Abstract
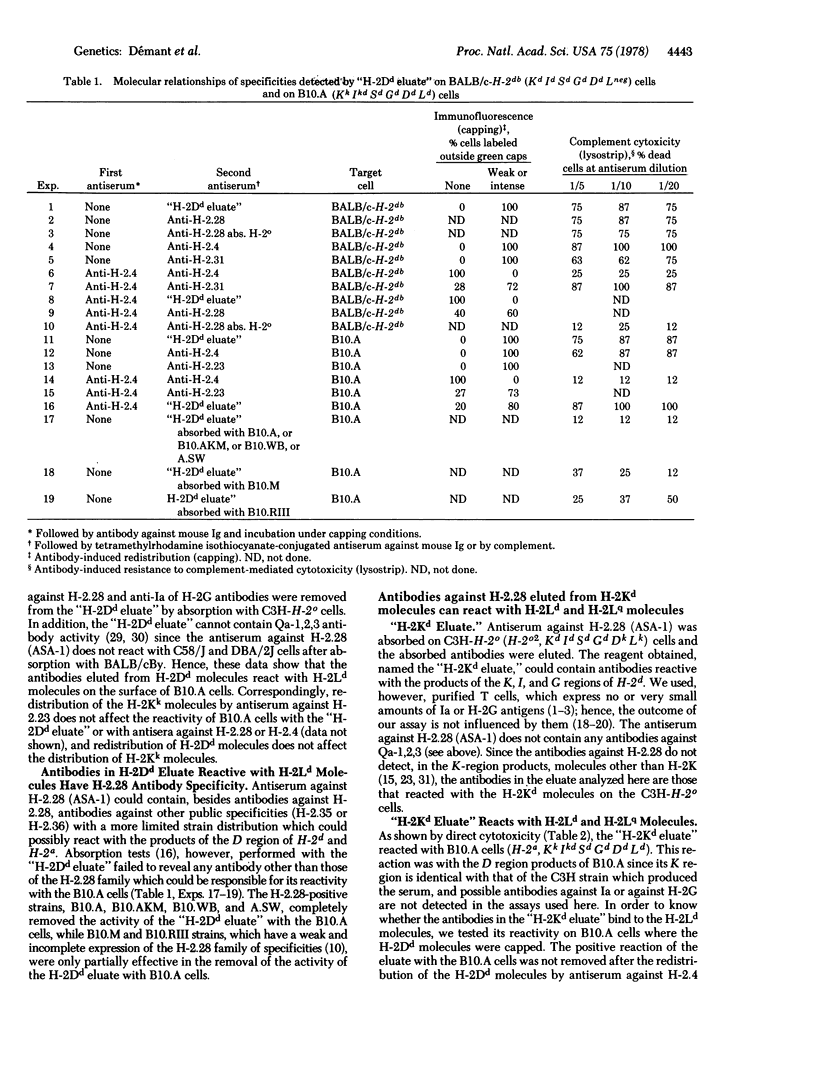
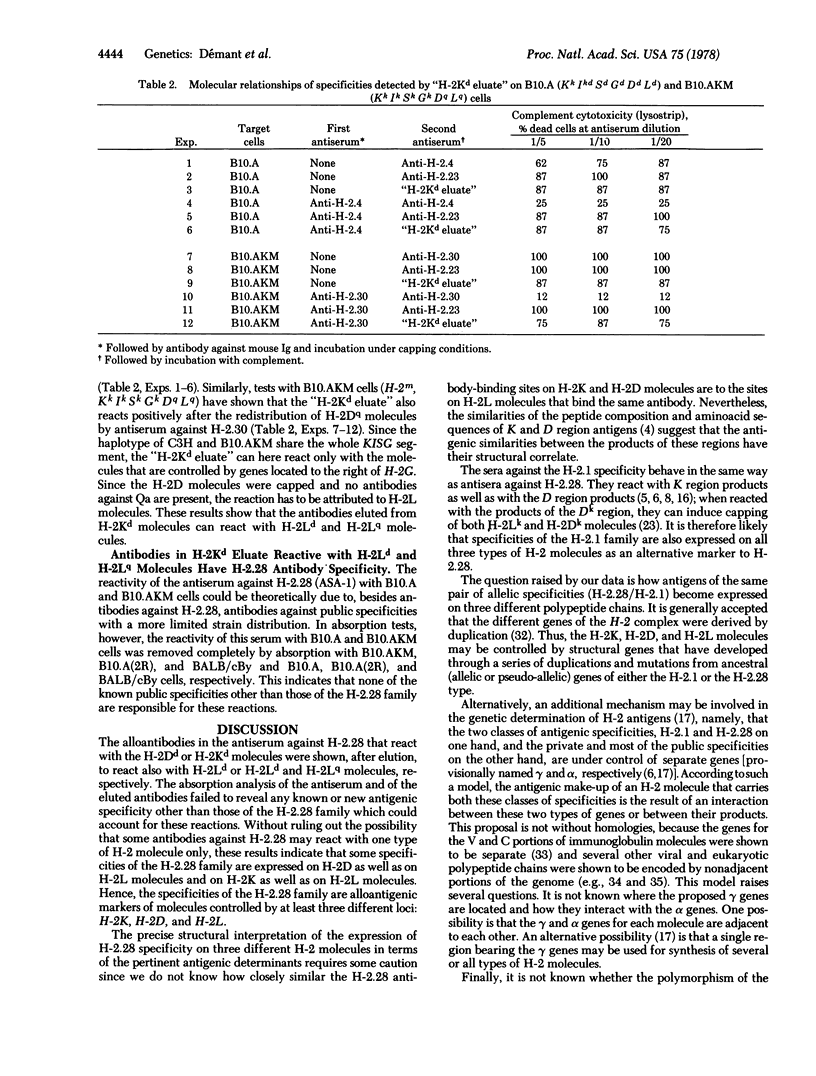
Each allele at the K or D region of the H-2 complex produces two kinds of "allelic" or mutually exclusive antigenic characteristics: its unique private specificity and a public specificity(ies) of either the H-2.28 or H-2.1 family. The private specificities of the K and D regions are expressed on H-2K and H-2D molecules, respectively. The D region produces another molecule, H-2L, which lacks the H-2K and H-2D private specificity but exhibits the H-2.28 or H-2.1 specificity. We analyzed the expression of the H-2.28 determinants on H-2K, H-2D, and H-2L molecules. When an antiserum against H-2.28 is used to sensitize cells where it can react with only H-2K molecules or H-2D molecules, by subsequent elution antibodies against H-2.28 are recovered that can also react with H-2L molecules. Hence, determinants reactive with antibodies against H-2.28 are present on H-2L as well as on H-2K and H-2D molecules. The expression of the H-2.28/H-2.1 polymorphism on all three known types of H-2 molecules, without some obvious relation to the private specificities, suggests that the antigenic determinants of these two kinds of allelic systems (private in contrast to H-2.28/H-2.1) may be controlled by separate genes, even when they are expressed on the same molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F. New genetic model for allelism at histocompatibility and other complex loci: polymorphism for control of gene expression. Transplant Proc. 1973 Dec;5(4):1471–1475. [PubMed] [Google Scholar]
- Colombani J., Colombani M., Shreffler D. C., David C. Separation of anti-Ia (I-region associated antigens) from anti-H-2 antibodies in complex sera, by absorption on blood platelets. description of three new Ia specificities. Tissue Antigens. 1976 Feb;7(2):74–85. doi: 10.1111/j.1399-0039.1976.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Nathenson S. G. Distribution of H-2 alloantigenic specificities on radiolabeled papain-solubilized antigen fragments. J Immunol. 1971 Aug;107(2):563–570. [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D., Nathenson S. G., Cherry M. The molecular basis of codominant expression of the histocompatibility-2 genetic region. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1394–1397. doi: 10.1073/pnas.69.6.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Démant P. H-2 gene complex and its role in alloimmune reactions. Transplant Rev. 1973;15:162–200. [PubMed] [Google Scholar]
- Démant P., Snell G. D., Cherry M. Hemagglutination and cytotoxic studies of H-2. 3. A family of 3-like specificities not in the C crossover region. Transplantation. 1971 Mar;11(3):242–259. [PubMed] [Google Scholar]
- Hansen T. H., Cullen S. E., Melvold R., Kohn H., Flaherty L., Sachs D. H. Mutation in a new H-2-associated histocompatibility gene closely linked to H-2D. J Exp Med. 1977 Jun 1;145(6):1550–1558. doi: 10.1084/jem.145.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. H., Cullen S. E., Sachs D. H. Immunochemical evidence for an additional H-2 region closely linked to H-2D. J Exp Med. 1977 Feb 1;145(2):438–442. doi: 10.1084/jem.145.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptfeld V., Klein J. Molecular relationship between private and public H-2 antigens as determined by antigen redistribution method. J Exp Med. 1975 Aug 1;142(2):288–298. doi: 10.1084/jem.142.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Klein J., Shreffler D. C. The H-2 model for the major histocompatibility systems. Transplant Rev. 1971;6:3–29. doi: 10.1111/j.1600-065x.1971.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Shreffler D. C. Cross reactivity between H-2K and H-2D products. II. Identification of the cross reacting specificities. Transplantation. 1975 Dec;20(6):443–456. doi: 10.1097/00007890-197512000-00002. [DOI] [PubMed] [Google Scholar]
- Neauport-Sautes C., Lilly F., Silvestre D., Kourilsky F. M. Independence of H-2K and H-2D antigenic determinants on the surface of mouse lymphocytes. J Exp Med. 1973 Feb 1;137(2):511–526. doi: 10.1084/jem.137.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neauport-Sautes C., Morello D., Freed J. H., Nathenson S. G., Démant P. The private specificity H-2.4 and the public specificity H-2.28 of the D region are expressed on two independent polypeptide chains. Eur J Immunol. 1977 Aug;7(8):511–515. doi: 10.1002/eji.1830070804. [DOI] [PubMed] [Google Scholar]
- Neauport-Sautes C., Morello D., Lemonnier F., Demant P. Relationships between private and public specificities controlled by the H-2D region. Transplant Proc. 1977 Mar;9(1):653–655. [PubMed] [Google Scholar]
- STIMPFLING J. H., RICHARDSON A. RECOMBINATION WITHIN THE HISTOCOMPATIBILITY LOCUS OF THE MOUSE. Genetics. 1965 May;51:831–846. doi: 10.1093/genetics/51.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Snell G. D., Cherry M., Démant P. Evidence that H-2 private specificities can be arranged in two mutually exclusive systems possibly homologous with two subsystems of HL-A. Transplant Proc. 1971 Mar;3(1):183–186. [PubMed] [Google Scholar]
- Snell G. D., Cherry M., Démant P. H-2: its structure and similarity to HL-A. Transplant Rev. 1973;15:3–25. doi: 10.1111/j.1600-065x.1973.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Snell G. D., Cherry M. Haemagglutination and cytotoxic studies of H-2 IV. Evidence that there are 3-like antigenic sites determined by both the K and the D crossover regions. Folia Biol (Praha) 1974;20(2):81–100. [PubMed] [Google Scholar]
- Snell G. D., Démant P., Cherry M. Haemagglutination and cytotoxic studies of H-2. V. T-e anti-27, 28, 29 family of antibodies. Folia Biol (Praha) 1974;20(3):145–167. [PubMed] [Google Scholar]
- Snell G. D., Démant P., Cherry M. Hemagglutination and cytotoxic studies of H-2. I. H-2.1 and related specificities in the EK crossover regions. Transplantation. 1971 Mar;11(3):210–237. doi: 10.1097/00007890-197103000-00002. [DOI] [PubMed] [Google Scholar]
- Wigzell H., Andersson B. Cell separation on antigen-coated columns. Elimination of high rate antibody-forming cells and immunological memory cells. J Exp Med. 1969 Jan 1;129(1):23–36. doi: 10.1084/jem.129.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]


