Abstract
Adenosine accumulates in inflammation and ischemia but it is more than an end-product of ATP catabolism. Signaling through different receptors with distinct, cell-specific cytoplasmic pathways, adenosine is now recognized as an inducible switch that regulates the immune system. By acting through the A2AAR, adenosine shapes T cell function, largely by conferring an anti-inflammatory tone on effector Th cells (Teff) and natural killer (NK)T cells. In contrast, both the A2AAR and A2BAR are expressed by antigen-presenting cells (APC) which have been shown to regulate innate responses and the transition to adaptive immunity. There is also emerging evidence that adenosine production is one mechanism that allows some pathogens as well as neoplasms to evade host defenses. This review discusses the immunoregulatory functions of adenosine and some of the interactions it may have in regulating host–microbial interactions.
Keywords: Adenosine, Adenosine receptor, Lymphocyte, Dendritic cell, Macrophage, Immune evasion
Extracellular adenosine concentrations are carefully regulated
Extracellular synthesis of adenosine is catalyzed mostly by two membrane-bound enzymes: nucleoside triphosphate diphosphohyrdolase (ecto-NTPDase-1 or CD39) and 5′-ribonucleotide phosphohydrolase (CD73). CD39 is the rate limiting enzyme that mediates the dephosphorylation of ATP to ADP and then to 5′-AMP; CD73 subsequently catalyzes the conversion of 5′-AMP to adenosine [1]. As ATP can be pro-inflammatory [2] its destruction complements the anti-inflammatory effects that may be associated with the accumulation of adenosine [3]. While these enzymes are expressed by regulatory helper T cells (Treg) and contribute to the control of inflammation [4–7], one or both (or functional homologues) may be expressed by some tumors [8] or bacteria [9]. As discussed in detail below, the expression by tumors and bacteria creates a local environment of immune suppression that allows these cells to inhibit host responses and thus, favor their own growth.
An extracellular membrane-bound or soluble adenosinolytic pathway is also available to regulate adenosine signaling. Adenosine deaminase (ADA) rapidly deaminates adenosine to inosine, which subsequently regenerates AMP via adenosine kinase. ADA1 and ADA2 isoforms, and a closely related protein [10] may all regulate adenosine concentrations. The primary role of ADA1 is to eliminate intracellular toxic derivatives of adenosine and deoxyadenosine. Although ADA1 does not express a signal sequence, it is measurable in extracellular fluids and can bind on cell surface via dipeptidyl peptidase IV (CD26) [11]. Moreover, a non-enzymatic co-stimulatory role for ADA1 within the immunological synapse has been suggested [12]. The second isoform—ADA2—is more soluble in water due to its extensive glycosylation and prefers an acidic pH requirement for adenosine deaminase activity [1, 13]. ADA2, like ADA1, has also been reported to bind proteoglycans and adenosine receptors and promotes T cell proliferation independently of its enzymatic activity [14]. Such an elaborate catalytic system suggests that adenosine is a mediator whose local concentration is carefully regulated in order to control the function of adjacent cells.
The pleiotropic effects of adenosine are mediated through distinct receptor isoforms
Adenosine binds through the P1 family of purine receptors. There are four known adenosine receptor subtypes encoded by separate genes. The A2AAR is a high affinity, Gs-protein-coupled receptor that directly stimulates adenylyl cyclase, thereby increasing intracellular cAMP levels [15, 16]. Downstream intracellular events include the activation of cAMP sensors, such as cAMP-dependent protein kinase (PKA). There are multiple substrates for PKA that confer the effects of cAMP on various transcription factors. While classically cAMP was thought to activate PKA, it is now apparent that it also regulates other cAMP sensors including the exchange protein directly activated by cAMP (Epac) [17, 18]. Epac initiates a large number of cAMP-mediated events including cell proliferation, cell survival, secretion, and Ca2+ metabolism [17, 18]. While cAMP may be a common change in a cell's signaling pattern, many different outcomes may emerge due to the subsequent signaling events mediated through PKA or Epac.
The A2AAR is expressed by different immune and inflammatory cells including neutrophils, antigen-presenting cells, and T and B cells and in general, confers an anti-inflammatory tone [19]. A2AAR are the most abundant adenosine receptors in human or murine CD4+ T cells [20–22] and activation of these cells causes an early A2AAR mRNA upregulation [23, 24]. A2AAR are also upregulated in human, gastric lymphocytes subsequent to activation [23]. As discussed in more detail below, engagement of these receptors inhibits the production of several proinflammatory cytokines.
The A2BAR is a low affinity receptor that appears to be absent in both peripheral and mucosal T cells [23]. This receptor can be coupled to two different G proteins—Gs and Gq—which enable it to induce cAMP and regulate host responses through other pathways controlled by the Gq protein [25, 26]. A2BAR are the most prominent subtype on dendritic cells (DC) after in vitro maturation using GM-CSF and IL-4 [27], but the expression of adenosine receptor subtypes is modified by several factors including LPS challenge. LPS induces an increase in both A2AAR and A3AR expression although the latter to a lesser extent while A2BAR mRNA decreases but remains present [27, 28]. One significant trigger of A2BAR expression is hypoxia [28, 29] which is a consequence of tissue injury. Despite the upregulation of A3AR, most of the effects of adenosine on DC appear to be mediated through the A2A or A2BAR. The net effect on cellular responses will reflect the relative number of each adenosine receptor isoform and the regulation of the downstream signaling molecules linked to these receptors.
Other cells also have been shown to express various receptor subtypes. For example, murine splenic and hepatic invariant NKT cells express all four adenosine receptor subtypes with A2AAR being the most abundant [30]. While some studies suggest the A1AR and A3AR play a role in immune regulation [31–33], others have not shown them to have a major impact on immune functions [34]. It should be remembered that the antibodies to these receptors are of limited use and most expression studies rely on studies of mRNA or in some cases, ligand-binding assays. These approaches are open to error due to contaminating cells or any limitations in receptor specificity that emerge attributable to the concentration of agonists used or their affinity for a particular receptor. Functional studies require careful examination of cells from mice that do or do not express the receptor as well as complementary pharmacological approaches.
All four receptor subtypes are expressed by lineages of cells other than immune or inflammatory so any effect of adenosine on the host response may include an indirect component. For example, the A3AR is abundant in the myenteric nerve plexus and it is involved in mechanisms regulating intestinal motility [35]. A3AR KO mice are protected from DSS-induced colitis and this in part, may be attributable to the effects of this receptor on nerve function [35]. In addition, adenosine receptors are found on fibroblasts, heart tissue, vascular smooth muscle, as well as nerves and can regulate fibrosis, blood pressure, and several neurological functions [25]. It is evident that any attempt to use adenosine analogs as a therapeutic strategy will have to consider the potential side effects due to a lack of receptor specificity or the effects of these drugs on other cell lineages.
Regulation of innate responses by adenosine
Following an infection or in response to ischemia, many of the innate responses are mediated by neutrophils, monocytes, and NK cells and adenosine has been shown to regulate several of their responses. For example, ischemia–reperfusion injury can be prevented in the liver [36], kidney [37], nervous system [38], and heart [38] by the administration of adenosine. The major target for adenosine in ischemia–reperfusion is the A2AAR expressed by NKT cells [39]. In DC, adenosine limits the inflammatory potential of LPS by inhibiting the release of pro-inflammatory cytokines such as IL-12 and TNF-α while increasing the expression of the anti-inflammatory cytokine IL-10 [27, 40]. The induction of IL-10 may account for some of the anti-inflammatory responses through negative feedback [27] although in other reports, the anti-inflammatory effects of adenosine are independent of IL-10 [41]. Genetic (A2BAR KO DC) and pharmacological (selective A2BAR blocking) approaches have shown that the effects of adenosine on TNF-α, IL-12, and IL-10 can be mediated by A2BAR [27]. The effects of adenosine on TNF-α, IL-12, and IL-10 have also been attributed to the A2AAR [28] or A1AR [31]. These differences in receptor-mediated effects of adenosine may be explained by the use of varied antigen-presenting cell preparations for example, using dissimilar DC maturation processes and/or different agonists. Alternatively, drugs with implied selectivity may be used at concentrations that interact with other receptor subtypes. Therefore, it is helpful when one approach is validated with another.
Adenosine modulates the intercellular signaling mediated by accessory molecules
In addition to cytokines, adenosine regulates the expression of surface molecules that modulate T cell responses and the transition from innate to adaptive immunity. While LPS stimulates the maturation of DC and an increase in the expression of CD80, CD86 as well as class I and II HLA/ MHC molecules, adenosine can inhibit these changes [27, 40]. Similarly, blocking ADA, which degrades adenosine, has been reported to enhance the expression of CD86, IL-12p70, and TNF-α in DC stimulated with poly(I:C) [31]. Interestingly, bone marrow-derived DC treated with adenosine analogs may be further divided according to CD86 expression level into two functionally distinct sets: a CD86lo population in which A2BAR is dominant, and responds to LPS and adenosine analogs by increasing IL-10, but not TNF-α and IL-12, and a CD86hi population which is A2AAR dominant and responds to these stimuli by increasing TNF-α and IL-12 [27]. These two subpopulations differ in their ability to activate Th cell proliferation and cytokine production with the CD86lo population being decidedly less immunogenic as they fail to trigger IL-2 secretion by OVA-specific CD4+ T cells [27]. Adenosine also suppresses DC-driven CD8+ T cell proliferation and differentiation to IFN-γ producing cells [42].
Other studies have shown that adenosine decreases the proliferative responses of Th cells in co-cultures with DC [43]. A2AAR expressed by the APC and the T cell contribute to the final effect, as suggested by co-culture experiments using A2AAR KO DC and/or A2AAR KO Th cells together with A2AAR agonist and/or antagonist [44]. A2AAR stimulation also prevented CD25 (IL-2R α chain) and co-stimulatory CD40L upregulation on the T cell surface [44]. In a complementary approach, the addition of the adenosine-degrading enzyme ADA causes similar responses supporting the conclusion that adenosine inhibits T proliferation and cytokine production [45]. Furthermore, there are data suggesting that adenosine imparts a functional memory on antigen-specific Th cell responses. This conclusion is supported by the observation that after priming DC-Th cell co-cultures with antigen and an A2AAR agonist, Th cells remain non-responsive upon restimulation with antigen alone [46].
Adenosine modulates T cell function directly
While adenosine can regulate APC and their ability to control T cell function, there are also direct effects of adenosine on T cells. The administration of A2AAR agonists during activation inhibits the induction of several pivotal mediators including IL-2, Th1 (IFN-γ, TNF-α) and Th2 (IL-4) cytokines in peripheral lymphocytes and gastric lamina propria CD4+ T cells [20, 23, 24, 46–49]. Similarly, it was shown that in the presence of either Th1 (IL-12, anti-IL-4) or Th2 (IL-4, anti-IFN-γ) polarizing conditions, selective A2AAR agonists decreased αCD3/CD28 stimulated IL-2, Th1 (IFN-γ) or Th2 (IL-4, IL-5, IL-10) responses [50–52]. Effects of A2AAR on IFN-γ and IL-2 are mediated at least in part, through a reduction in mRNA stability [53]. A2AAR were also implicated in limiting proliferation based on results obtained using selective antagonists or A2AAR KO mice [46].
In CD8+ cytotoxic T lymphocytes, A2AAR and A2BAR, but not A1AR or A3AR mRNA, have been detected. The A2AAR inhibits cytotoxic T lymphocyte degranulation and cytotoxicity by upregulating intracellular cAMP and suppressing TCR-triggered FasL mRNA upregulation [16, 22, 54, 55]. Data from splenic T cells show that A2AAR stimulation inhibits the activation of the Zap-70 tyrosine kinase T cell activation pathway and upregulates inhibitory PD-1 and CTLA-4 [44]. Indeed, data on Zap-70 were verified and attributed mainly to CD8+ T cells [42]. The increase in cAMP and the subsequent activation of PKA appears to account for these actions of adenosine [54]. Furthermore, adenosine or specific A2AAR agonists also downregulate IFN-γ secretion [42, 56] in CD8+ T cells. It is noteworthy that late addition of non-specific adenosine or specific A2AAR agonist days after culture with allogenic spleen cell suspension cannot suppress CD8+ cell proliferation and cytokine secretion probably due to the fact that the cytotoxic phenotype has already been induced [57]. There is also evidence that exposure to adenosine drives CD8+ cells toward a phenotype resistant to adenosine [57].
The association of adenosine with regulatory T cell function
Regulatory helper T cells (Treg) have emerged as important cells in the control of host responses and autoimmunity. The role of adenosine in Treg was first studied using an adoptive transfer model in which naïve, splenic CD4+CD45RBhi Th cells placed into immunodeficient mice cause colitis [58]. Co-transfer of Treg of a CD4+CD45RBlo or a CD4+CD25+ phenotype protects from colitis [59]. Using this adoptive transfer model, Treg failed to protect recipients from colitis when the pathogenic Teff cell lacked the A2AAR [53]. Furthermore, Treg also express the A2AAR and respond to adenosine with an increase in their number and/or their function [46]. Again using the adoptive transfer model, Treg failed to protect recipients from colitis if they lacked the A2AAR [53]. These data support the notion that adenosine regulates host responses to infection since the colitis in this model is driven by the luminal microbiome. A role for adenosine in the selection of Treg is supported by other studies showing that T cells activated by allogenic cells are driven by adenosine acting through the A2AAR to a Treg phenotype, expressing surface CD25 and LAG3 [44, 46]. Thus, signaling through A2AAR on both Treg and Teff is crucial for optimal anti-inflammatory action conferred by Treg.
The role for adenosine in Treg function was advanced significantly by several laboratories with data demonstrating that most Treg in mice or humans express CD39 and CD73 which would enable them to synthesize adenosine as an anti-inflammatory mediator [4–6, 43] (Fig. 1). In other studies, undifferentiated/naive CD4+CD44lo mouse spleen T cells (IL-2, MIP-1α but not IFN-γ secretors) lack CD73, and thus cannot synthesize adenosine, while minimally differentiated CD4+CD44+CCD25- Thpp cells—which are believed to be the next step toward Th or Treg differentiation—do express CD73 [51]. Robust CD73 expression coincides with differentiated Treg defined by the presence of cytoplasmic Foxp3 and membrane CD25 expression, TGF-β1 (but not IL-2 or IFN-γ) secretion and the ability to inhibit lymphocyte proliferation [4, 43, 51, 60]. Nearly all spleen and peripheral blood CD4+CD39+ Th cells resemble the phenotype of Treg by being CD25hi, CD45RBlo, mostly CD62Llo, express high levels of Foxp3, GITR, CTLA-4, IL-10 mRNA, and suppress Teff proliferation, while CD4+CD39- do not [4, 61–63]. CD73 and CD39 are often co-expressed by T cells in mouse spleen or lymph nodes as well as by T cells from human tissues [4–6, 51, 64]. The CD39/CD73 machinery is also functional in Tr1 cells, a subset of Th induced by IL-10 that do not express neither CD25 nor Foxp3, but have immunoregulatory properties [65]. Together, these observations suggest that CD39 is an important marker of Treg and portends the role of adenosine in the immunosuppressive functions of these cells [62].
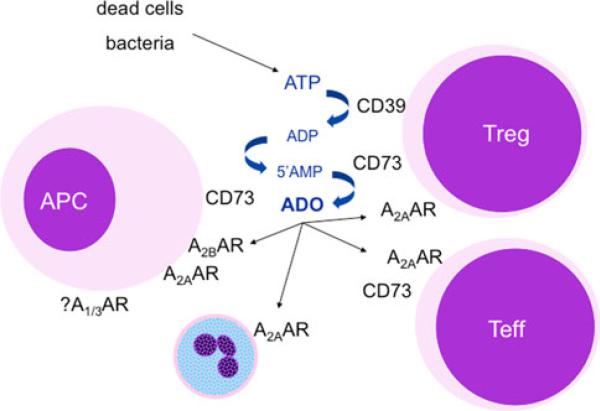
Fig. 1.
Adenosine synthesis and control of host responses to limit tissue damage. ATP can accumulate in areas of inflammation or hypoxia from dead cells or bacteria [2]. The metabolism of ATP can be achieved by CD39 and CD73 leading to the accumulation of adenosine (ADO). These enzymes are expressed differentially by various cells but both are found on Treg and contribute to their regulatory function. Subsequently, the adenosine can interact with any of the four receptor subtypes, primarily the A2A and A2BAR but possibly A1AR and A3AR as well. The short half-life of adenosine requires a close juxtaposition among cells producing and responding to its signals suggesting its effects are largely paracrine or autocrine. This figure is modified from [26]
Production of adenosine as a mechanism of immune evasion
While the concept that adenosine provides a feedback to limit the tissue damage mediated by a host response, it is possible, and indeed likely, that it can confer a detrimental degree of immunosuppression. For example, pioneering work by Sitkovsky and colleagues suggest adenosine offers protection to various neoplasms by inhibiting tumor immunity [8, 22]. Moreover, CD73 knockdown with si-RNA, as well as CD73 or A2AAR blocking, reverses the inhibition of T cell proliferation and prevents T cell apoptosis caused by ovarian carcinoma cells. These treatments targeting CD73 render the tumors vulnerable to cytotoxic lymphocytes [66]. Results were verified in vivo with adoptive transfer experiments that showed improvement on both mouse survival and tumor burden if tumor cells did not express CD73 [66]. CD39+ adenosine-producing cells in follicular lymphomas have also been implicated in T cell anergy observed in those tumors [67].
The immunosuppressive effects of potential detriment to the host are not limited to tumor growth. As described by Dr. Stanley Falkow, there are several criteria that determine if a microbe is a pathogen [68]. These include their ability to: breach host cell barriers; evade, subvert, or circumvent host responses and; cause damage. It is increasingly apparent that host factors enabling infection or causing tissue damage are key elements of the pathogenesis. Thus, infections that are harmless in some cohorts are more severe, or even devastating in those with altered immune reactivity.
Since the immune response contributes to microbial pathogenesis, it follows that treatment with anti-inflammatory drugs may decrease tissue damage and disease. For example, A2AAR agonists prevent inflammation and disease induced by C. difficile [69]. One study reported that an A2AAR agonist conferred dose-sensitive protection of mice against lethal challenge with endotoxin [70]. However, adenosine has been suggested to exacerbate sepsis [71]. This leads to the possibility that adenosine may favor the pathogenesis of a pathogen if it is present at the correct time or context.
The adenosine-A2AAR axis has been implicated as having an important role in immune regulation. However, it should be pointed out that mice lacking the A2AAR or CD73 are remarkably healthy—until provoked. Infection with Helicobacter pylori provides an interesting example of the interaction between an organism and the host adenosine response. H. pylori infection occurs in the majority of humanity with relatively mild consequences [72–75]. However, in individuals with genetic traits that exacerbate the inflammatory response, gastric cancer is more likely to occur [73, 76]. The onset of disease caused by gastroenteric infections is often associated with genes that enhance the expression or function of bacterial receptors or host cytokines [77]. CD73 KO mice appear quite healthy but when infected with Helicobacter felis—a model of H. pylori infection—they develop a more severe gastritis with increased levels of IL-1, TNF-α, and IFN-γ mRNA in gastric tissue than wildtype mice. Moreover, adoptive transfer of Treg from CD73 KO mice that cannot produce adenosine failed to prevent gastritis caused by the simultaneous transfer of Teff, while wildtype Treg effectively prevented disease [6]. Further evidence for the protective role of adenosine in bacterial gastritis comes from data indicating that gastric pathology associated with Helicobacter felis is more severe in A2AAR KO mice [23]. In addition, feeding an A2AAR agonist to IL-10 deficient mice suffering from gastritis due to Helicobacter pylori, attenuated gastritis and lowered TNF-α and IFN-γ in the gastric mucosa, but led to increased bacterial colonization [23]. These data suggested that when adenosine inhibits host responses, it favors persistent infection.
Other recent studies examining the pathogenesis of an array of bacteria suggest that manipulation of the host's adenosine signaling pathways to benefit the pathogen may be more common than previously appreciated. The Gram-positive pathogens Staphylococcus aureus, Bacillus anthracis, Enterococcus faecilis, and S. epidermidis all produce adenosine from AMP via adenosine synthase A (AdsA), an extracellular 5′-nucleotidase that is covalently linked to the cell wall of the bacterium [9] (Fig. 2). Relative to AdsA-knockout mutants, wildtype S. aureus survive better in the blood, resist killing by neutrophils and ultimately, induce greater abscess formation in the kidneys of intravenously infected mice. Similarly, B. anthracis that produce adenosine with AdsA also resist killing within blood. From these observations it has been hypothesized that bacterially produced adenosine alters the function of cells of the innate immune system, likely signaling through the A2AAR to reduce neutrophil and/or macrophage bactericidal activation to promote the early establishment of infection. As noted above, adenosine signaling through A2AAR has been demonstrated to inhibit secretion of IL-12, TNF-α, neutrophil degranulation [78], adhesion of phagocytes to vascular surfaces [79], and the oxidative burst [80]; all of which have been implicated in the clearance of bacterial pathogens. Interestingly, adenosine is also a germinant that promotes the transition of dormant B. anthracis spores into rapidly dividing vegetative cells [81]. Dormant spores are unable to be destroyed by phagocytes, but are quickly eliminated upon germination [82], thus B. anthracis spores can remain protected in a dormant state in host tissues for weeks to months. Resultantly, one may speculate that the spores remain quiescent until an adenosine-rich environment is sensed, signaling a convalescent anti-inflammatory state within the host. Within this anti-inflammatory environment, it would be predicted that B. anthracis would be less likely to be killed by highly activated phagocytes, thus promoting early bacterial growth by properly timing bacterial outgrowth.
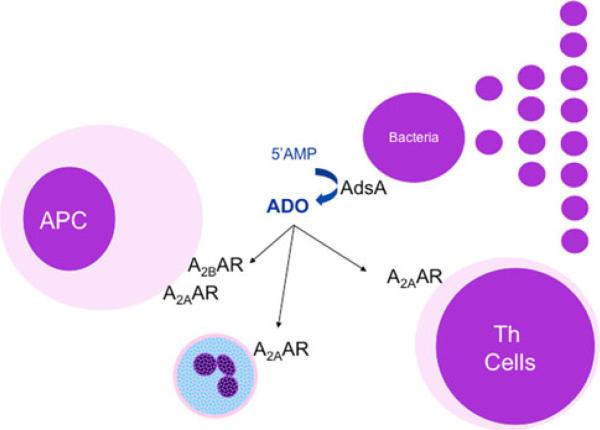
Fig. 2.
Control of host responses by adenosine-producing bacteria. As the immunological functions include the impairment of host responses, one might predict that microbes have developed ways to synthesize adenosine and favor their colonization. Indeed, Gram-positive bacteria express an extracellular 5′-nucleotidase, AdsA, that converts 5′AMP to adenosine (ADO) in a manner similar to CD73. Subsequently, local immune/inflammatory cells are inhibited and this local immune suppression favors the expansion and colonization of the organism
There is also evidence that some pathogens utilize extracellular ATP and its derivatives to cause disease and/ or subvert the immune system. Both Aeromonas sobria and enteropathogenic E. coli (EPEC) have been found to increase extracellular ATP release from cultured T84 monolayers [83, 84]. ATP is released from host cells by the secretion of a hemolysin by Aeromonas, whereas the mechanism for EPEC is less well understood, but is frequently attributed to its type III secretion system. The extracellular ATP can be converted by host 5′-ectonucleotidases to adenosine which causes A2BAR signaling and raises host intracellular cAMP levels. High levels of cAMP subsequently activate the cystic fibrosis transmembrane conductance regulator, which increases Cl- secretion into the lumen of the intestine [85], causing diarrhea through its effects on the A2BAR [86]. The cAMP response was independent of cell death, as both low concentrations of hemolysin and non-cytotoxic mutants of EPEC are still able to stimulate ATP release. In a similar fashion, several other pathogens have been shown to secrete enzymes which modify purinergic signaling for both adenosine receptors and the P2X7 receptors. The latter receptors sense extracellular ATP and can lead to macrophage lysis via pore formation. Burkholderia cepacia, Pseudomonas aeruginosa, and Vibrio cholerae all secrete 5′-nucleotidases, nucleoside diphosphate kinases, and adenylate kinases [87–89], which can alter the balance between adenosine and its multiple-related phosphorylated metabolites. The main function for the secreted enzymes is to alter the concentration of ATP to accelerate macrophage death via P2X7. These studies also demonstrate that the adenosine concentrations are greatly increased by these enzymes, but the authors did not investigate the role of adenosine receptor signaling in pathogenesis [87]. It is interesting to note that many of the pathogens that stimulate adenosine signaling can also raise cAMP levels through adenylate cyclase toxins, such as ExoY in P. aeruginosa, edema toxin in B. anthracis, and cholera toxin. It remains to be determined if and how these toxins are leading to independent effects from adenosine receptor-mediated cAMP production, but the findings suggest that there may be convergence between the targeting of cAMP production by toxins with that of adenosine receptors.
Since adenosine is involved with the pathogenesis of many bacteria, several groups have begun to investigate nucleoside analogs as a potential therapeutic. Since B. anthracis spores germinate in response to purines, including adenosine, work from Akoachere et al. explored germination inhibitors based on purine analogs [90]. Their work identified several analogs with alterations at the 2 and 6 positions that inhibited spore germination in liquid culture. However, the germination environment encountered by a spore interacting with a phagocyte is more complex than within liquid culture, potentially explaining why only one of these inhibitors—6-thioguanosine—was able to protect RAW264.7 macrophage-like cells from being killed by bacteria arising from spores. Yet, adding germinants after infection significantly exacerbated the infection. Additionally, pretreatment of mice with nebulized l-alanine + adenosine + casamino acids 1 h before infection to promote precocious spore germination also led to greater mouse mortality. The authors suggest this is due to toxin production from the recently germinated spores, leading to a breakdown in the pulmonary tissue and ultimately allowing more bacteria to reach the bloodstream. Indeed, many published works have shown that spores generally do not germinate on the mucosal surface of the lung except when delivered in a large bolus [91, 92]. It should be noted, however, that Cote et al. did not look at the effect of inhibiting germination with 6-thioguanosine, but with d-alanine [93].
The recent reports of bacteria utilizing host adenosine to cause damage and increase survival have opened new potential to use adenosine receptor antagonists as a treatment. Although, several Gram-positive bacteria have been implicated as using ectonucleotidases as a virulence factor, the targeted adenosine receptors have not been identified. It has been reported that the pathogenesis of Salmonella is attenuated in mice lacking the A2BAR or treated with A2BAR antagonists [94, 95]. No studies have investigated using A2BAR antagonists to prevent diarrhea from either EPEC or Aeromonas infection. Thus, there is the potential for adenosine receptor antagonists to ameliorate bacterial burden or disease in situations where the bacteria are manipulating the host's adenosine signaling pathways. The use of A2AAR agonists to decrease inflammation, tissue damage, and disease in models of C. difficile [69] and sepsis [96] will have to be used with the caveat that their ability to inhibit the host response may favor colonization by other bacteria.
Conclusions
Adenosine has pleiotropic effects on most levels of the immune and inflammatory response. Membrane-bound adenosine producing and catabolizing enzymes add to the local control of adenosine concentrations. Four different receptors are linked to cytoplasmic pathways with A2AAR and A2BAR being predominant and responsive on cells involved in regulating the host response. Adenosine limits exaggerated effects of host responses that contribute to tissue damage. In animal models, targeting the A2AAR has had profound beneficial effects as an anti-inflammatory and preventing ischemia–reperfusion injury that could be of enormous benefit in transplantation or the management of ischemic heart disease. However, some cells, such as tumors and bacteria, may produce adenosine as a means to evade the immune system and favor their survival. Thus, in developing adenosine receptor agonists or antagonists, more information is needed so their design will maximize the intended effect without incurring adverse reactions that might include fibrosis, tumor survival, or enhanced colonization of pathogens.
Acknowledgments
This work was supported by NIH grants AI 070491 to PBE and IJG and AI 079145 to PBE. DL is supported by T32AI007046.
Abbreviations
- AdsA
Adenosine synthase A
- αCD3/CD28
Anti-CD3/anti-CD28
- ADA
Adenosine deaminase
- APC
Antigen-presenting cells
- AR
Adenosine receptor
- DC
Dendritic cell
- KO
Knock-out
- NKT
Natural killer T cell
- TcR
T cell receptor
- Teff
Effector T cell
- Th
Helper T cell
- Thpp
Primed precursor helper T cell
Contributor Information
Ioannis Drygiannakis, Division of Gastroenterology and Hepatology, Department of Medicine, University of Virginia, Box 800708, Charlottesville, VA 22908-0708, USA.
Peter B. Ernst, Division of Gastroenterology and Hepatology, Department of Medicine, University of Virginia, Box 800708, Charlottesville, VA 22908-0708, USA Department of Microbiology, University of Virginia, Box 800708, Charlottesville, VA 22908-0708, USA.
David Lowe, Department of Microbiology, University of Virginia, Box 800708, Charlottesville, VA 22908-0708, USA.
Ian J. Glomski, Department of Microbiology, University of Virginia, Box 800708, Charlottesville, VA 22908-0708, USA
References
- 1.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–82. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 2.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 3.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–87. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 4.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borsellino G, Kleinewietfeld M, DiMitri D, et al. Expression of ectonucleotidase CD39 by Foxp3 + Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 6.Alam MS, Kurtz CC, Rowlett RM, et al. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J Infect Dis. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vukmanovic-Stejic M, Agius E, Booth N, et al. The kinetics of CD4 + Foxp3 + T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin Invest. 2008;118:3639–50. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukashev D, Sitkovsky M, Ohta A. From “Hellstrom Paradox” to anti-adenosinergic cancer immunotherapy. Purinergic Signal. 2007;3:129–34. doi: 10.1007/s11302-006-9044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206:2417–27. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maier SA, Galellis JR, McDermid HE. Phylogenetic analysis reveals a novel protein family closely related to adenosine deaminase. J Mol Evol. 2005;61:776–94. doi: 10.1007/s00239-005-0046-y. [DOI] [PubMed] [Google Scholar]
- 11.Weihofen WA, Liu J, Reutter W, Saenger W, Fan H. Crystal structure of CD26/dipeptidyl-peptidase IV in complex with adenosine deaminase reveals a highly amphiphilic interface. J Biol Chem. 2004;279:43330–5. doi: 10.1074/jbc.M405001200. [DOI] [PubMed] [Google Scholar]
- 12.Pacheco R, Martinez-Navio JM, Lejeune M, et al. CD26, aden-osine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc Natl Acad Sci U S A. 2005;102:9583–8. doi: 10.1073/pnas.0501050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zavialov AV, Engstrom A. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem J. 2005;391:51–7. doi: 10.1042/BJ20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zavialov AV, Gracia E, Glaichenhaus N, Franco R, Zavialov AV, Lauvau G. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol. 2010;88:279–90. doi: 10.1189/jlb.1109764. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong JM, Chen JF, Schwarzschild MA, et al. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J. 2001;354:123–30. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem. 1997;272:25881–9. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 17.Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:345–57. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- 18.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–62. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linden J. New insights into the regulation of inflammation by adenosine. J Clin Invest. 2006;116:1835–7. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol Pharmacol. 1999;55:614–24. [PubMed] [Google Scholar]
- 21.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–10. [PubMed] [Google Scholar]
- 22.Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–7. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alam MS, Kurtz CC, Wilson JM, et al. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4 + helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol. 2009;2:232–42. doi: 10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4 + T cells. J Immunol. 2005;174:1073–80. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 25.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine. Adenosine synthesis and function in regulatory T cell biology. J Immunol. 2010;185:1993–8. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson JM, Ross WG, Agbai ON, et al. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J Immunol. 2009;182:4616–23. doi: 10.4049/jimmunol.0801279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Ma C, Liu S, et al. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunol Cell Biol. 2009;88:165–71. doi: 10.1038/icb.2009.77. [DOI] [PubMed] [Google Scholar]
- 29.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–50. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 30.Nowak M, Lynch L, Yue S, et al. The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur J Immunol. 2010;40:682–7. doi: 10.1002/eji.200939897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desrosiers MD, Cembrola KM, Fakir MJ, et al. Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol. 2007;179:1884–92. doi: 10.4049/jimmunol.179.3.1884. [DOI] [PubMed] [Google Scholar]
- 32.Dickenson JM, Reeder S, Rees B, Alexander S, Kendall D. Functional expression of adenosine A2A and A3 receptors in the mouse dendritic cell line XS-106. Eur J Pharmacol. 2003;474:43–51. doi: 10.1016/s0014-2999(03)02041-7. [DOI] [PubMed] [Google Scholar]
- 33.Hasko G, Nemeth ZH, Vizi ES, Salzman AL, Szabo C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur J Pharmacol. 1998;358:261–8. doi: 10.1016/s0014-2999(98)00619-0. [DOI] [PubMed] [Google Scholar]
- 34.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–80. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 35.Ren T, Grants I, Alhaj M, et al. Impact of disrupting adenosine A(3) receptors (A(3) (-/-)AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21553. doi: 10.1002/ibd.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol. 2004;286:G285–93. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 37.Day YJ, Huang L, McDuffie MJ, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–91. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rork TH, Wallace KL, Kennedy DP, Marshall MA, Lankford AR, Linden J. Adenosine A2A receptor activation reduces infarct size in the isolated, perfused mouse heart by inhibiting resident cardiac mast cell degranulation. Am J Physiol Heart Circ Physiol. 2008;295:H1825–33. doi: 10.1152/ajpheart.495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–48. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panther E, Corinti S, Idzko M, et al. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–90. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 41.Hasko G, Kuhel DG, Chen JF, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–74. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 42.Linnemann C, Schildberg FA, Schurich A, et al. Adenosine regulates CD8 T-cell priming by inhibition of membrane-proximal T-cell receptor signalling. Immunology. 2009;128:e728–37. doi: 10.1111/j.1365-2567.2009.03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ring S, Oliver SJ, Cronstein BN, Enk AH, Mahnke K. CD4 + CD25 + regulatory T cells suppress contact hypersensitivity reactions through a CD39, adenosine-dependent mechanism. J Allergy Clin Immunol. 2009;123:1287–96. doi: 10.1016/j.jaci.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Sevigny CP, Li L, Awad AS, et al. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol. 2007;178:4240–9. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- 45.Climent N, Martinez-Navio JM, Gil C, et al. Adenosine deaminase enhances T-cell response elicited by dendritic cells loaded with inactivated HIV. Immunol Cell Biol. 2009;87:634–9. doi: 10.1038/icb.2009.53. [DOI] [PubMed] [Google Scholar]
- 46.Zarek PE, Huang CT, Lutz ER, et al. A2A receptor signaling promotes peripheral tolerance by inducing T cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–9. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chehata VJ, Domeier PP, Weilnau JN, Lappas CM. Adenosine A(2A) receptor activation limits chronic granulomatous disease-induced hyperinflammation. Cell Immunol. 2011;267:39–49. doi: 10.1016/j.cellimm.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Odashima M, Otaka M, Jin M, et al. A selective adenosine A2A receptor agonist, ATL-146e, prevents concanavalin A-induced acute liver injury in mice. Biochem Biophys Res Commun. 2006;347:949–54. doi: 10.1016/j.bbrc.2006.06.185. [DOI] [PubMed] [Google Scholar]
- 49.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–20. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 50.Csoka B, Himer L, Selmeczy Z, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008;22:3491–9. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 52.Yip L, Cheung CW, Corriden R, Chen Y, Insel PA, Junger WG. Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock. 2007;27:242–50. doi: 10.1097/01.shk.0000245014.96419.3a. [DOI] [PubMed] [Google Scholar]
- 53.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for adenosine A2A receptors in the T cell mediated regulation of colitis. J Immunol. 2006;177:2765–9. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 54.Raskovalova T, Lokshin A, Huang X, et al. Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res. 2007;67:5949–56. doi: 10.1158/0008-5472.CAN-06-4249. [DOI] [PubMed] [Google Scholar]
- 55.Ohta A, Ohta A, Madasu M, et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extra-cellular adenosine-rich microenvironments. J Immunol. 2009;183:5487–93. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 56.Parish ST, Kim S, Sekhon RK, Wu JE, Kawakatsu Y, Effros RB. Adenosine deaminase modulation of telomerase activity and replicative senescence in human CD8 T lymphocytes. J Immunol. 2010;184:2847–54. doi: 10.4049/jimmunol.0903647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohta A, Kjaergaard J, Sharma S, et al. In vitro induction of T cells that are resistant to A(2) adenosine receptor-mediated immunosuppression. Br J Pharmacol. 2008;156:306. doi: 10.1111/j.1476-5381.2008.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4 + T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 59.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 60.Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009;346:55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fletcher JM, Lonergan R, Costelloe L, et al. CD39+ Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–10. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Q, Yan J, Putheti P, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transpl. 2009;9:2303–11. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFoxp3+ regulatory T cells. J Biol Chem. 2010;285:7176–86. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bynoe MS, Viret C. Foxp3+CD4+ T cell-mediated immuno-suppression involves extracellular nucleotide catabolism. Trends Immunol. 2008;29:99–102. doi: 10.1016/j.it.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. 2010;285:27571–80. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin D, Fan J, Wang L, et al. CD73 on tumor cells impairs anti-tumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–55. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hilchey SP, Kobie JJ, Cochran MR, et al. Human follicular lymphoma CD39 + -infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–66. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falkow S. What is a pathogen? ASM News. 1997;63:359–65. [Google Scholar]
- 69.Cavalcante IC, Castro MV, Barreto ARF, et al. Effect of a novel A2A adenosine receptor agonist (ATL 313) on Clostridium difficile toxin induced murine ileal enteritis. Infect Immun. 2006;74:2606–12. doi: 10.1128/IAI.74.5.2606-2612.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189:1897–904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 71.Nemeth ZH, Csoka B, Wilmanski J, et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–26. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suerbaum S, Josenhans C. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol. 2007;5:441–52. doi: 10.1038/nrmicro1658. [DOI] [PubMed] [Google Scholar]
- 73.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 74.Algood HM, Cover TL. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev. 2006;19:597–613. doi: 10.1128/CMR.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blaser MJ. The biology of cag in the Helicobacter pylori-human interaction. Gastroenterology. 2005;128:1512–5. doi: 10.1053/j.gastro.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 76.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–9. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flores J, Okhuysen PC. Genetics of susceptibility to infection with enteric pathogens. Curr Opin Infect Dis. 2009;22:471–6. doi: 10.1097/QCO.0b013e3283304eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouma MG, Jeunhomme TM, Boyle DL, et al. Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J Immunol. 1997;158:5400–8. [PubMed] [Google Scholar]
- 79.Firestein GS, Bullough DA, Erion MD, et al. Inhibition of neutrophil adhesion by adenosine and an adenosine kinase inhibitor. The role of selectins. J Immunol. 1995;154:326–34. [PubMed] [Google Scholar]
- 80.Kaufmann I, Hoelzl A, Schliephake F, et al. Effects of adenosine on functions of polymorphonuclear leukocytes from patients with septic shock. Shock. 2007;27:25–31. doi: 10.1097/01.shk.0000238066.00074.90. [DOI] [PubMed] [Google Scholar]
- 81.Ireland JA, Hanna PC. Macrophage-enhanced germination of Bacillus anthracis endospores requires gerS. Infect Immun. 2002;70:5870–2. doi: 10.1128/IAI.70.10.5870-5872.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang TJ, Fenton MJ, Weiner MA, et al. Murine macrophages kill the vegetative form of Bacillus anthracis. Infect Immun. 2005;73:7495–501. doi: 10.1128/IAI.73.11.7495-7501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crane JK, Shulgina I, Naeher TM. Ecto-5′-nucleotidase and intestinal ion secretion by enteropathogenic Escherichia coli. Purinergic Signal. 2007;3:233–46. doi: 10.1007/s11302-007-9056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujii Y, Nomura T, Yokoyama R, Shinoda S, Okamoto K. Studies of the mechanism of action of the aerolysin-like hemolysin of Aeromonas sobria in stimulating T84 cells to produce cyclic AMP. Infect Immun. 2003;71:1557–60. doi: 10.1128/IAI.71.3.1557-1560.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994;266:107–9. doi: 10.1126/science.7524148. [DOI] [PubMed] [Google Scholar]
- 86.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387–94. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 87.Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, Chakrabarty AM. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun. 2000;68:4930–7. doi: 10.1128/iai.68.9.4930-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melnikov A, Zaborina O, Dhiman N, Prabhakar BS, Chakrabarty AM, Hendrickson W. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol Microbiol. 2000;36:1481–93. doi: 10.1046/j.1365-2958.2000.01976.x. [DOI] [PubMed] [Google Scholar]
- 89.Zaborina O, Misra N, Kostal J, et al. P2Z-independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1999;67:5231–42. doi: 10.1128/iai.67.10.5231-5242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akoachere M, Squires RC, Nour AM, Angelov L, Brojatsch J, bel-Santos E. Identification of an in vivo inhibitor of Bacillus anthracis spore germination. J Biol Chem. 2007;282:12112–8. doi: 10.1074/jbc.M611432200. [DOI] [PubMed] [Google Scholar]
- 91.Glomski IJ, Piris-Gimenez A, Huerre M, Mock M, Goossens PL. Primary involvement of pharynx, peyer's patch in inhalational, intestinal anthrax. PLoS Pathog. 2007;3:e76. doi: 10.1371/journal.ppat.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanz P, Teel LD, Alem F, Carvalho HM, Darnell SC, O'Brien AD. Detection of Bacillus anthracis spore germination in vivo by bioluminescence imaging. Infect Immun. 2008;76:1036–47. doi: 10.1128/IAI.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cote CK, Bozue J, Twenhafel N, Welkos SL. Effects of altering the germination potential of Bacillus anthracis spores by exogenous means in a mouse model. J Med Microbiol. 2009;58:816–25. doi: 10.1099/jmm.0.008656-0. [DOI] [PubMed] [Google Scholar]
- 94.Kolachala VL, Vijay-Kumar M, Dalmasso G, et al. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–70. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kolachala V, Ruble B, Vijay-Kumar M, et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155:127–37. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM. An A2A adenosine receptor agonist, ATL313, reduces inflammation, improves survival in murine sepsis models. BMC Infect Dis. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]