Abstract
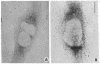
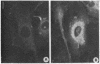
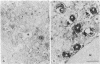
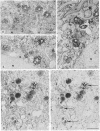
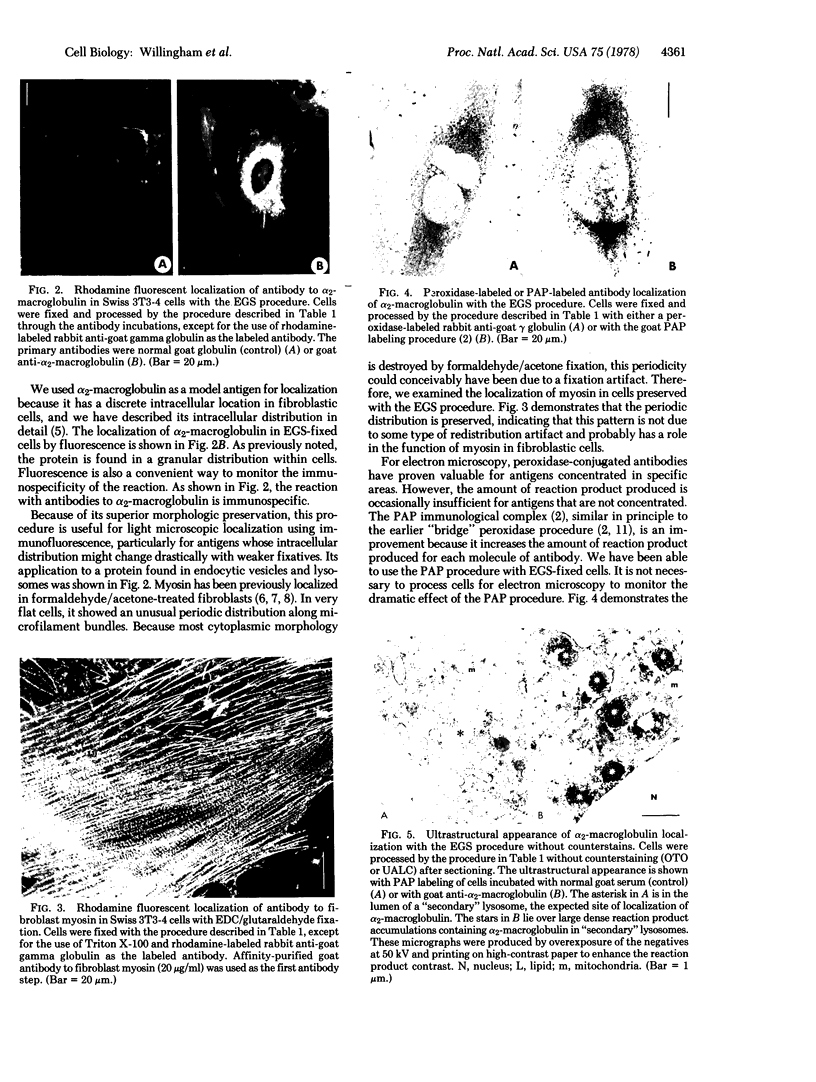
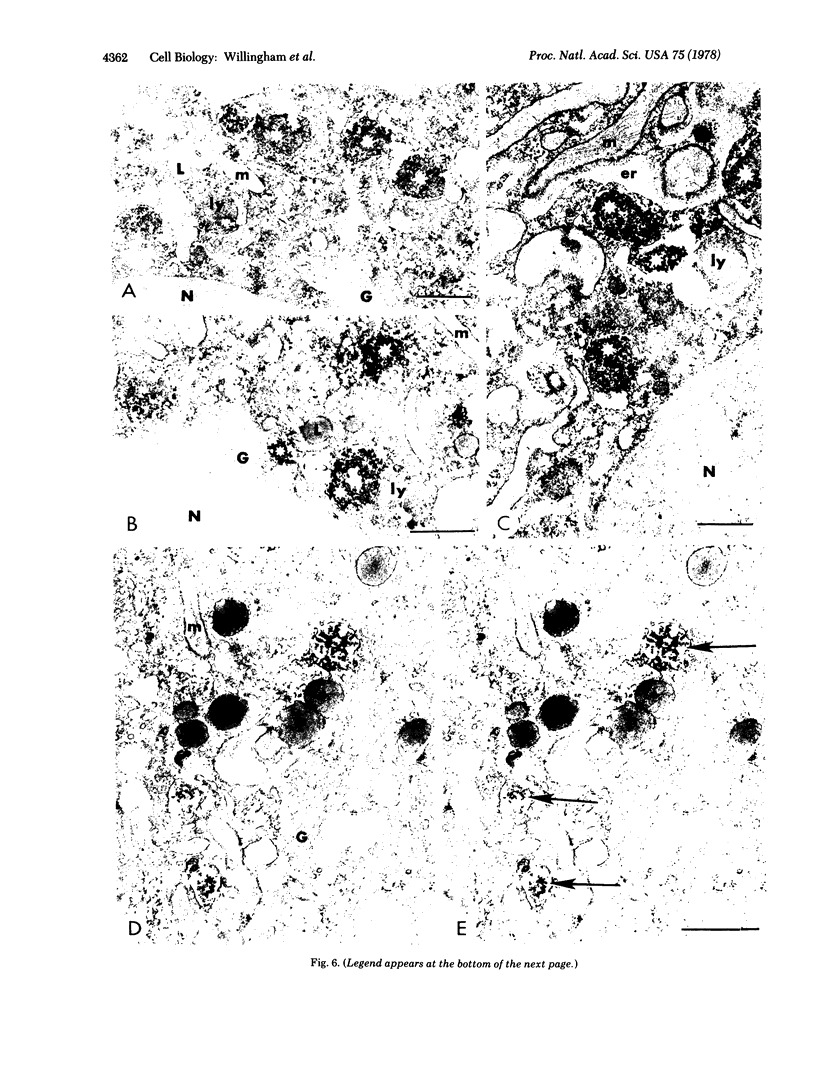
We have been developing a procedure for localizing intracellular antigens in cultured cells, by using peroxidase-labeled antibodies, that allows good morphologic preservation. Although useful, our previous technique did not preserve the morphology of membranes, and the location of the peroxidase reaction product was difficult to establish. In this paper, we report major improvements on the basic technique that markedly enhance the quality of localization and of morphology. Saponin is used to permeabilize membranes without destroying their morphology. The amount of reaction product is enhanced with a peroxidase-antiperoxidase label. The clarity of morphologic detail and contrast of reaction product density are increased by using postsectioning staining with the osmium/thiocarbohydrazide/osmium and uranyl acetate/lead citrate procedures. We have applied this technique to the ultrastructural localization of alpha2-macroglobulin and demonstrated that it is localized in membrane-limited vesicles. We have also used this method to improve the preservation of structures for localization by fluorescence microscopy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Mason T. E., Phifer R. F., Spicer S. S., Swallow R. A., Dreskin R. B. An immunoglobulin-enzyme bridge method for localizing tissue antigens. J Histochem Cytochem. 1969 Sep;17(9):563–569. doi: 10.1177/17.9.563. [DOI] [PubMed] [Google Scholar]
- Pastan I., Willingham M., Anderson W., Gallo M. Localization of serum-derived alpha 2 macroglobulin in cultured cells and decrease after Moloney sarcoma virus transformation. Cell. 1977 Nov;12(3):609–617. doi: 10.1016/0092-8674(77)90261-6. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman A. M., Wasserkrug H. L., Hanker J. S. A new staining method (OTO) for enhancing contrast of lipid--containing membranes and droplets in osmium tetroxide--fixed tissue with osmiophilic thiocarbohydrazide(TCH). J Cell Biol. 1966 Aug;30(2):424–432. doi: 10.1083/jcb.30.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Ostlund R. E., Pastan I. Myosin is a component of the cell surface of cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4144–4148. doi: 10.1073/pnas.71.10.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Cyclic amp and cell morphology in cultured fibroblasts. Effects on cell shape, microfilament and microtubule distribution, and orientation to substratum. J Cell Biol. 1975 Oct;67(1):146–159. doi: 10.1083/jcb.67.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Yamada K. M., Yamada S. S., Pouysségur J., Pastan I. Microfilament bundles and cell shape are related to adhesiveness to substratum and are dissociable from growth control in cultured fibroblasts. Cell. 1977 Mar;10(3):375–380. doi: 10.1016/0092-8674(77)90024-1. [DOI] [PubMed] [Google Scholar]