Abstract
The NMDA receptor (NMDAR) family of L-glutamate receptors are well known to have diverse roles in CNS function as well as in various neuropathological and psychiatric conditions. Until recently, the types of agents available to pharmacologically regulate NMDAR function have been quite limited in terms of mechanism of action and subtype selectivity. This has changed significantly in the past two years. The purpose of this review is to summarize the many drug classes now available for modulating NMDAR activity. Previously, this included competitive antagonists at the L-glutamate and glycine binding sites, high and low affinity channel blockers, and GluN2B-selective N-terminal domain binding site antagonists. More recently, we and others have identifed new classes of NMDAR agents that are either positive or negative allosteric modulators (PAMs and NAMs, respectively). These compounds include the pan potentiator UBP646, the GluN2A-selective potentiator/GluN2C & GluN2D inhibitor UBP512, the GluN2D-selective potentiator UBP551, the GluN2C/GluN2D-selective potentiator CIQ as well as the new NMDAR-NAMs such as the pan-inhibitor UBP618, the GluN2C/GluN2D-selective inhibitor QZN46 and the GluN2A inhibitors UBP608 and TCN201. These new agents do not bind within the L-glutamate or glycine binding sites, the ion channel pore or the N-terminal regulatory domain. Collectively, these new allosteric modulators appear to be acting at multiple novel sites on the NMDAR complex. Importantly, these agents display improved subtype-selectivity and as NMDAR PAMs and NAMs, they represent a new generation of potential NMDAR therapeutics.
Keywords: NMDA receptors, allosteric modulators, glycine, potentiators, competitive inhibitors, channel blockers, antagonists
1. Introduction
Rapid synaptic excitation throughout most of the vertebrate central nervous system (CNS) is mediated by L-glutamate-activated ion channels belonging to the three receptor families so named for agonists by which they are selectively activated, the N-methyl-D-aspartate (NMDA) receptors, the AMPA receptors (for the agonist α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and the kainate receptors (Dingledine et al., 1999; Monaghan et al., 1989; Watkins and Evans, 1981). Of these, the NMDA receptor family has received special attention because of its distinct role in the regulation of synaptic plasticity (long-term potentiation (LTP), long-term depression (LTD) and experience-dependent synaptic refinement) (Collingridge, 1987; Cotman et al., 1988) and because of its critical role in neurological and psychiatric disorders (Choi, 1992; Kalia et al., 2008). Hypo- or hyper-activation of NMDA receptors is critically involved in pain amplification, stroke, epilepsy, schizophrenia, post-traumatic stress disorder, dementia, depression and various neurodegenerative diseases (e.g. Alzheimer’s and Parkinson’s)(Kalia et al., 2008). Consequently, the pharmaceutical industry has spent on the order of a billion dollars over the past 25 years developing NMDA receptor antagonists and agonists for several of these therapeutic applications. Despite these high expectations, NMDA receptor agents have failed in most clinical trials (Kalia et al., 2008; O’Collins et al., 2006; Villmann and Becker, 2007). Nevertheless, there remains much potential for improved NMDA receptor therapeutics. Very recently, multiple classes of positive and negative allosteric modulators of NMDA receptors have been identifed. Much work remains, but there are now new opportunities for developing effective NMDA receptor therapeutics. In this review, we provide an overview of the many drug target sites on the NMDA receptor complex and describe the corresponding prototype compounds for the modulation of NMDA receptor activity.
2. The NMDA receptor complex
2.1 NMDA receptor subunits
NMDA receptors are heterotetrameric complexes composed of subunits from seven homologous genes - GluN1, GluN2A-GluN2D, and GluN3A-GluN3B(Dingledine et al., 1999; Monyer et al., 1994; Monyer et al., 1992; Mori and Mishina, 1995). The majority of NMDA receptors are thought to be composed of two GluN1 subunits and two GluN2 subunits (Laube et al., 1998). In recombinant systems, GluN3 subunits are capable of combining with GluN1 subunits or with both GluN1 and GluN2 subunits. GluN3 incorporation into a complex with GluN1 and GluN2 subunits reduces receptor-gated currents and magnesium sensitivity (Cavara and Hollmann, 2008; Henson et al., 2010). In contrast to AMPA and kainate receptors, NMDA receptors also require glycine (or D-serine) to act as a co-agonist with L-glutamate (Johnson and Ascher, 1987). Glutamate binds to GluN2 subunits and glycine binds to a homologous site on GluN1 and GluN3 subunits, to cause the opening of the receptor’s Na+/K+/Ca++-permeable ion channel (Dingledine et al., 1999). It is the influx of Ca++ ions through this channel that initiates many of the actions of NMDA receptors.
Importantly, the GluN2 subunits confer distinct physiological, biochemical, and pharmacological properties to the NMDA receptor complex (Buller et al., 1994; Cull-Candy et al., 2001; Monyer et al., 1994). The various GluN2-containing receptors differ in their single channel conductance, open probability, the ability to desensitize, decay rate, sensitivity to L-glutamate and glycine, and the ability to bind to various intracellular signaling proteins (Traynelis et al., 2010). Furthermore, these subunits have different spatio-temporal patterns of expression in the CNS and even different distributions within individual dendritic spines. This diversity of GluN1/GluN2 function very likely contributes to findings that differing NMDA receptor populations have distinct, and sometimes even opposing, roles in various physiological and pathophysiological actions (Chen et al., 2008a; DeRidder et al., 2006; Hrabetova et al., 2000). Consequently, agents that have differing NMDA receptor specificities are likely to have significantly different therapeutic and adverse effect profiles. To date, however, relatively little is known about the function and therapeutic potential of different NMDA receptor subtypes. Their specific roles in CNS function and disease have been difficult to study in the absence of highly-selective antagonists.
2.2 NMDA receptor subunit structure
NMDA receptor subunits have distinct domains that interact with one another upon the binding of L-glutamate and glycine to cause channel activation, modulation of channel open probability and receptor desensitization (Figure 1). The N-terminal domain (NTD) comprises the first ~400 amino acids following the signal peptide and has a bilobed structure (or sometimes described as a clam shell or venus fly-trap structure) (Dingledine et al., 1999). This domain has homology to aleucine-isoleucine-valine binding protein from bacteria (O’Hara et al., 1993) in which amino acids bind within the cleft formed by the two lobes. For NMDA receptors the NTD can be in either the open or shut conformation, with the shut conformation inhibiting receptor function. In the case of GluN1/GLuN2A receptors, Zn++ is thought to bind between the two lobes of the GluN2A NTD to stabilize the closed conformation and thus inhibit receptor activity (Paoletti et al., 2000).
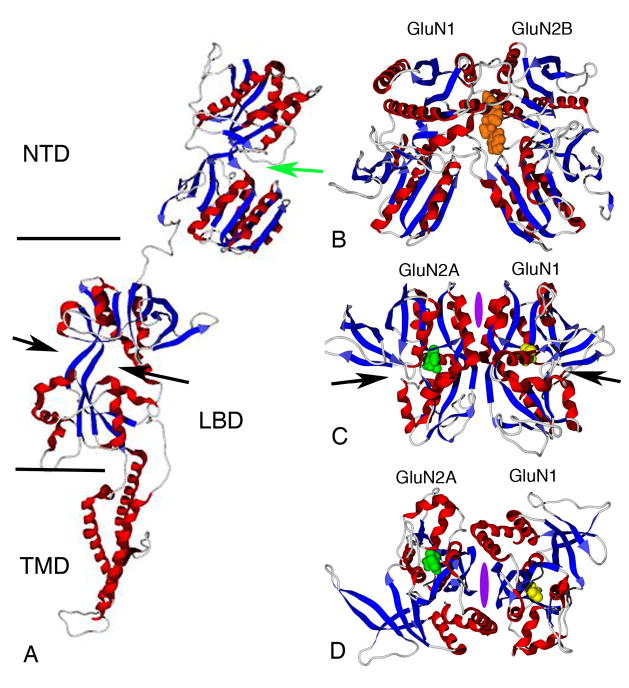
Figure 1.
NMDA receptor tetra-heteromeric structure. Space-filled (left) and backbone ribbon (right) representations of a homology model of the GluN1 (blue/green)/GluN2A (magenta/yellow) receptor complex based upon crystallographic structures of the GluA2 tetramer, the GluN1/GluN2A LBD dimer, and the GluN1/GluN2 NTD dimer. The layers of N-terminal domains (NTD), ligand-binding domains (LBD), and transmembrane domains (TMD) are indicated. Figures were generated using Molegro Molecular Viewer (Aarhus, Denmark).
The NTD is attached through a short peptide sequence to the agonist ligand binding domains (LBD) for L-glutamate and glycine (Figures 1 & 2). The LBDs are formed from two extracellular segments (segment 1 or S1 and segment 2 or S2) that together have homology to other bilobed bacterial amino acid binding proteins, the LAOBP (lysine-alanine-ornithine binding protein) and QBP (glutamine binding protein)(Moriyoshi et al., 1991; O’Hara et al., 1993). Crystallographic studies of agonist and antagonist bound inotropic glutamate receptor LBD structures reveal that agonist activity is associated with binding within the LBD bilobed cleft that stabilizes a closed conformation while antagonists prevent interlobe closure (Armstrong and Gouaux, 2000). In the peptide sequence, the S1 and S2 regions are separated by 3 membrane-associated domains (M1, M2, and M3), thus S1 is between the NTD and M1, and S2 is an extracellular loop that is between the two transmembrane domains M3 and M4 (Dingledine et al., 1999). The NMDA receptor ion channel is formed by the three transmembrane domains, M1, M3 and M4 plus the reentrant loop of M2, a “P-loop” structure, which enters and exits the membrane from the cytoplasmic side. The C-terminal is located within the cytoplasm and is the site of various protein-protein interactions and regulation by posttranslational modifications.
Figure 2.
Drug binding sites on the NMDA receptor complex. A. Ribbon representation of a GluN2A subunit showing the domain structure and the NTD cleft where Zn++ is thought to bind (green arrow) and the LBD cleft (black arrows). B. Crystal structure (3QEL, Karakas et al., 2011) of the GluN1/GluN2B NTD dimer with ifenprodil (orange) bound to the dimer interface. C. and D. GluN1/GluN2A LBD dimer - 2A5T, (Furukawa et al., 2005) with glutamate (green) and glycine (yellow) bound in the LBD clefts (black arrows). Purple ovals represent one of multiple potential sites for NAM/PAM binding based upon homology to cyclothiazide and CX614 binding to AMPA receptors. View of LBD dimer is parallel to membrane (C) or perpendicular to the membrane from above (D). Figures were generated using MolegroMolecular Viewer (Aarhus, Denmark) and the Protein Data Bank (PDB) files 3QEL and 2A5T.
3. Classic inhibitory drug targets on the NMDA receptor complex
Over the past 20–30 years, most compounds that act at NMDA receptors were found to interact with one of 4 drug binding sites on the NMDA receptor complex, the glutamate or glycine binding sites, the ion channel pore, or a binding site on the regulatory NTD. The first compounds identified were agonists and antagonists at the glutamate binding site (Watkins and Evans, 1981). These compounds, such as D-α-aminoadipic acid and D-2-amino-5-phosphonopentanoic acid (D-AP5) (Figure 3) allowed for the initial proposal that the compound NMDA interacted with its own class of receptors and that these so-called NMDA receptors have a role in synaptic transmission and synaptic plasticity (Monaghan et al., 1989; Watkins and Evans, 1981). Soon after the finding that NMDA receptor activation requires glycine for activation (Johnson and Ascher, 1987), glycine competitive antagonists and partial agonists were identified (Hood et al., 1989; Kemp et al., 1988; Kessler et al., 1989) (Figure 3). It was also soon recognized that some dissociative anesthetics (e.g. ketamine and phencyclidine, Figure 3) were NMDA receptor blockers and that these did so in a use- and voltage-dependent manner by binding within the ion channel (Anis et al., 1990; Lodge and Johnson, 1990). Subsequently, the widely used inhibitor MK-801 (dizocilpine maleate, Figure 3) was shown to be a potent NMDA receptor channel blocker (Wong et al., 1986).
Figure 3.
Structures of antagonists that bind to the glutamate binding site (A), the glycine binding site (B), the ion channel (C), or the NTD (D) on the NMDA receptor.
The fourth class of NMDA receptor antagonists came from the compound ifenprodil (Figure 3)(Carter et al., 1988) which binds at an interface between NTDs (Karakas et al., 2011). Ifenprodil displays a strong selectivity for the GluN1/GluN2B receptor (Williams, 1993) which explains the observation that ifenprodil is more effective early in CNS development (Zeevalk and Nicklas, 1992). This compound was followed by many derivatives that generally show a high degree of selectivity for GluN1/GluN2B receptors (Borza and Domany, 2006). Until recently, there has been relatively little further progress in the identification of compounds with different sites of action or with a different pattern of subtype-selectivity. Given the close homology between the different GluN2 subunits within the agonist binding sites and the ion channel, it is not surprising that it has been difficult to develop highly-selective NMDA receptor antagonists or agonists.
3.1 Glutamate binding site antagonists
In general, competitive glutamate binding site antagonists display a weak GluN2A>GluN2B>GluN2C>GluN2D pattern of selectivity, while the glycine and channel blockers display little to no selectivity. For compounds such as CPP (4-(3-phosphonopropyl)piperazine-2-carboxylic acid, Figure 3, Table 1) there can be up to a 50-fold selectivity of GluN2A over GluN2D and this may account for some pharmacological observations of native NMDA receptor heterogeneity in the literature (Feng et al., 2005). Although the glutamate binding site on GluN2 subunits is found on 4 genetically-distinct proteins, their binding site is highly conserved within the glutamate binding pocket and hence there is little opportunity for agents to display subtype-selective binding between the different GluN2 subunits (Kinarsky et al., 2005). However, adding bulky multi-aromatic ring substituents onto anaspartate-binding moiety, resulted in a series of antagonists which begin to probe regions outside of the glutamate-binding pocket that display subunit-specificity. These agents, such as PPDA ((2R*,3S*)-1-(phenanthrene-2-carbonyl)piperazine-2,3-dicarboxylic acid), UBP141 ((2R*,3S*)-1-(phenanthrene-3-carbonyl)piperazine-2,3-dicarboxylic acid) and UBP145 ((2R*,3S*)-1-(9-bromophenanthrene-3-carbonyl)piperazine-2,3-dicarboxylic acid) (Figure 3) display a slight (PPDA) or up to a 17-fold selectivity (UBP145) in terms of Ki for receptors containing GluN2C or GluN2D subunits over receptors containing GluN2A or GluN2B subunits (Costa et al., 2009; Feng et al., 2004; Hrabetova et al., 2000; Morley et al., 2005). Other related derivatives, e.g. UBP161, have additional kainate receptor activity and intriguingly, can be highly selective for subtypes of kainate receptors while retaining some NMDA receptor subtype selectivity as well (Irvine et al., 2012). PPDA, UBP141, and UBP145 have now been used by a number of laboratories to define the role of GluN2C and GluN2D subunits in NMDA receptor signaling (see further discussion below).
Table 1.
Pharmacological data for the activity of compounds on NMDAR subtypes
| Compound | GluN1/GluN2A | GluN1/GluN2B | GluN1/GluN2C | GluN1/GluN2D | |
|---|---|---|---|---|---|
| L-Glutamate Binding Site | D-AP5a | 0.28 ± 0.02 | 0.46 ± 0.14 | 1.64 ± 0.14 | 3.71 ± 0.67 |
| CPPa | 0.041 ± 0.003 | 0.27 ± 0.02 | 0.63 ± 0.05 | 1.99 ± 0.20 | |
| UBP141b | 44.1 ± 1.7 | 10.4 ± 2.1 | 3.22 ± 0.31 | 2.57 ± 0.16 | |
| UBP145b | 16.1 ± 2.3 | 6.32 ± 0.20 | 2.22 ± 0.55 | 0.94 ± 0.34 | |
| NVP-AAM077c | 0.0054 ± 0.0004 | 0.067 ± 0.003 | 0.012 ± 0.001 | 0.037 ± 0.004 | |
| Channel Blocker | Memantined | 13.4 ± 1.3 | 10.4 ± 0.4 | 1.61 ± 0.06 | 1.76 ± 0.06 |
| NTD binding | Ifenprodile | 40 | 0.11 | 29 | 76 |
| Ro 25-6981f | 52 | 0.009 | |||
| Allosteric Modulators | TCN 201g | 0.16 | >50 | >30 | >30 |
| QNZ46h | 182 ± 24 | 193 ± 45 | 7.1 ± 0.4 | 3.9 ± 0.2 | |
| UBP512i | ≫300i | ≫300 | 51 ± 11j | 46 ± 6j | |
| UBP608j | 18.6 ± 1.4 | 90 ± 4 | 68 ± 9 | 426 ± 40 | |
| UBP618j | 1.8 ± 0.2 | 2.4 ± 0.1 | 2.0 ± 0.1 | 2.4 ± 0.3 | |
| UBP710k | (24 ± 1)l | (51 ± 3) | (3.6 ± 1.5) | (2.6 ± 1.4) | |
| UBP551 | 9.7 ± 0.2j | 9.4 ± 0.6j | 15 ± 6j | (50 ± 4)k | |
| UBP646k | (32 ± 10) | (34 ± 9) | (22 ± 8) | (45 ± 8) | |
| CIQm | >10 | >10 | (2.7) | (2.8) |
Data represents Ki values (μM) ± s.e.m., (Feng et al., 2005)
Data represents KB values (μM) ± s.e.m., (Costa et al., 2009)
Data represents Ki values (μM) ± s.e.m., (Feng et al., 2004; therein referred to as PEAQX). Schild plot KB values: GluN1/GluN2A 0.015 μM; GluN1/GluN2B, 0.078 μM (Frizelle et al., 2006)
Data represents IC50 values (μM) ± s.e.m, obtained in the presence of 1 mM Mg2+, Kotermanski and Johnson, 2009
Data represents IC50 values (μM) at human receptors, Hess et al., 1996
Data represents IC50 values (μM), Fischer et al., 1997
Data represents IC50 values (μM), Bettini et al., 2010
Data represents IC50 values (μM) ± s.e.m, Hansen and Traynelis, 2011
UBP512 is a weak potentiator of agonist-induced effects on GluN1/GluN2A and inactive at GluN1/GluN2B when tested at 100–300 μM.
Data represents IC50 values (μM) ± s.e.m, Costa et al., 2010
Data represents % potentiation ± s.e.m. of agonist-induced responses by 100 μM compound, Costa et al., 2010
Potentiation of responses are indicated by values in parentheses.
Data represents EC50 values (μM), Mullasseril et al., 2010
The quinoxalinedione derivative (1RS,1′S)-PEAQX ([(RS)-[(S)-1-(4-bromophenyl)ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)methyl]-phosphonic acid (Figure 3), is a competitive antagonist with a reported 100-fold selectivity for human GluN1/GluN2A receptors over GluN1/GluN2B receptors in terms of IC50 (Auberson et al., 2002). (1RS,1′S)-PEAQX is an equimolecular mixture of the (1R,1′S)- and (1S,1′S)-diastereoisomers and this was subsequently resolved to provide the pure (1R,1′S) stereoisomer (NVP-AAM077, Figure 3). Subsequent studies with NVP-AAM077 using rodent NMDA receptors find only a ~10-fold selectivity in terms of Ki value (Berberich et al., 2005; Feng et al., 2004; Neyton and Paoletti, 2006) and only 2- and 6-fold selectivity for GluN2A over GluN2C or GluN2D-containing receptors, respectively (Feng et al., 2004). Nonetheless, this compound has been used in numerous studies to gain insight into the differential role of receptors containing GluN2A or GluN2B subunits.
3.2 Glycine binding site antagonists
A variety of NMDA receptor antagonists were developed that bind at the glycine binding site on GluN1 subunits. One of the originally identified glycine antagonists was HA966 (Figure 3) which was known to be a NMDA receptor antagonist well before the discovery that the NMDA receptor had a glycine binding site (Davies and Watkins, 1972; Foster and Kemp, 1989). Other important glycine site antagonists (Figure 3) were developed by the pharmaceutical industry, such as MDL 105,519 (Baron et al., 1997), L-701,324 (Priestley et al., 1996), and 7-chloro-5-iodokynurenic acid (Leeson et al., 1991). Since the glycine binding site is on the GluN1 subunit, it would be expected that glycine site agonists and antagonists would display no GluN2 subunit selectivity. However, competitive glycine site antagonists generally display a slightly higher affinity at GluN2A-containing receptors (Jane et al., 2000). And, as found for the glutamate binding site, agonists at the glycine binding site display highest affinities for the GluN2D-containing receptors and progressively lower potency at those containing GluN2C, GluN2B, and GluN2A subunits (Priestley et al., 1995). The ability of the GluN2 subunit to influence the glycine binding pocket appears to be due to specific residues within the S2 domain of the GluN2 subunit (Chen et al., 2008b). Overall, however, it appears unlikely that it will be possible to develop glycine binding site antagonists with a high degree of subtype-selectivity.
3.3 NMDA receptor channel blockers
With the finding that activity at the psychosis producing “phencyclidine receptor” correlates to the blockade of NMDA receptors (Martin and Lodge, 1988), it was apparent that psychosis may be a concern for NMDA receptor antagonists as therapeutic agents. These concerns were confirmed in clinical studies with high affinity NMDA receptor channel blockers. However, it was later discovered that low affinity channel blockers such as ketamine and memantine (Figure 3) were better tolerated in the clinic (Kalia et al., 2008; Rogawski and Wenk, 2003). The reduced adverse effect profile is thought to be due to the fast dissociation of the low affinity channel blockers out of the channel upon receptor inactivation, thus leaving unblocked channels when a subsequent synaptic response occurs (Lipton, 2005; Rogawski and Wenk, 2003). Consistent with this hypothesis, the clinically-tolerated NMDA receptor compounds are low affinity NMDA receptor channel blockers (memantine, ketamine, dextromethorphan, and amantadine, Figure 3) (Kalia et al., 2008).
As the NMDA receptor channel is also highly conserved among the different GluN1/GluN2 receptors, one would expect little subtype-selectivity by channel blockers. Indeed, the variation in channel blocker potency is of low selectivity (Beaton et al., 1992; Ebert et al., 1991; Monaghan and Larson, 1997). Evaluation of numerous channel blockers found that some display a subtype-selectivity of up to 10-fold (Dravid et al., 2007). Additionally, magnesium ions reduce the affinity of memantine for NMDA receptor channels, but especially so at GluN2A and GluN2B-containing receptors. Thus, in the presence of physiological levels of Mg++ ions, memantine displays approximately 10-fold selectivity for GluN1/GluN2C and GluN1/GluN2D receptors (Kotermanski and Johnson, 2009).
3.4 Ifenprodil-like agents
Ifenprodil (Figure 3) was originally studied in the mid-1970’s as a cardiovascular modulator. In this context it was tested in animal models as an anti-stroke agent and found to have some therapeutic benefit. Further studies showed that this agent was an NMDA receptor blocker with an unusual mechanism of action (Carter et al., 1989; Carter et al., 1990; Legendre and Westbrook, 1991). The blockade is neither use-dependent nor voltage-dependent and is selective for GluN1/GluN2B receptors (Hess et al., 1996; Legendre and Westbrook, 1991; Williams, 1993). Ifenprodil does not bind at either the L-glutamate or the glycine binding site but rather binds to the NTD (Perin-Dureau et al., 2002). Crystallographic studies indicate that the ifenprodil binding site is in the interface formed between the NTDs of the GluN1 and GluN2 heterodimers (Karakas et al., 2011).
Over the next two decades, many ifenprodil-like agents were developed that are highly specific for the GluN1/GluN2B receptor complex. Such compounds include CP101,606 (Chenard et al., 1995) and Ro 25-6981 (Fischer et al., 1997)(Figure 3) which along with ifenprodil have been useful in a large number of studies. The results from much of this work has led to the general impression that GluN2B-containing receptors have a predominant role in excitotoxicity and neuropathic pain; thus this class of agents has been actively pursued for their potential clinical applications (Gogas, 2006; Nikam and Meltzer, 2002).
4.0 Recently identified negative allosteric modulators of NMDA receptors
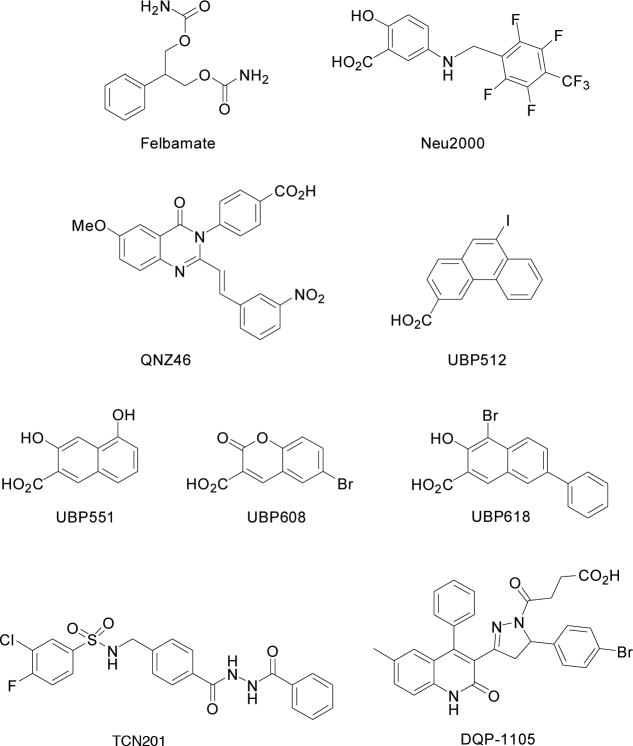
In the field of G-protein coupled receptors, the development of negative allosteric modulators (NAMs) and positive allosteric modulators (PAMs) has resulted in new classes of compounds with therapeutic advantages over the orthosteric, competitive inhibitors or the use of receptor agonists, respectively (Keov et al., 2011; Urwyler, 2011). Similarly, AMPA receptor modulation by its NAMs, e.g. compounds such as GYKI 52466 (Maj et al., 1995) and AMPA receptor PAMs, e.g. cyclothiazide (Bertolino et al., 1993) is presenting new therapeutic opportunities for AMPA receptor modulation. In the area of NMDA receptors, development of allosteric modulators, other than the NTD-binding GluN2B-selective agents, has been lagging. Individual compounds such as felbamate (Figure 4) (Chang and Kuo, 2008; Kleckner et al., 1999) and neu2000 (Figure 4) (Cho et al., 2010) appear to also represent NAMs and further work is necessary to better define their sites of action. In the past year, however, three new classes of drugs have emerged that can negatively modulate NMDA receptor function. Importantly, these agents also show enhanced subtype-selectivity thus resulting in multiple new patterns of activity.
Figure 4.
Structures of NMDA receptor negative allosteric modulators.
4.1 Quinazolin-4-one derivatives
Using a high-throughput screening assay of a diverse chemical library, Traynelis and colleagues identified NMDA receptor inhibitory activity in a compound with a quinazoline-4-one scaffold. During structural optimization, many compounds were identified that had additional AMPA receptor activity, but QNZ46 (Figure 4) emerged as a reasonably potent (low micromolar) and selective NMDA receptor antagonist that displays a ~50-fold selectivity for GluN2D-containing receptors over GluN2A and GluN2B (Hansen and Traynelis, 2011; Mosley et al., 2010). QNZ46 displays a non-competitive, voltage-independent block of NMDA receptor responses. Using GluN2A/GluN2D chimeras and point mutations, it was determined that the GluN2 S2 domain, and specific amino acid residues therein, are critical for antagonist activity (Hansen and Traynelis, 2011). The mode of blockade is unusual in that blockade requires the binding of L-glutamate thus giving a partial use-dependent antagonist activity.
4.2 Phenanthroic and naphthaloic acid NAMs
Derivatives of naphthoic acid and phenanthroic acid (Figure 4) represent a functionally diverse family of NMDA receptor allosteric modulators that have novel activities and novel mechanisms of action (Costa et al., 2012; Costa et al., 2010). These compounds display varied subunit-selectivity and have potentiating activity as well as inhibitory activity. The NMDA receptor inhibitory compounds, NAMs, are not competitive L-glutamate or glycine site antagonists, do not block in a voltage-dependent manner as expected for channel blockers, and do not require the regulatory N-terminal domain (NTD) for activity. Thus, they are mechanistically distinct from the compounds that have been tested in the clinic to date.
The compound UBP512 (Figure 4) selectively inhibits GluN2C/GluN2D-containing receptors (IC50 ~ 50 μM). It has virtually no activity at GluN2B-containing receptors at concentrations up to 300 μM and causes a small potentiation of GluN2A-containing receptors at high concentrations. UBP551 (Figure 4) is distinctive in that it potentiates GluN1/GluN2D receptor responses (maximally at ~30 μM) while inhibiting the other GluN1/GluN2 receptors with IC50s around 10 μM. Thus, it is the only compound to be reported which distinguishes between GluN2C and GluN2D. In a related structure, the coumarin UBP608 (Figure 4) has exclusively inhibitory activity with a preference for GluN2A-containing receptors. The 2-naphthoic acid derivative, UBP618 (Figure 4) is also a purely inhibitory, non-selective compound with an IC50 ~ 2 μM at each of the four GluN1/GluN2 receptors. Under low agonist concentrations (10 μM L-glutamate and 10 μM glycine) UBP618 does not fully inhibit NMDA receptor responses; UBP618 maximally inhibits 80 – 90% of the response.
The inhibitory actions of these compounds appear to be mechanistically distinct from those of QNZ46. Chimeric GluN2 studies indicate that the subtype-selective activity of UBP608 is more greatly influenced by the S1 domain than the S2 domain (Costa et al., 2010)– a result that contrasts with that found for QNZ46 (Hansen and Traynelis, 2011). Another distinction is the effect of agonist concentration on modulator activity. Increasing the concentration of L-glutamate and glycine increases the inhibitory potency and maximal inhibitory effect of UBP512 and UBP608 at GluN1/GluN2D receptors and reduces the maximal inhibitory effect of UBP608 at GluN1/GluN2A receptors (Costa et al., 2010). In contrast, increasing agonist concentration has no effect on the extent of QNZ46 inhibition (Hansen and Traynelis, 2011).
4.3 Sulfonamide derivatives
Bettini and colleagues, identified a series of potent compounds with distinctive NMDA receptor selectivity (Bettini et al., 2010). In particular, “compound 1” from this study (3-chloro-4-fluoro-N-[(4-{[2-(phenylcarbonyl)hydrazino]carbonyl}phenyl)methyl]benzenesulfonamide) (also known as TCN 201, Figure 4) displays more than a 300-fold selectivity for GluN1/GluN2A receptors over GluN1/GluN2B receptors. This is not a competitive glutamate binding site antagonist since 1 mM concentrations of L-glutamate did not reverse the blockade. While high glycine concentrations did block the antagonist effect, this does not appear to be due to competition for the same binding site, TCN 201 only weakly inhibited the binding of the glycine binding site antagonist [3H]MDL 105,519 at high concentrations. Since the antagonist effect of TCN 201 is eliminated by high glycine concentrations, the actions of these antagonists appear to be distinct from both the phenanthrene/naphthalene NAMs and QNZ46.
5.0 Enhancement of NMDA receptor function: Glycine agonists and positive allosteric modulators
While most efforts in NMDA receptor therapeutics has focused on the development of antagonists, there are therapeutic indications that instead would be expected to benefit from the enhancement of NMDA receptor function (e.g. schizophrenia or cognitive enhancement). In general, this can be achieved by either increasing glycine agonist concentrations at NMDA receptors or by an NMDA receptor PAM.
5.1 Glycine site agonists and partial agonists
Since the acute, phasic gating of NMDA receptor activity is mediated by the release of L-glutamate from synaptic vesicles, the ambient concentration of the necessary co-agonist glycine or D-serine would act as an allosteric modulator. Thus, if extracellular levels of glycine/D-serine are not saturating, then administration of a glycine agonist should increase the activity of NMDA receptors upon the release of L-glutamate. To this end, glycine, D-serine, and the partial agonist D-cycloserine have been evaluated for the treatment of schizophrenia and autism. Although the results are not entirely supportive, the studies do suggest that there may be some therapeutic benefit to augmenting NMDA receptor responses with glycine site agonists (Kalia et al., 2008; Shim et al., 2008; Singh and Singh, 2011; Tsai and Lin, 2010). Glycine agonists are also being considered for accelerating the reversal of post-traumatic stress disorder (Heresco-Levy et al., 2009). Two alternative approaches for increasing glycine/D-serine agonist activity are through the use of glycine transporter-1 inhibitors, for a review see (Hashimoto, 2011) and D-amino acid oxidase inhibitors (Strick et al., 2011).
An important limitation to this general approach is that the degree of glycine-site enhancement of NMDA receptor function depends upon the extracellular concentration of glycine and D-serine. There would be no effect if the glycine binding site is already saturated. Additionally, one would expect that the function of GluN2A-containing receptors would be preferentially enhanced since they display the lowest affinity for glycine and hence are the least likely to have their glycine binding site saturated. Conversely, receptors containing GluN2D subunits might be the least affected. A related factor to consider is the degree of extracellular compartmentalization of glycine/D-serine near the synapse.
5.2 Endogenous NMDA receptor PAMs: histamine, ATP, spermine, Mg++, and neurosteroids
The endogenous compounds histamine (Williams, 1994), ATP (Kloda et al., 2004), polyamines such as spermine (Williams et al., 1994) (for other references see (Dingledine et al., 1999), and the neurosteroid, pregnenolone sulfate (Sedlacek et al., 2008), have all been shown to potentiate the actions of agonists on the activation of NMDA receptors (see Figure 5 for structures). Spermine potentiates by two mechanisms, by increasing glycine affinity (glycine-dependent potentiation) and by an allosteric interaction that can be seen in saturating glycine conditions (glycine-independent potentiation). This latter action of spermine has the same subunit specificity as that displayed by potentiation due to histamine and Mg++; potentiation is specific to the combination of GluN1 subunits that lack exon 5 and GluN2B subunits (Paoletti et al., 1995; Williams, 1994; Williams et al., 1994). The potentiation by ATP and histamine have in common that their potentiating actions are enhanced in the presence of high glutamate concentrations. For ATP, potentiation at low mM concentrations occurs at each of GluN1/GluN2A, GluN1/GluN2B, and GluN1/GluN2C receptors (Kloda et al., 2004). ATP is also reported to act as a competitive inhibitor at GluN1/GluN2A and GluN1/GluN2B receptors, hence part of the L-glutamate-enhanced potentiation seen at these receptors may be due to the reversal of ATP-inhibition (Kloda et al., 2004).
Figure 5.
Structures of NMDA receptor positive allosteric modulators.
Pregnenolone sulfate (PS) (Figure 5) in micromolar concentrations also potentiates NMDA receptor responses (Wu et al., 1991; Sedlacek et al., 2008). PS specifically potentiates responses at receptors containing GluN2A or GluN2B subunits while weakly inhibiting responses at GluN2C or GluN2D-containing receptors (Malayev et al., 2002). By using a drug pre-application protocol, it was possible to show that PS causes a small degree of potentiation at both GluN2C and GluN2D-containing receptors which is masked by inhibition when agonist and PS are co-applied (Horak et al., 2006). Likewise, it is possible to show that PS has an additional inhibitory activity at GluN1/GluN2A and GluN1/GluN2B receptors. Modeling of receptor kinetics suggests that PS increases the open probability of NMDA receptor responses (Horak et al., 2004). The ability to potentiate responses may be dependent upon the phosphorylation state of the receptor (Petrovic et al., 2009).
Chimera and point mutation studies have identified a putative binding site for PS potentiation. Replacing the S2 domain of GluN2A with that of GluN2C significantly reduces PS potentiation (Horak et al., 2004). In particular, replacing S2’s helixes J and K of GluN2B plus the last transmembrane domain, M4, with the corresponding segments in GluN2D eliminate potentiation (Jang et al., 2004). Of individual amino acid residues mutated, GluN2B’s Q812 that precedes M4, had the strongest effect on potentiation. Thus, these regions are critical to ligand binding or to transducing the effect of ligand binding.
5.3 Naphthoic and phenanthroic acid NMDA receptor PAMs
As discussed above (section 4.2), some derivatives containing naphthoic acid or phenanthroic acid (Figure 5) have NMDA receptor potentiating activity (Costa et al., 2010). In common with the neurosteroids, some of the naphthoic and phenanthroic acid compounds discriminate between GluN2A/GluN2B vs GluN2C/GluN2D receptors. For example, UBP710 (Figure 5) potentiates the responses of receptors containing GluN2A or GluN2B subunits and weakly inhibits GluN2C and GluN2D-containing receptors. Conversely, NSC339614 selectively potentiates GluN1/GluN2C and GluN1/GluN2D receptor responses (Costa et al., 2010). However, some compounds distinguish between receptors having GluN2A and GluN2B subunits while other compounds can distinguish between GluN2C- and GluN2D-containing receptors. For example the 9-iodo derivative of phenanthroic acid (UBP512) (Figure 5) potentiates GluN1/GluN2A but not GluN1/GluN2B receptors and the compound UBP551 (Figure 5) selectively potentiates GluN1/GluN2D receptors and not the other 3 GluN1/GluN2 receptors.
A critical structural feature for PAM activity is the size and nature of substituents at the 9-position of 3-phenanthroic acid. Adding a large hydrophobic group as in UBP646, results in a pan NR1/NR2 potentiator. For the GluN2A-potentiator UBP512, replacement of the 9-iodo group with a bromo group converts the compound to an inhibitor at GluN1/GluN2A receptors (unpublished observations).
The binding site for the PAM activity of these compounds is unknown. These compounds do not require the NTD for activity, thus the binding site is presumably associated with the agonist-binding domain, the linkers of this domain to the transmembrane domains, and/or within the transmembrane region. Chimeras between GluN2A and GluN2C show that the potentiating activity requires elements in the S2 domain; UBP512 and UBP710 inhibit rather than potentiate responses of GluN1/GluN2A receptors in which the GluN2A subunit has the S2 domain of a GluN2C subunit. Thus, the potentiating binding/effector site may possibly overlap with that of the neurosteroid PS which also requires elements of the S2 domain. This result is in contrast to the NAM activity of UBP608 whose GluN2A-selective inhibitory activity is associated with the S1 domain (Costa et al., 2010). Like that found for ATP and histamine potentiation, UBP512 potentiation of GluN1/GluN2A receptor responses is enhanced in the presence of high agonist concentrations.
The AMPA receptor PAMs, cyclothiazide, CX614 and others, have been shown to bind in the intersubunit interface of the LBD dimer. Thus, it is possible that some NMDA receptor PAMs bind at the homologous site within the NMDA receptor complex at the GluN1/GluN2 LBD dimer interface (purple oval in Figure 2C and 2D). Binding of an AMPAR PAM in this interface can slow desensitization and/or slow deactivation depending upon the precise position within the interface (Ahmed and Oswald, 2010; Arai and Kessler, 2007; Jin et al., 2005; Ptak et al., 2009). It is also possible that some PAMs bind within or near the transmembrane domain and stabilize the open channel conformation.
5.4 CIQ
CIQ (3-chlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-ylmethanone (Figure 5) selectively potentiates receptors containing GluN2C and GluN2D subunits with a low micromolar potency (Mullasseril et al., 2010). Single channel analysis indicates that CIQ increases open probability by decreasing mean shut time. Chimera and point mutation studies identify two sites on the NMDA receptor complex as being important for the activity of CIQ, Thr592 within the M1 region and the linker between the NTD and the ligand binding domain (Mullasseril et al., 2010). Perhaps, some of these sites are necessary for mediating the downstream transduction activity of this compound while other sites are important specifically for the binding of CIQ. Overall, chimera studies suggest that the CIQ has a different site of action than the naphthalene/phenanthrene PAMs and is also different from the site of PS potentiation.
6. Differential roles of GluN2 subunits in NMDAR function
Studies regarding the function of pharmacologically-distinct NMDA receptors have not yet incorporated the newer NAMs and PAMs. Thus functional studies have relied on the previously available agents and the use of siRNA or knockout animals. Pharmacologically, CPP, UBP141/UBP145, NVP-AAM077, and the ifenprodil-like compounds have proven the most useful for distinguishing NMDAR subtypes in studies thus far.
PPDA and other glutamate site antagonists provided the first evidence of a differential role of receptors containing different GluN2 subunits in LTP and LTD with GluN2A and/or GluN2B largely contributing to LTP and GluN2C/GluN2D contributing to LTD (Hrabetova et al., 2000). Several subsequent studies have focused specifically on the roles of GluN2A and GluN2B subunits in synaptic plasticity using NVP-AAM077 and ifenprodil (and related compounds). One study suggested that GluN2A-containing receptors are specifically responsible for LTP and GluN2B for LTD (Liu et al., 2004). In a manner consistent with this hypothesis, cortical LTP and LTD can also be pharmacologically distinguished (Massey et al., 2004). However, other studies indicate a role for GluN2B in LTP and GluN2A is not essential for LTP (Bartlett et al., 2007; Berberich et al., 2005; Liu et al., 2004; Morishita et al., 2007; Weitlauf et al., 2005). Likewise, GluN2B subunits are not essential for LTD (Morishita et al., 2007).
More recently, studies using UBP141 have suggested a role for GluN2D in LTD in the cerebral cortex (Banerjee et al., 2009). Furthermore, in hippocampal synaptic plasticity, the role of GluN2D appears more complex. Short-term potentiation, a component of LTP, is more sensitive to Ro 25-6981 and UBP145 than is the stable component of LTP (Volianskis, 2010) thus suggesting a special role for GluN2B and GluN2D in short-term potentiation. In contrast, GluN2A-preferring ligands, AP5 and NVP-AAM077 did not selectively inhibit short-term potentiation.
There is also evidence for a differential role of GluN2A and GluN2B subunits in excitotoxicity and neuroprotection. This work has led to the intriguing hypothesis that activation of GluN2A-containing receptors leads to neuroprotection whereas activation of receptors containing GluN2B subunits cause cell death (DeRidder et al., 2006; Kinney et al., 2006; Liu et al., 2007; Chen et al., 2008a). This hypothesis, if confirmed, would have significant therapeutic implications. However others using neuronal cell cultures do not see a differential role in cell survival (von Engelhardt et al., 2007). The highly selective GluN2B antagonists such as ifenprodil and Ro-25-6981 have been used in a variety of other preclinical studies which support a potential role of these agents for neuroprotection and in treating pain (Gogas, 2006; Nikam and Meltzer, 2002).
The functional roles of GluN2C and GluN2D have been examined using PPDA, UBP141, and UBP145. These studies support a role for GluN2C/GluN2D-containing NMDA receptor subpopulations in hippocampal extrasynaptic NMDA receptors (Costa et al., 2009; Harney et al., 2008; Lozovaya et al., 2004), astrocytes (Palygin et al., 2011), pain pathways (Shiokawa et al., 2010; Tong et al., 2008), and substantianigrasynaptic responses (Brothwell et al., 2008; Suarez et al., 2010). As the native prion protein has been found to selectively inhibit GluN2D-containing receptors (Khosravani et al., 2008), and GluN2D disinhibition by tissue plasminogen activator (TPA) can account for augmentation of stroke damage by TPA (Jullienne et al., 2011), the role of GluN2D in cortical cell death should be further evaluated.
Resolution of the function of discrete NMDA receptor subtypes will require both the further improvement in subtype-selective agents and the ability to define the function of heterotrimeric NMDA receptors. Other than the ifenprodil-like compounds, those that have been used in functional studies have on the order of a ten-fold degree of selectivity (e.g. UBP141 and NVP-AAM077). Use of these agents can provide useful information if they are used at multiple concentrations, compared in the same preparation to other drugs, and, preferably, when two-different processes (e.g. LTP vs LTD) can be directly compared. Thus, an important goal of developing subtype-selective agents is to identify compounds with well over a 100-fold degree of selectivity, so that meaningful conclusions can be made with single dose applications.
The presence of heterotrimeric NMDA receptors, such as those containing GluN1/GluN2A/GluN2B subunits significantly increases the difficulty in resolving the function of different NMDA receptor subtypes. The highly GluN2B-selective compound ifenprodil, displays a high affinity but lowered efficacy at GluN1/GluN2A/GluN2B receptors (Hatton and Paoletti, 2005). Thus if the relative contribution of the different di- and tri-heteromeric receptor complexes is unknown, it may not be possible to make quantitative conclusions regarding subunit contribution to receptor function. Careful analysis of NMDAR synaptic currents in the well-studied hippocampal CA3-CA1 synapse suggests that GluN1/GluN2A/GluN2B receptors provide the majority of the NMDAR synaptic response (Rauner and Kohr, 2011).
7. Conclusions
The first 30 years of NMDA receptor pharmacology generated many compounds that for the most part work at 4 binding sites within the receptor complex, the glutamate and glycine binding sites, the channel blocker site, and the ifenprodil binding site in the NTD interface between GluN1 and GluN2B. After many clinical trials using NMDA receptor agents that target these original drug binding sites, most have failed although some have shown therapeutic benefit (Kalia et al., 2008). Results from the past year suggest the presence of potentially 5 new drug target sites, three NAMs and two PAMs. The existence of NAMs and PAMs of NMDA receptors was to be expected from the activity of AMPA receptor allosteric modulators such as cyclothiazide and GYKI 52466.
One potential value of allosteric modulators is that they probe different portions of the receptor complex than that occupied by the orthosteric ligands and the channel blockers. If the NAM and PAM binding pockets are in regions with low sequence homology between subunits, then it may possible to develop compounds with yet greater selectivity. The early success in developing subunit-selective agents with these compounds suggests that this is indeed possible. Another potential advantage of these new categories of compounds is that they vary in their uncompetitive/noncompetitive behavior and they vary in the effect that agonists have on their activity. These properties may provide a therapeutic advantage for individual compounds in particular situations. For example, specific antagonists may be more suitable for blocking chronically low agonist concentrations that might be found in pathological conditions while not blocking phasic, high agonist concentrations found in neurotransmission. These compounds can also vary in their maximal effects. Ifenprodil-like compounds and the naphthalene/phenanthrene NAMs vary in their maximal inhibition. Thus, it might be possible to avoid some of the adverse effects of excessive NMDA receptor blockade by using antagonists that can only maximally inhibit a portion of the receptor response. The newly identified PAM activities offer novel leads for further developing PAMs for new therapeutic applications such as schizophrenia and cognitive enhancement.
Acknowledgments
The authors would like to thank the NIH (grant MH60252), the BBSRC (grant BB/F012519/1) and the MRC (Grants G0601509, G0601812) for funding their work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed AH, Oswald RE. Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J Med Chem. 2010;53:2197–2203. doi: 10.1021/jm901905j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis N, Sherby S, Goodnow R, Jr, Niwa M, Konno K, Kallimopoulos T, Bukownik R, Nakanishi K, Usherwood P, Eldefrawi A, et al. Structure-activity relationships of philanthotoxin analogs and polyamines on N-methyl-D-aspartate and nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1990;254:764–773. [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Meredith RM, Rodriguez-Moreno A, Mierau SB, Auberson YP, Paulsen O. Double dissociation of spike timing-dependent potentiation and depression by subunit-preferring NMDA receptor antagonists in mouse barrel cortex. Cereb Cortex. 2009;19:2959–2969. doi: 10.1093/cercor/bhp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Baron BM, Harrison BL, Kehne JH, Schmidt CJ, van Giersbergen PL, White HS, Siegel BW, Senyah Y, McCloskey TC, Fadayel GM, Taylor VL, Murawsky MK, Nyce P, Salituro FG. Pharmacological characterization of MDL 105,519, an NMDA receptor glycine site antagonist. Eur J Pharmacol. 1997;323:181–192. doi: 10.1016/s0014-2999(97)00045-9. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Beaton JA, Stemsrud K, Monaghan DT. Identification of a novel N-methyl-D-aspartate receptor population in the rat medial thalamus. J Neurochem. 1992;59:754–757. doi: 10.1111/j.1471-4159.1992.tb09433.x. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino M, Baraldi M, Parenti C, Braghiroli D, DiBella M, Vicini S, Costa E. Modulation of AMPA/kainate receptors by analogues of diazoxide and cyclothiazide in thin slices of rat hippocampus. Receptors Channels. 1993;1:267–278. [PubMed] [Google Scholar]
- Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, Cardullo F. Identification and characterisation of novel NMDA receptor antagonists selective for NR2A-over NR2B-containing receptors. J Pharmacol Exp Ther. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- Borza I, Domany G. NR2B selective NMDA antagonists: the evolution of the ifenprodil-type pharmacophore. Curr Top Med Chem. 2006;6:687–695. doi: 10.2174/156802606776894456. [DOI] [PubMed] [Google Scholar]
- Brothwell SL, Barber JL, Monaghan DT, Jane DE, Gibb AJ, Jones S. NR2B- and NR2D-containing synaptic NMDA receptors in developing rat substantia nigra pars compacta dopaminergic neurones. J Physiol. 2008;586:739–750. doi: 10.1113/jphysiol.2007.144618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Benavides J, Legendre P, Vincent JD, Noel F, Thuret F, Lloyd KG, Arbilla S, Zivkovic B, MacKenzie ET, et al. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. II. Evidence for N-methyl-D-aspartate receptor antagonist properties. J Pharmacol Exp Ther. 1988;247:1222–1232. [PubMed] [Google Scholar]
- Carter C, Rivy JP, Scatton B. Ifenprodil and SL 82.0715 are antagonists at the polyamine site of the N-methyl-D-aspartate (NMDA) receptor. Eur J Pharmacol. 1989;164:611–612. doi: 10.1016/0014-2999(89)90275-6. [DOI] [PubMed] [Google Scholar]
- Carter CJ, Lloyd KG, Zivkovic B, Scatton B. Ifenprodil and SL 82.0715 as cerebral antiischemic agents. III. Evidence for antagonistic effects at the polyamine modulatory site within the N-methyl-D-aspartate receptor complex. J Pharmacol Exp Ther. 1990;253:475–482. [PubMed] [Google Scholar]
- Cavara NA, Hollmann M. Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol Neurobiol. 2008;38:16–26. doi: 10.1007/s12035-008-8029-9. [DOI] [PubMed] [Google Scholar]
- Chang HR, Kuo CC. Molecular determinants of the anticonvulsant felbamate binding site in the N-methyl-D-aspartate receptor. J Med Chem. 2008;51:1534–1545. doi: 10.1021/jm0706618. [DOI] [PubMed] [Google Scholar]
- Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, Xu L, Duan WH, Xiong ZQ. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke. 2008a;39:3042–3048. doi: 10.1161/STROKEAHA.108.521898. [DOI] [PubMed] [Google Scholar]
- Chen PE, Geballe MT, Katz E, Erreger K, Livesey MR, O’Toole KK, Le P, Lee CJ, Snyder JP, Traynelis SF, Wyllie DJ. Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J Physiol. 2008b;586:227–245. doi: 10.1113/jphysiol.2007.143172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, Ducat MF, Dumont ML, Fox CB, Mena EE, et al. (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem. 1995;38:3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- Cho SI, Park UJ, Chung JM, Gwag BJ. Neu2000, an NR2B-selective, moderate NMDA receptor antagonist and potent spin trapping molecule for stroke. Drug News Perspect. 2010;23:549–556. doi: 10.1358/dnp.2010.23.9.1513493. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Collingridge G. Synaptic plasticity. The role of NMDA receptors in learning and memory. Nature. 1987;330:604–605. doi: 10.1038/330604a0. [DOI] [PubMed] [Google Scholar]
- Costa BM, Feng B, Tsintsadze TS, Morley RM, Irvine MW, Tsintsadze V, Lozovaya NA, Jane DE, Monaghan DT. N-methyl-D-aspartate (NMDA) receptor NR2 subunit selectivity of a series of novel piperazine-2,3-dicarboxylate derivatives: preferential blockade of extrasynaptic NMDA receptors in the rat hippocampal CA3-CA1 synapse. J Pharmacol Exp Ther. 2009;331:618–626. doi: 10.1124/jpet.109.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Laube B, Jane DE, Monaghan DT. Structure-activity relationships for allosteric NMDA receptor inhibitors based on 2-naphthoic acid. Neuropharmacology. 2012;62:1730–1736. doi: 10.1016/j.neuropharm.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE, Monaghan DT. A novel family of negative and positive allosteric modulators of NMDA receptors. J Pharmacol Exp Ther. 2010;335:614–621. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Monaghan DT, Ganong AH. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu Rev Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Davies J, Watkins JC. Is 1-hydroxy-3-aminopyrrolidone-2 (HA-966) a selective excitatory amino-acid antagonist? Nat New Biol. 1972;238:61–63. doi: 10.1038/newbio238061a0. [DOI] [PubMed] [Google Scholar]
- DeRidder MN, Simon MJ, Siman R, Auberson YP, Raghupathi R, Meaney DF. Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol Dis. 2006;22:165–176. doi: 10.1016/j.nbd.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Mrray E, Mosely C, Barber J, French A, Balster R, Murray TF, Traynelis SF. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Wong EH, Krogsgaard Larsen P. Identification of a novel NMDA receptor in rat cerebellum. Eur J Pharmacol. 1991;208:49–52. doi: 10.1016/0922-4106(91)90050-r. [DOI] [PubMed] [Google Scholar]
- Feng B, Morley RM, Jane DE, Monaghan DT. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48:354–359. doi: 10.1016/j.neuropharm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25–6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- Foster AC, Kemp JA. HA-966 antagonizes N-methyl-D-aspartate receptors through a selective interaction with the glycine modulatory site. J Neurosci. 1989;9:2191–2196. doi: 10.1523/JNEUROSCI.09-06-02191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJ. Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquino xalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-D-aspartate receptors: Implications for studies of synaptic transmission. Mol Pharmacol. 2006;70:1022–1032. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Gogas KR. Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol. 2006;6:68–74. doi: 10.1016/j.coph.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Hansen KB, Traynelis SF. Structral and Mechanistic Determinants of a Novel Site for Noncompetitive Inhibition of GluN2D-Containing NMDA Receptors. J Neurosci. 2011;31:3650–3661. doi: 10.1523/JNEUROSCI.5565-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci. 2008;28:11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Glycine transporter-1: a new potential therapeutic target for schizophrenia. Curr Pharm Des. 2011;17:112–120. doi: 10.2174/138161211795049598. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Perez-Otano I, Philpot BD. Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol. 2010;91:23–37. doi: 10.1016/j.pneurobio.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U, Vass A, Bloch B, Wolosker H, Dumin E, Balan L, Deutsch L, Kremer I. Pilot controlled trial of D-serine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2009;12:1275–1282. doi: 10.1017/S1461145709000339. [DOI] [PubMed] [Google Scholar]
- Hess SD, Daggett LP, Crona J, Deal C, Lu CC, Urrutia A, Chavez Noriega L, Ellis SB, Johnson EC, Velicelebi G. Cloning and functional characterization of human heteromeric N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1996;278:808–816. [PubMed] [Google Scholar]
- Hood WF, Sun ET, Compton RP, Monahan JB. 1-Aminocyclobutane-1-carboxylate (ACBC): a specific antagonist of the N-methyl-D-aspartate receptor coupled glycine receptor. Eur J Pharmacol. 1989;161:281–282. doi: 10.1016/0014-2999(89)90861-3. [DOI] [PubMed] [Google Scholar]
- Horak M, Vlcek K, Chodounska H, Vyklicky L., Jr Subtype-dependence of N-methyl-D-aspartate receptor modulation by pregnenolone sulfate. Neuroscience. 2006;137:93–102. doi: 10.1016/j.neuroscience.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Horak M, Vlcek K, Petrovic M, Chodounska H, Vyklicky L., Jr Molecular mechanism of pregnenolone sulfate action at NR1/NR2B receptors. J Neurosci. 2004;24:10318–10325. doi: 10.1523/JNEUROSCI.2099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA Receptor Subpopulations Contribute to Long-Term Potentiation and Long-Term Depression Induction. J Neurosci (Online) 2000;20:RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine MW, Costa BM, Dlaboga D, Culley GR, Hulse R, Scholefield CL, Atlason P, Fang G, Eaves R, Morley R, Mayo-Martin MB, Amici M, Bortolotto ZA, Donaldson L, Collingridge GL, Molnar E, Monaghan DT, Jane DE. Piperazine-2,3-dicarboxylic Acid Derivatives as Dual Antagonists of NMDA and GluK1-Containing Kainate Receptors. J Med Chem. 2012;55:327–341. doi: 10.1021/jm201230z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane DE, Tse H-W, Skifter DA, Christie JM, Monaghan DT. Glutamate receptor ion channels: Activators and inhibitors. In: Endo, Mishina, Kurachi, editors. Handbook of Experimental Pharmacology: Pharmacology of Ionic Channel Function: Activators and Inhibitors. Springer; Berlin: 2000. pp. 415–478. [Google Scholar]
- Jang MK, Mierke DF, Russek SJ, Farb DH. A steroid modulatory domain on NR2B controls N-methyl-D-aspartate receptor proton sensitivity. Proc Natl Acad Sci U S A. 2004;101:8198–8203. doi: 10.1073/pnas.0401838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM. Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci. 2005;25:9027–9036. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jullienne A, Montagne A, Orset C, Lesept F, Jane DE, Monaghan DT, Maubert E, Vivien D, Ali C. Selective inhibition of GluN2D-containing N-methyl-D-aspartate receptors prevents tissue plasminogen activator-promoted neurotoxicity both in vitro and in vivo. Mol Neurodegener. 2011;6:68. doi: 10.1186/1750-1326-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475:249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JA, Foster AC, Leeson PD, Priestley T, Tridgett R, Iversen LL, Woodruff GN. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988;85:6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology. 2011;60:24–35. doi: 10.1016/j.neuropharm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, Hamid J, Chen L, Villemaire M, Ali Z, Jirik FR, Zamponi GW. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J Gen Physiol. 2008;131:i5. doi: 10.1085/JGP1316OIA5. [DOI] [PubMed] [Google Scholar]
- Kinarsky L, Feng B, Skifter DA, Morley RM, Sherman S, Jane DE, Monaghan DT. Identification of subunit- and antagonist-specific amino acid residues in the N-Methyl-D-aspartate receptor glutamate-binding pocket. J Pharmacol Exp Ther. 2005;313:1066–1074. doi: 10.1124/jpet.104.082990. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW, Glazewski JC, Chen CC, Moscrip TD. Subtype-selective antagonism of N-methyl-D-aspartate receptors by felbamate: insights into the mechanism of action. J Pharmacol Exp Ther. 1999;289:886–894. [PubMed] [Google Scholar]
- Kloda A, Clements JD, Lewis RJ, Adams DJ. Adenosine triphosphate acts as both a competitive antagonist and a positive allosteric modulator at recombinant N-methyl-D-aspartate receptors. Mol Pharmacol. 2004;65:1386–1396. doi: 10.1124/mol.65.6.1386. [DOI] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson PD, Baker R, Carling RW, Curtis NR, Moore KW, Williams BJ, Foster AC, Donald AE, Kemp JA, Marshall GR. Kynurenic acid derivatives. Structure-activity relationships for excitatory amino acid antagonism and identification of potent and selective antagonists at the glycine site on the N-methyl-D-aspartate receptor. J Med Chem. 1991;34:1243–1252. doi: 10.1021/jm00108a002. [DOI] [PubMed] [Google Scholar]
- Legendre P, Westbrook GL. Ifenprodil blocks N-methyl-D-aspartate receptors by a two-component mechanism. Mol Pharmacol. 1991;40:289–298. [PubMed] [Google Scholar]
- Lipton SA. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res. 2005;2:155–165. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D, Johnson KM. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol Sci. 1990;11:81–86. doi: 10.1016/0165-6147(90)90323-z. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj J, Rogoz Z, Skuza G, Kolodziejczyk K. Some central effects of GYKI 52466, a non-competitive AMPA receptor antagonist. Pol J Pharmacol. 1995;47:501–507. [PubMed] [Google Scholar]
- Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Lodge D. Phencyclidine receptors and N-methyl-D-aspartate antagonism: electrophysiologic data correlates with known behaviours. Pharmacol Biochem Behav. 1988;31:279–286. doi: 10.1016/0091-3057(88)90346-2. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2Aand NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Larson H. NR1 and NR2 subunit contributions to N-methyl-D-aspartate receptor channel blocker pharmacology. J Pharmacol Exp Ther. 1997;280:614–620. [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Morley RM, Tse HW, Feng B, Miller JC, Monaghan DT, Jane DE. Synthesis and pharmacology of N1-substituted piperazine-2,3-dicarboxylic acid derivatives acting as NMDA receptor antagonists. J Med Chem. 2005;48:2627–2637. doi: 10.1021/jm0492498. [DOI] [PubMed] [Google Scholar]
- Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, Vellano KM, Brauner-Osborne H, Liotta DC, Traynelis SF. Quinazolin-4-one derivatives: A novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J Med Chem. 2010;53:5476–5490. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, Santangelo R, Orr AG, Le P, Vellano KM, Liotta DC, Traynelis SF. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat Commun. 2010;1:1–8. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikam SS, Meltzer LT. NR2B selective NMDA receptor antagonists. Curr Pharm Des. 2002;8:845–855. doi: 10.2174/1381612024607072. [DOI] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- O’Hara PJ, Sheppard PO, Thogersen H, Venezia D, Haldeman BA, McGrane V, Houamed KM, Thomsen C, Gilbert TL, Mulvihill ER. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron. 1993;11:41–52. doi: 10.1016/0896-6273(93)90269-w. [DOI] [PubMed] [Google Scholar]
- Palygin O, Lalo U, Pankratov Y. Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br J Pharmacol. 2011;163:1755–1766. doi: 10.1111/j.1476-5381.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Neyton J, Ascher P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+ Neuron. 1995;15:1109–1120. doi: 10.1016/0896-6273(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Perin-Dureau F, Rachline J, Neyton J, Paoletti P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci. 2002;22:5955–5965. doi: 10.1523/JNEUROSCI.22-14-05955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic M, Sedlacek M, Cais O, Horak M, Chodounska H, Vyklicky L., Jr Pregnenolone sulfate modulation of N-methyl-D-aspartate receptors is phosphorylation dependent. Neuroscience. 2009;160:616–628. doi: 10.1016/j.neuroscience.2009.02.052. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Macaulay AJ, Hill RG, Kemp JA. Electrophysiological characterisation of the antagonist properties of two novel NMDA receptor glycine site antagonists, L-695,902 and L-701,324. Neuropharmacology. 1996;35:1573–1581. doi: 10.1016/s0028-3908(96)00141-4. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Ptak CP, Ahmed AH, Oswald RE. Probing the allosteric modulator binding site of GluR2 with thiazide derivatives. Biochemistry. 2009;48:8594–8602. doi: 10.1021/bi901127s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner C, Kohr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286:7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Rev. 2003;9:275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlacek M, Korinek M, Petrovic M, Cais O, Adamusova E, Chodounska H, Vyklicky L., Jr Neurosteroid modulation of ionotropic glutamate receptors and excitatory synaptic transmission. Physiol Res. 2008;57(Suppl 3):S49–57. doi: 10.33549/physiolres.931600. [DOI] [PubMed] [Google Scholar]
- Shim SS, Hammonds MD, Kee BS. Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site. Eur Arch Psychiatry Clin Neurosci. 2008;258:16–27. doi: 10.1007/s00406-007-0757-8. [DOI] [PubMed] [Google Scholar]
- Shiokawa H, Kaftan EJ, MacDermott AB, Tong CK. NR2 subunits and NMDA receptors on lamina II inhibitory and excitatory interneurons of the mouse dorsal horn. Mol Pain. 2010;6:26. doi: 10.1186/1744-8069-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Singh V. Meta-Analysis of the Efficacy of Adjunctive NMDA Receptor Modulators in Chronic Schizophrenia. CNS Drugs. 2011;25:859–885. doi: 10.2165/11586650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Strick CA, Li C, Scott L, Harvey B, Hajos M, Steyn SJ, Piotrowski MA, James LC, Downs JT, Rago B, Becker SL, El-Kattan A, Xu Y, Ganong AH, Tingley FD, 3rd, Ramirez AD, Seymour PA, Guanowsky V, Majchrzak MJ, Fox CB, Schmidt CJ, Duplantier AJ. Modulation of NMDA receptor function by inhibition of D-amino acid oxidase in rodent brain. Neuropharmacology. 2011;61:1001–1015. doi: 10.1016/j.neuropharm.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Suarez F, Zhao Q, Monaghan DT, Jane DE, Jones S, Gibb AJ. Functional heterogeneity of NMDA receptors in rat substantia nigra pars compacta and reticulata neurones. Eur J Neurosci. 2010;32:359–367. doi: 10.1111/j.1460-9568.2010.07298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CK, Kaftan EJ, Macdermott AB. Functional identification of NR2 subunits contributing to NMDA receptors on substance P receptor-expressing dorsal horn neurons. Mol Pain. 2008;4:44. doi: 10.1186/1744-8069-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev. 2011;63:59–126. doi: 10.1124/pr.109.002501. [DOI] [PubMed] [Google Scholar]
- Villmann C, Becker CM. On the hypes and falls in neuroprotection: targeting the NMDA receptor. Neuroscientist. 2007;13:594–615. doi: 10.1177/1073858406296259. [DOI] [PubMed] [Google Scholar]
- Volianskis A, Bannister N, Collett V, Irvine MW, Monaghan DT, Fitzjohn SM, Jensen MS, Jane DE, Collingridge GL. Induction of STP and LTP at CA1 synapses in the hippocampus is mediated by different NMDAR subtypes. Soc for Neuroscience (abstract) 2010:526.10. [Google Scholar]
- von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Kohr G, Seeburg PH, Monyer H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 2007;53:10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Williams K. Subunit-specific potentiation of recombinant N-methyl-D-aspartate receptors by histamine. Mol Pharmacol. 1994;46:531–541. [PubMed] [Google Scholar]
- Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol. 1994;45:803–809. [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- Zeevalk GD, Nicklas WJ. Developmental differences in antagonism of NMDA toxicity by the polyamine site antagonist ifenprodil. Brain Res Dev Brain Res. 1992;65:147–155. doi: 10.1016/0165-3806(92)90173-t. [DOI] [PubMed] [Google Scholar]