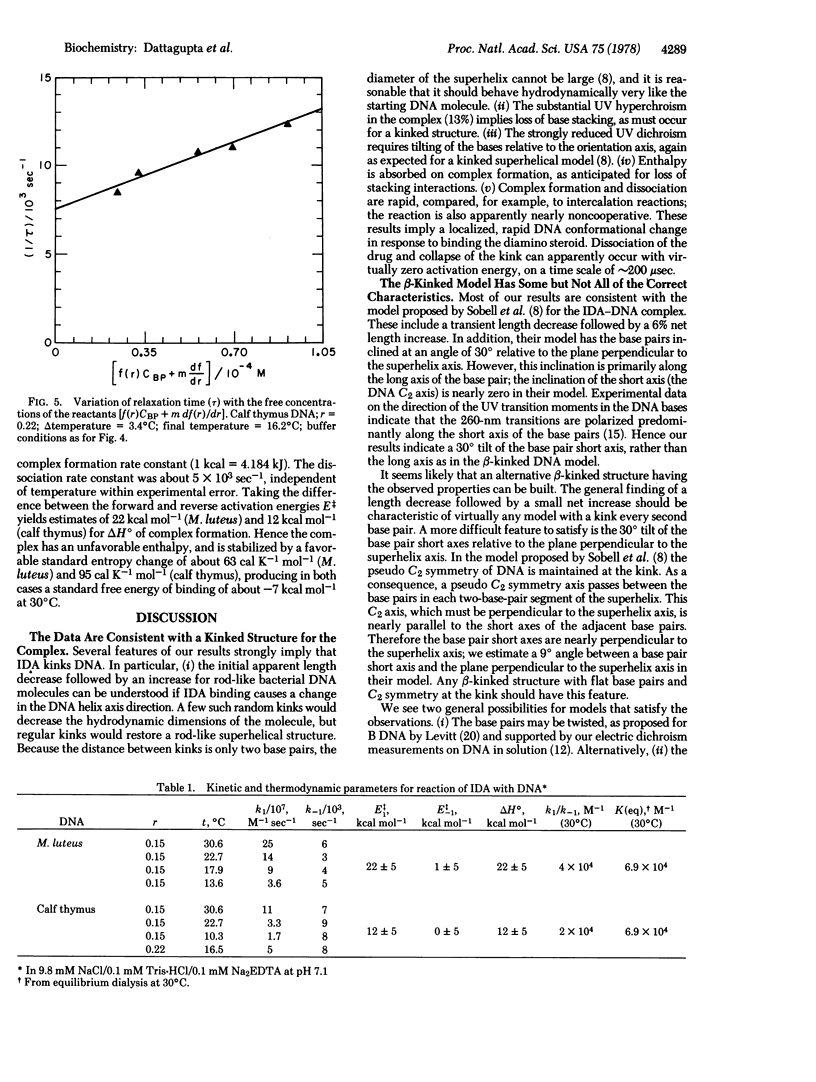

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen F. S., Van Holde K. E. Dichroism of TMV in pulsed electric fields. Biopolymers. 1971;10(5):865–881. doi: 10.1002/bip.360100510. [DOI] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. DNA-ethidium reaction kinetics: demonstration of direct ligand transfer between DNA binding sites. J Mol Biol. 1975 Jun 15;95(1):103–123. doi: 10.1016/0022-2836(75)90339-3. [DOI] [PubMed] [Google Scholar]
- Crick F. H., Klug A. Kinky helix. Nature. 1975 Jun 12;255(5509):530–533. doi: 10.1038/255530a0. [DOI] [PubMed] [Google Scholar]
- Crothers D. M. Calculation of binding isotherms for heterogenous polymers. Biopolymers. 1968 Apr;6(4):575–584. doi: 10.1002/bip.1968.360060411. [DOI] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transient electric dichroism of rod-like DNA molecules. Proc Natl Acad Sci U S A. 1978 Jan;75(1):195–199. doi: 10.1073/pnas.75.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. How many base-pairs per turn does DNA have in solution and in chromatin? Some theoretical calculations. Proc Natl Acad Sci U S A. 1978 Feb;75(2):640–644. doi: 10.1073/pnas.75.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. J., Crothers D. M. Relaxation studies of the proflavine-DNA complex: the kinetics of an intercalation reaction. J Mol Biol. 1969 Feb 14;39(3):461–477. doi: 10.1016/0022-2836(69)90138-7. [DOI] [PubMed] [Google Scholar]
- Mahler H. R., Goutarel R., Khuong-Huu Q., Truong HO M. Nucleic acid interactions. VI. Effects of steroidal diamines. Biochemistry. 1966 Jul;5(7):2177–2191. doi: 10.1021/bi00871a005. [DOI] [PubMed] [Google Scholar]
- Mahler H. R., Green G., Goutarel R., Khuong-Huu Q. Nucleic acid-small molecule interactions. VII. Further characterization of deoxyribonucleic acid-diamino steroid complexes. Biochemistry. 1968 Apr;7(4):1568–1582. doi: 10.1021/bi00844a046. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. J Mol Biol. 1970 Nov 14;53(3):401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Lefresne P., Paoletti C. Etudes sur l'interaction d'un steroïde diamine, l'irehdiamine A, avec l'acide desoxyribonucléique. C R Acad Sci Hebd Seances Acad Sci D. 1968 Feb 12;266(7):731–734. [PubMed] [Google Scholar]
- Saucier J. M. Physicochemical studies on the interaction of irehdiamine A with bihelical DNA. Biochemistry. 1977 Dec 27;16(26):5879–5889. doi: 10.1021/bi00645a036. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Gilbert S. G., Jain S. C., Sakore T. D. Organization of DNA in chromatin. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3068–3072. doi: 10.1073/pnas.73.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Jain S. C., Gilbert S. G. Visualization of drug-nucleic acid interactions at atomic resolution. III. Unifying structural concepts in understanding drug-DNA interactions and their broader implications in understanding protein-DNA interactions. J Mol Biol. 1977 Aug 15;114(3):333–365. doi: 10.1016/0022-2836(77)90254-6. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Chisholm J. W. Uncoiling of bacteriophage PM2 DNA by binding of steroidal diamines. Biochim Biophys Acta. 1972 Feb 23;262(1):18–23. doi: 10.1016/0005-2787(72)90214-6. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Henley S. M. Stereochemical aspects of the interaction between steroidal diamines and DNA. Nucleic Acids Res. 1975 Apr;2(4):567–586. doi: 10.1093/nar/2.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]


