Abstract
DNA base excision repair is essential for maintaining genomic integrity and for active DNA demethylation, a central element of epigenetic regulation. A key player is thymine DNA glycosylase (TDG), which excises thymine from mutagenic G·T mispairs that arise by deamination of 5-methylcytosine (mC). TDG also removes 5-formylcytosine and 5-carboxylcytosine, oxidized forms of mC produced by Tet enzymes. Recent studies show that the glycosylase activity of TDG is essential for active DNA demethylation and for embryonic development. Our understanding of how repair enzymes excise modified bases without acting on undamaged DNA remains incomplete, particularly for mismatch glycosylases such as TDG. We solved a crystal structure of TDG (catalytic domain) bound to a substrate analog and characterized active-site residues by mutagenesis, kinetics, and molecular dynamics simulations. The studies reveal how TDG binds and positions the nucleophile (water) and uncover a previously unrecognized catalytic residue (Thr197). Remarkably, mutation of two active-site residues (Ala145 and His151) causes a dramatic enhancement in G·T glycosylase activity but confers even greater increases in the aberrant removal of thymine from normal A·T base pairs. The strict conservation of these residues may reflect a mechanism used to strike a tolerable balance between the requirement for efficient repair of G·T lesions and the need to minimize aberrant action on undamaged DNA, which can be mutagenic and cytotoxic. Such a compromise in G·T activity can account in part for the relatively weak G·T activity of TDG, a trait that could potentially contribute to the hypermutability of CpG sites in cancer and genetic disease.
DNA base excision repair (BER) plays an established role in maintaining genomic integrity, and recent studies indicate that BER is also essential for active DNA demethylation, a key element of epigenetic gene regulation (1–3). A central player in both processes is thymine DNA glycosylase (TDG), which initiates BER by excising damaged or modified forms of 5-methylcytosine (mC) that arise at 5′-CpG-3′ sites. TDG was discovered for its ability to selectively remove T from G·T mispairs, mutagenic lesions that can arise from deamination of mC to T (4, 5). TDG also excises 5-formylcytosine (fC) and 5-carboxylcytosine (caC), oxidized forms of mC that are generated by Tet enzymes (6, 7). In addition, TDG was shown to be essential for active DNA demethylation and for embryonic development (8, 9), a role that likely involves excision of deaminated or oxidized forms of mC generated by a deaminase or Tet enzyme (7, 10–12). Thus, the ability of TDG to remove deaminated and oxidized forms of mC is important for genetic and epigenetic integrity.
The question of how DNA glycosylases remove modified bases without acting upon the huge excess of undamaged DNA remains largely unresolved, particularly for mismatch glycosylases such as TDG and MBD4 (methyl binding domain IV) (13–15). These enzymes face the formidable task of removing a normal base, thymine, from G·T mispairs but not from A·T base pairs. Previous studies show that TDG activity is weak for G·T mispairs compared with most in vitro substrates (e.g., G·U), even though G·T mispairs are an important biological substrate for TDG (8, 9, 16–19). This might reflect a mechanism used by TDG to strike a balance between the requirement for efficient repair of mutagenic G·T lesions and the need for avoiding aberrant removal of T from A·T pairs, which can be mutagenic and cytotoxic (13, 20, 21). Notably, such a compromise in G·T repair activity could account in part for the high frequency of C→T transitions at CpG sites in cancer and genetic disease (20, 22–24). A mechanism for limiting thymine excision could be needed if the capacity of TDG to discriminate between G·T and A·T pairs, which is approximately 104.3-fold (17), is not sufficient to allow for highly efficient G·T repair in the absence of aberrant A·T activity. Consistent with this possibility, our previous studies strongly suggest that steric hindrance involving the methyl of thymine weakens substrate binding and slows base excision for G·T mispairs (11, 17, 19, 25, 26), but direct structural evidence of such a mechanism was lacking. We address these and other questions regarding the basis of TDG specificity and catalysis here, using a combination of structural, biochemical, and computational approaches.
Results and Discussion
Overall Structure.
To advance our understanding of the specificity and catalytic mechanism of TDG, we sought to determine the crystal structure of a lesion recognition complex (i.e., a structure of TDG bound to DNA with a target nucleotide flipped productively into its active site but not cleaved). Previous studies show that 2′-deoxy-2′-fluoroarabinouridine (or UF; Fig. S1) is an excellent mimic of deoxyuridine (dU), an in vitro TDG substrate, because DNA containing a G·UF site binds productively but is not cleaved by TDG (11, 19, 26, 27). We obtained crystals of TDG (catalytic domain, residues 111–308) bound to DNA containing a G·UF site, solved the structure by molecular replacement, and refined it to a crystallographic R factor of 26.3% and an Rfree of 30.4% at a resolution of 2.97 Å (Table S1).
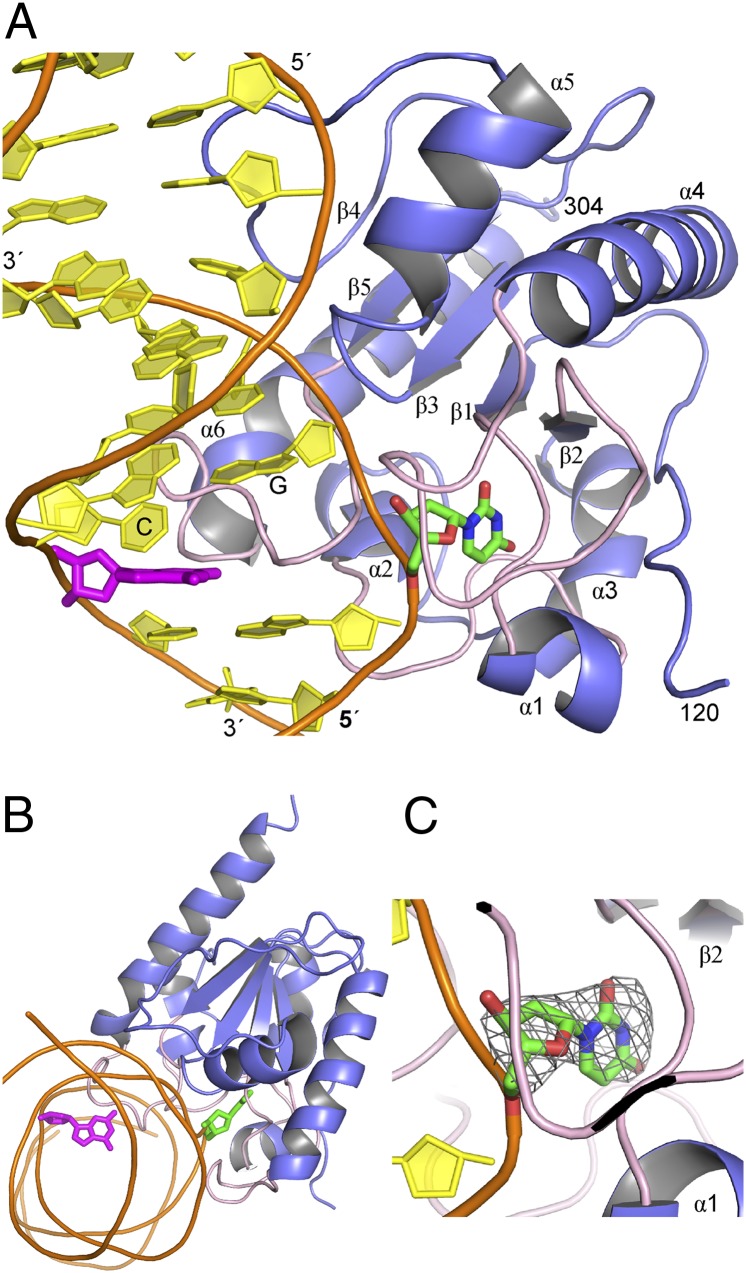
As shown in Fig. 1, the UF substrate analog is flipped completely out of the DNA duplex and buried deep in the TDG active site, with the base–sugar bond intact. As detailed below, the structure reveals many important details regarding the specificity and catalytic mechanism of TDG. Some of our results have implications for the mechanism of closely related prokaryotic MUG enzymes (mismatch specific uracil glycosylase) (28), although many key differences between TDG and MUG enzymes are also revealed.
Fig. 1.
Structure of TDG lesion recognition complex. (A) Overall structure of TDG (catalytic domain) bound to a G·UF substrate analog shows the dUF nucleotide (green) flipped deep into the active site, whereas the mismatched G (magenta) remains stacked in the duplex. Loops containing key catalytic residues are colored pink. The G located 3′ of the target nucleotide (UF) and the C located 5′ of mismatched G are labeled. (B) Orthogonal view of the overall structure. (C) Close-up view of the active site showing the UF analog with the 2fo-fc electron density map contoured at the 1σ level.
First, we briefly discuss features of the structure that are consistent with those observed in a previous structure of TDG bound to abasic DNA (an enzyme–product or E·P complex) (29). Overall, the conformation of TDG is highly similar in the enzyme–substrate (E·S) and E·P complexes, with an overall percentile-based spread of 0.6 Å (30). As seen in the E·P complex, TDG contacts numerous backbone phosphates in the target DNA strand, and it compresses together (“pinches”) the phosphates of the two nucleotides that flank the UF nucleotide, which is thought to facilitate and/or stabilize nucleotide flipping. In addition, TDG imposes a severe bend in the DNA, predominantly at the target site, as illustrated by the severely disrupted stacking between the mismatched guanine (magenta) and the 3′ cytosine (Fig. 1A). These features are also observed for the TDG product complex (29) and for many other glycosylases (31, 32). As shown in Fig. 1 A and B, the mismatched guanine remains fully stacked in the DNA duplex; its recognition by TDG has been previously described (29). We note that TDG crystallized in a 2:1 complex with the DNA, with one subunit bound at the G·UF site and an adjacent subunit bound to nonspecific DNA, as seen previously for the TDG E·P complex (29). We have previously shown that 2:1 binding arises under conditions of high and saturating concentrations of TDG and is dispensable for lesion recognition and base excision (26, 29). Thus, our focus here is on the lesion recognition complex (Fig. 1).
Nucleophile Binding for TDG and MUG Enzymes.
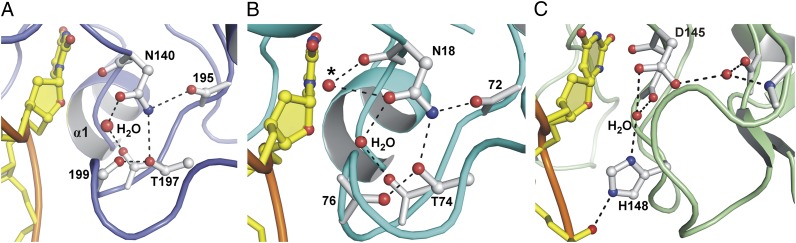
A key question regarding the catalytic mechanism of DNA glycosylases is how they bind and position the nucleophilic water molecule. Previous structural studies indicated that Asn18 of Escherichia coli MUG coordinates the nucleophile (33, 34), and biochemical studies showed that the corresponding residue in TDG, Asn140, is essential for G·T glycosylase activity, consistent with a major role in catalysis (19, 35). Although a mechanism of nucleophile binding involving the catalytic Asn was proposed for MUG and TDG enzymes (33), it has remained unsubstantiated because a putative nucleophilic water molecule has not been directly observed in any of the existing DNA-bound structures of TDG or MUG enzymes (29, 33, 34). The structure of TDG bound to a G·UF substrate analog reported here reveals the putative water nucleophile, confirming a key role for Asn140 and revealing a binding mechanism that differs from the previously proposed mechanism for MUG-TDG enzymes (33). As shown in Fig. 2A, TDG coordinates the putative water nucleophile via contacts with the Asn140 side chain oxygen (Oδ1) and the Thr197 backbone oxygen. Although a nucleophile was not seen in the MUG G·UF complex, two water molecules contact the Asn18 side chain Oδ1 in the free enzyme (33, 34). One of these water molecules also contacts the Asn18 backbone oxygen, and it was proposed to represent the nucleophile, as modeled in Fig. 2B (33). In contrast, the second water molecule in free MUG contacts the Thr74 backbone oxygen and Asn18 Oδ1 (Fig. 2B). Remarkably, this binding mechanism corresponds exactly to that observed here for TDG (Fig. 2A).
Fig. 2.
Coordination of the water nucleophile in TDG-MUG enzymes. (A) Close-up view of the TDG active site shows that the putative water nucleophile is coordinated by the Asn140 side chain oxygen (Oδ1) and the backbone oxygen of Thr197. Also shown are interactions that position the Asn140 side chain, and contacts with the Thr197 hydroxyl side chain. (B) A revised mechanism for nucleophile coordination is proposed for MUG enzymes, involving the Asn18 side chain Oδ1 and the Thr74 backbone oxygen (E. coli MUG numbering). The previous proposal that MUG coordinates the nucleophile using the backbone oxygen and side chain Oδ1 of Asn18 (33) results in substantially different position for the nucleophile (marked by *). Note that a nucleophile is not observed in the eMUG E·S complex (PDB ID: 1MWJ), but two water molecules contact Asn18 in the free enzyme (PDB ID: 1MUG). The figure shows free MUG (with both water molecules) and DNA from the MUG E·S complex (proteins aligned). (C) A similar view of the E·S complex for human UNG (PDB ID: 1EMH) (31) shows that the position of the nucleophile relative to the electrophile (C1′) is very similar to that observed here for TDG (and proposed for eMUG).
Our revised mechanism of nucleophile binding by TDG-MUG enzymes is supported by previous findings for UNG (uracil DNA glycosylase), which belongs to the same enzyme superfamily. Indeed, the position of the nucleophile relative to the catalytic Asn and ribose C1′ observed here for TDG (Fig. 2A) is highly similar to that observed in high-resolution structures (≤1.9 Å) of UNG bound to a substrate analog (Fig. 2C) (31) and a transition-state analog (36), even as the coordinating residues differ. As shown in Fig. 2A, the putative water nucleophile is separated from the electrophile (ribose C1′) by approximately 4 Å in the TDG E·S complex. Previous studies of UNG reveal a similar nucelophile–electrophile distance (Fig. 2C) and indicate that the gap is reduced by electrophile migration after cleavage of the base–sugar bond (36). Notably, our structure indicates that the backbone carbonyl oxygen of Asn140 is well positioned to stabilize the glycosyl cation that is generated upon base–sugar bond cleavage (37, 38).
As shown in Fig. 2A, our structure also indicates an important role for Thr197, a residue that was not previously recognized as important for catalysis by TDG or MUG enzymes. The hydroxyl of Thr197 helps to position the Asn140 side chain (by contacting Nδ2), and it seems to stabilize a turn in the β2–α4 catalytic loop by contacting the Gly199 backbone oxygen. To examine the role of Thr197 in the glycosylase activity of TDG, we produced a T197A-TDG variant and performed kinetics experiments with a G·T substrate. The activity, kobs = 0.005 ± 0.001 min−1, is 32-fold lower than for native TDG (Table S2 and Fig. S2). This finding confirms a key role for Thr197 in catalysis, consistent with our structure and the strict conservation of Thr197 in TDG-MUG enzymes.
Interactions with the Flipped Uracil Base.
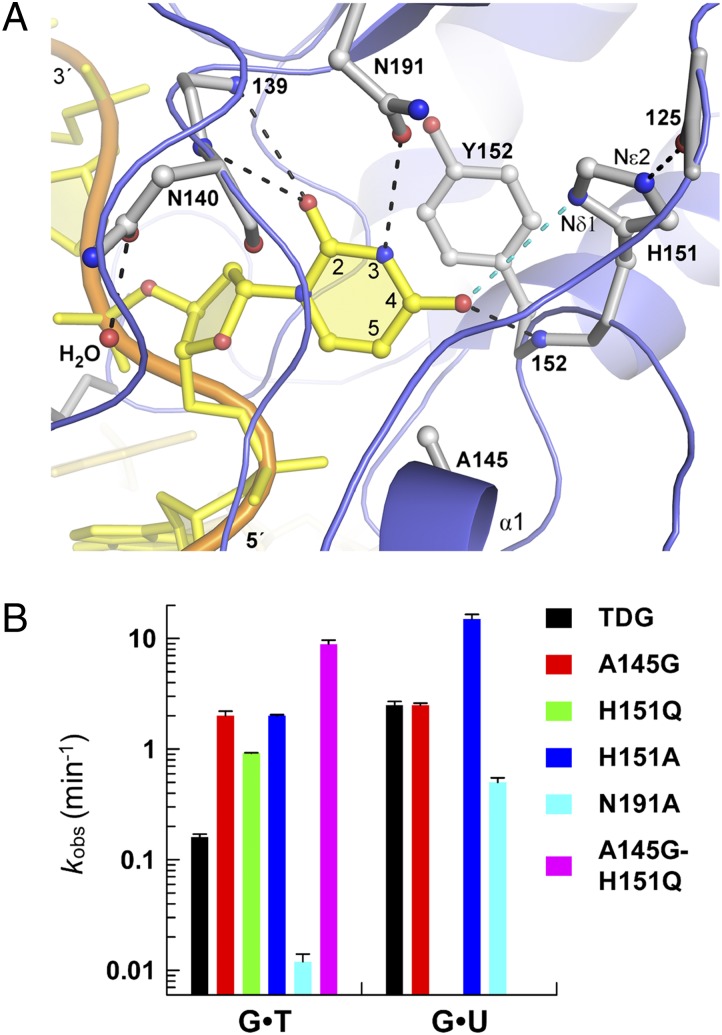
As shown in Fig. 3A, TDG forms numerous interactions with the flipped base. Notably, these interactions differ remarkably from those observed in the MUG G·UF complex, consistent with the substantial differences in specificity between these enzymes (which exhibit 33% sequence identity) (29). TDG contacts the O2 oxygen of uracil from the backbone NH of both Ile139 and Asn140, and it contacts uracil O4 using the backbone NH of Tyr152. The backbone interactions involving Ile139 and Tyr152 are conserved in MUG (33). Previous studies indicate that these backbone interactions could be important for retaining the flipped nucleotide in the active site, and they may contribute to the chemical step by stabilizing negative charge that develops on O2 and O4 upon cleavage of the base–sugar bond (39, 40). Notably, the interactions formed with O2 could potentially stabilize the flipping and/or excision of fC, caC, and other cytosine analogs (6, 25). As shown in Fig. 3A, Tyr152 partially stacks with the flipped base, which may limit the extent of nucleotide flipping and help to position the flipped base to optimize the catalytic interactions described above and below.
Fig. 3.
Interactions with the flipped base and mutational effects on glycosylase activity. (A) Close-up of the TDG active site shows hydrogen bond interactions with the flipped uracil base (black dashed lines). The imidazole nitrogens of H151 are labeled. The cyan dashed line indicates a potential repulsive electrostatic interaction. (B) Bar graph of rate constants (kobs) obtained from kinetics experiments.
The crystal structure reveals that the Asn191 side chain (Oδ1) contacts uracil N3H, providing the sole hydrogen bond to the base involving a side chain. Notably, Asn191 is strictly conserved in vertebrate TDGs, whereas MUG enzymes have a conserved Lys in the corresponding position, which does not contact the flipped base (33). The N191A mutation in TDG causes 6- and 15-fold losses in G·U and G·T activity (Fig. 3B and Fig. S2), indicating that Asn191 is important for processing these lesions, particularly G·T. Notably, other UNG superfamily members, including UNG and SMUG1, have a conserved Asn at the position corresponding to Asn191 of TDG (31, 41, 42). The Asn side chain of UNG and SMUG1 provides two hydrogen bonds to the flipped uracil base, contacting the N3H and O4 positions (31, 41, 42). The molecular dynamics studies below (Fig. 4) suggest that Asn191 of TDG could also contact both the N3H and O4 positions of the flipped U or T base, although this likely disrupts the O4 to Tyr152-NH interaction. Notably, for UNG and SMUG1, the position and orientation of the Asn side chain is restricted by contacts with other residues, which likely precludes the excision of cytosine analogs by these enzymes (31, 42). In contrast, the Asn191 side chain of TDG is not restricted by contacts with other active-site groups, and TDG can readily excise cytosine analogs, including fC and caC (6, 7). It seems likely that a flexible Asn191 side chain is necessary to allow removal of both uracil and cytosine analogs by TDG.
Fig. 4.
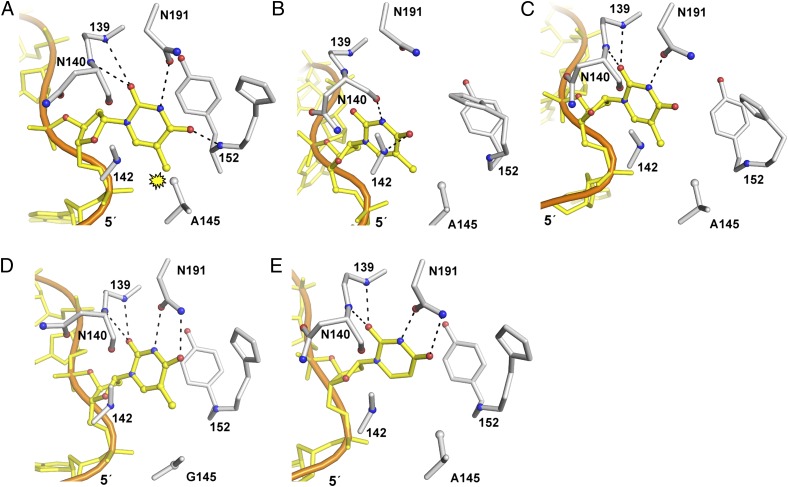
Crystal structure and MD simulations of TDG and A145G-TDG with G·T and G·U substrates. (A) To illustrate the potential steric clash of the methyl groups from dT and Ala145, dU from the TDG-G·UF structure was replaced with dT (interactions shown are from TDG-G·UF structure). The remaining panels are representative structures from the MD simulations, which were selected using the 2D distance plots for active-site interactions (Fig. S3). (B) TDG-G·T with a partially flipped dT nucleotide; (C) TDG-G·T with fully flipped dT, as indicated by interactions seen in the TDG-G·UF crystal structure; (D) A145G-TDG with G·T exhibits complete flipping and active-site contacts; (E) TDG-G·U illustrates complete nucleotide flipping.
Ala145 and His151 Reduce G·T Activity.
As shown in Fig. 3A, the structure suggests a potential catalytic role for His151, another highly conserved residue in vertebrate TDGs. Although the distance from the His151 imidazole Nδ1 to O4 of uracil is long for a hydrogen bond (4 Å), we investigated the role of His151 by mutagenesis. Remarkably, the H151A variant has 6- and 13-fold higher activity for G·U and G·T substrates, respectively, compared with native TDG (Fig. 3B and Table S2). In addition, H151Q-TDG has sixfold higher G·T activity than TDG. Because the kinetics experiments were collected under saturating enzyme conditions, the rate constant (kobs) reports on the nucleotide flipping and chemical steps of the reaction, and is not impacted by subsequent steps such as product release or product inhibition (which are rate-limiting under excess substrate conditions for TDG) (18). Thus, one potential explanation for the enhanced activity of His151 variants is that the imidazole side chain destabilizes nucleotide flipping. Another possibility is that the His151 imidazole slows the chemical step by destabilizing the partial negative charge that develops on O4 of the base upon glycosylic bond cleavage (39, 40). Such a destabilizing electrostatic interaction could occur if the His151 imidazole ring is neutral and Nδ1 carries a lone electron pair (i.e., >N: rather than >N-H). Accordingly, the other His151 imidazole nitrogen (Nε2) provides a short hydrogen bond (2.8 Å) to the backbone oxygen of Pro125, an interaction observed in all three previous structures of TDG (29, 43, 44). Notably, a neutral rather than protonated His151 imidazole would likely be best suited for excision of fC and caC, which have an NH2 rather than carbonyl oxygen at C4 of the nucleobase.
Previous studies indicate that steric hindrance limits the active-site access of thymine and other bases with a bulky C-5 substituent (i.e., 5-BrUra), and that steric effects largely account for the much weaker binding and base-excision activity for G·T relative to G·U mispairs (11, 17, 19, 25). However, residue(s) that could potentially impose this steric hindrance were unknown. Fig. 4A shows a model, derived from the TDG-G·UF structure, in which the flipped uracil base is replaced with thymine. Remarkably, the model predicts a steric clash between the methyl of thymine and the methyl of Ala145, a residue that is strictly conserved in vertebrate TDG. Accordingly, a TDG variant designed to relieve this steric hindrance, A145G-TDG, exhibits a dramatic 13-fold increase in G·T activity compared with native TDG (Fig. 3B and Fig. S2). The TDG-G·UF structure indicates no steric hindrance between uracil and Ala145 (Fig. 3A), suggesting that the A145G mutation should not affect the activity for a G·U mispair. Indeed, we find identical G·U activity for A145G-TDG and native TDG (Fig. S2). Together, these findings indicate that Ala145 adversely impacts the nucleotide flipping rather than the chemical step of the TDG reaction for G·T mispairs.
Given our finding that two highly conserved residues dramatically reduce the G·T glycosylase activity of TDG, we examined the effect of a double mutant. Remarkably, the A145G-H151Q double mutant exhibits 101.8-fold (56-fold) greater G·T activity relative to native TDG (Fig. 3B and Fig. S2). Thus, the effects of the individual mutations for A145G (101.1-fold) and H151Q (100.8-fold) are additive, indicating that Ala145 and His151 act independently to suppress TDG activity for G·T mispairs. Observation of such large increases in repair activity upon mutation of conserved active-site residues is, to our knowledge, unprecedented for a repair enzyme.
Molecular Dynamics Simulations of Nucleotide Flipping.
To examine the role of Ala145 on nucleotide flipping for TDG, we performed molecular dynamics (MD) simulations, an approach used previously to study spontaneous and enzyme-mediated nucleotide flipping (45–47). The MD simulations were initiated from the TDG-G·UF structure, modified as needed to replace the UF analog with deoxythymidine (dT) or dU, or generate TDG variants. After solvent overlay and MD equilibration including harmonic restraints on the protein–DNA complex (13 ns), production simulations were performed (40 ns). As shown in Fig. 4B and Fig. S3, MD simulations of the TDG-G·T system indicate that the target dT nucleotide does not flip stably into the active-site pocket, as judged by the small population of MD conformers that form key hydrogen bond interactions observed in the TDG-G·UF crystal structure, including Thy-O2 to Ile139-NH, and Thy-N3H to Asn191-Oδ1. Instead, the dT nucleotide is flipped nearly but not completely into the active site. Notably, this conformation is stabilized by contacts not seen in the crystal structure, including Thy N3H to Asn140 CO, and Thy O4 to Gly142 NH (Fig. 4B). In addition, the Asn140 backbone and side chain carbonyl oxygens seem to be improperly positioned for catalysis (relative to the flipped dT), as defined by the crystal structure (Fig. 2A). Thus, it is unlikely that the incompletely flipped TDG-G·T complex represents a productive (Michaelis) complex. Rather, it may reflect a transient nucleotide flipping intermediate. Previous studies of UNG and OGG1 (8-oxoguanine DNA glycosylase) reveal that nucleotide flipping involves a series of transient intermediates rather than a single concerted step, consistent with the major conformational changes required for the enzyme and DNA (48–50). Although the target dT nucleotide is incompletely flipped for most TDG-G·T conformers, it appears to be fully flipped for some, as indicated by the presence of contacts that are observed in the crystal structure (Fig. 4C and Fig. S3), and this state seems likely to be catalytically competent. In contrast to the result for wild-type TDG, the MD simulations for A145G-TDG indicate that the dT nucleotide flips completely and stably into the active site (Fig. 4D and Fig. S3). MD simulations of TDG-G·U indicate that the dU nucleotide does the same (Fig. 4E and Fig. S3), as expected.
Thus, the results of the MD simulations are consistent with the structural and biochemical findings indicating that the methyl of Ala145 destabilizes the flipping of dT, but not dU, into the TDG active site, and that the A145G mutation relieves this steric effect. The MD results are also consistent with our previous findings that nucleotide flipping is destabilized for G·T relative to G·U substrates (17, 19, 25, 26). The MD simulations suggest a transient flipping intermediate that is substantially populated for TDG with G·T but not G·U substrates. Studies of UNG and OGG1 indicate that the transient flipping intermediates provide kinetic or thermodynamic barriers that hinder the flipping of undamaged nucleotides into the active site (48–50). For TDG, the flipping intermediate may serve to transiently trap dT nucleotides as they flip out of the active site back toward the DNA helix. This could afford rejected dT nucleotides an opportunity to flip a very short distance back into the active site, rather than starting anew from the DNA helix (or a less-flipped transient state). Such a mechanism could dampen or “fine-tune” the destabilizing effect of Ala145 on dT flipping, and might be important for obtaining the proper balance between the needs for efficient G·T repair and for minimizing excision of T from A·T pairs.
Ala145 and His151 Variants Exhibit Aberrant Activity on A·T Base Pairs.
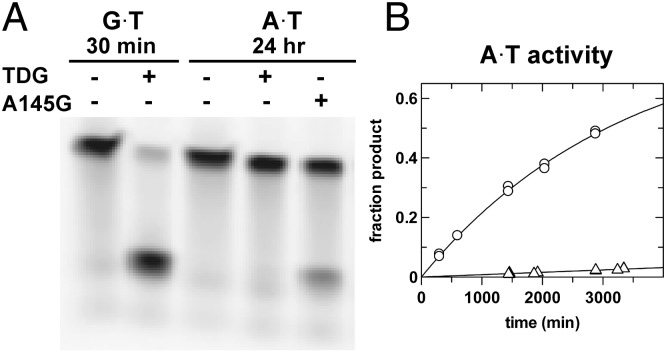
One reasonable explanation for the strict conservation of active-site residues that dramatically suppress G·T repair activity is that the residues are needed to minimize aberrant excision of T from the vast excess of A·T base pairs. Accordingly, we sought to determine whether the TDG variants that exhibit greatly enhanced G·T activity also exhibit an increase in A·T activity. As shown in Fig. 5A, the A145G TDG variant possesses substantial activity for excising T from an A·T base pair, whereas TDG does not. A more quantitative HPLC assay reveals that A145G-TDG exhibits 38-fold greater A·T activity than native TDG (Fig. 5B and Table S2). Notably, the A·T activity of A145G-TDG is even fivefold higher at 37 °C (kobs = 0.0015 min−1) vs. 22 °C, such that nearly 10% of the A·T substrate is converted to abasic DNA product in 60 min (Fig. S2). We also find that the A·T activity for H151A is 34-fold greater than native TDG. Moreover, the A·T activity is 100-fold higher for the A145G-H151Q double mutant compared with native TDG (Table S2 and Fig. S2). Thus, the Ala145 and His151 mutations cause dramatic increases in aberrant action on A·T pairs, at least twofold greater than the substantial increases observed for G·T activity. The high affinity of TDG for nonspecific DNA (Kd < 0.3 μM) (26) suggests these variants could exhibit substantial aberrant A·T activity in vivo. Electrophoretic mobility shift assays indicate that the A145G variant binds undamaged DNA with the same affinity as wild-type TDG, and H151A binds modestly weaker (Fig. S4). This result is consistent with the expectation that, although the A145G mutation facilitates dT nucleotide flipping (Fig. 4), the population of the fully flipped state is probably quite low for undamaged DNA, such that the mutation is unlikely to substantially enhance DNA binding affinity.
Fig. 5.
Ala145 limits aberrant A·T activity of TDG. (A) A145G mutant removes T from A·T pairs as shown by an electrophoretic mobility assay. (B) HPLC analysis of A·T glycosylase activity for A145G-TDG (○) relative to TDG (Δ).
It is of interest to consider the results of similar studies for other glycosylases. Previous studies of AAG (alkadenine DNA glycosylase) revealed a mutation (N169S) that gives 33-fold faster excision of G from G·C pairs, but marginally (≤ twofold) increased activity for damaged bases (Hx and εA) (51, 52). For UNG, mutations that cause large increases in excision of T from A·T pairs or C from G·C pairs lead to decreased rather than increased activity for the target lesion (uracil) (53). In contrast, for TDG, mutation of two conserved residues gives large increases in repair activity (G·T) and confers even greater increases in aberrant action on undamaged DNA (A·T).
Conclusions
The crystal structure of a TDG lesion recognition complex, together with biochemical and computational studies, provides much insight into the molecular basis of specificity and catalysis for TDG, a repair enzyme with an essential role in active DNA demethylation and embryonic development (8, 9). The studies reveal a modified mechanism for binding the nucleophile (water) that is likely shared by MUG enzymes, and uncover a previously unrecognized catalytic residue, Thr197, which is highly conserved in TDG-MUG enzymes. The results show that Asn191 is important for G·T activity and suggest that the absence of interactions that could restrain the Asn191 side chain may allow for excision of both thymine and cytosine analogs by TDG.
Remarkably, we find that two highly conserved residues (Ala145, His151) severely curtail the G·T repair activity of TDG, but they are needed to minimize aberrant removal of T from A·T base pairs. The results indicate that Ala145 destabilizes nucleotide flipping for dT (but not dU) via steric hindrance, as suggested by previous studies (17, 19, 25, 26). In addition, our findings suggest that His151 forms a repulsive electrostatic interaction with thymine (or uracil) that slows the chemical step of the reaction. MD simulations support these findings and suggest the presence of a transient flipping intermediate that could moderate the destabilizing effect of Ala145 on dT nucleotide flipping.
Our findings offer an explanation for the relatively weak G·T glycosylase activity of TDG, a trait that may contribute to the high mutational frequency at CpG sites in cancer and genetic disease (13, 20). Although enhanced G·T repair activity by TDG could potentially be beneficial to the cell, we find that active-site mutations that confer enhanced G·T activity cause even larger increases in aberrant A·T activity. This result suggests that specificity may already be optimized for wild-type TDG, which exhibits approximately 104.3-fold higher activity for G·T relative to A·T pairs (17). It seems that the G·T repair activity of TDG may be restrained by (i) its limited capacity to distinguish between G·T and A·T pairs, and (ii) the tolerance for aberrant excision of T from A·T pairs, which can be mutagenic and cytotoxic (21). Thus, the strict conservation of residues that severely restrain G·T activity may reflect a mechanism used to strike a tolerable balance between the conflicting needs for efficient damage repair and avoidance of action on undamaged DNA.
Materials and Methods
Details regarding the production of enzymes and DNA, determination of glycosylase activity, and crystal growth and structure determination are provided in the SI Materials and Methods. Figures were prepared with PyMOL (http://www.pymol.org). MD simulations were performed with CHARMM (54) and NAMD (55) using the CHARMM additive force field (56–58). Additional details are in SI Materials and Methods.
Note Added in Proof.
While this manuscript was under review, crystal structures of TDG bound DNA containing to G⋅caC pair were reported (59).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants R01-GM072711 (to A.C.D.) and R01-GM051501 (to A.D.M.). X-ray diffraction data were collected at the Stanford Synchrotron Radiation Lightsource (SSRL), a national user facility operated by Stanford University on behalf of the Department of Energy (DOE). The SSRL Structural Molecular Biology Program is supported by the DOE and the NIH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors reported in this paper have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3UFJ).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201010109/-/DCSupplemental.
References
- 1.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 2.Wu SC, Zhang Y. Active DNA demethylation: Many roads lead to Rome. Nat Rev Mol Cell Biol. 2010;11:607–620. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabel CS, Manning SA, Kohli RM. The curious chemical biology of cytosine: Deamination, methylation, and oxidation as modulators of genomic potential. ACS Chem Biol. 2012;7:20–30. doi: 10.1021/cb2002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiebauer K, Jiricny J. Mismatch-specific thymine DNA glycosylase and DNA polymerase beta mediate the correction of G.T mispairs in nuclear extracts from human cells. Proc Natl Acad Sci USA. 1990;87:5842–5845. doi: 10.1073/pnas.87.15.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neddermann P, et al. Cloning and expression of human G/T mismatch-specific thymine-DNA glycosylase. J Biol Chem. 1996;271:12767–12774. doi: 10.1074/jbc.271.22.12767. [DOI] [PubMed] [Google Scholar]
- 6.Maiti A, Drohat AC. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: Potential implications for active demethylation of CpG sites. J Biol Chem. 2011;286:35334–35338. doi: 10.1074/jbc.C111.284620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He YF, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortázar D, et al. Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature. 2011;470:419–423. doi: 10.1038/nature09672. [DOI] [PubMed] [Google Scholar]
- 9.Cortellino S, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiti A, Drohat AC. Dependence of substrate binding and catalysis on pH, ionic strength, and temperature for thymine DNA glycosylase: Insights into recognition and processing of G·T mispairs. DNA Repair (Amst) 2011;10:545–553. doi: 10.1016/j.dnarep.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito S, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millar CB, et al. Enhanced CpG mutability and tumorigenesis in MBD4-deficient mice. Science. 2002;297:403–405. doi: 10.1126/science.1073354. [DOI] [PubMed] [Google Scholar]
- 14.Hendrich B, Hardeland U, Ng HH, Jiricny J, Bird A. The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites. Nature. 1999;401:301–304. doi: 10.1038/45843. [DOI] [PubMed] [Google Scholar]
- 15.Wu P, et al. Mismatch repair in methylated DNA. Structure and activity of the mismatch-specific thymine glycosylase domain of methyl-CpG-binding protein MBD4. J Biol Chem. 2003;278:5285–5291. doi: 10.1074/jbc.M210884200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters TR, Swann PF. Kinetics of the action of thymine DNA glycosylase. J Biol Chem. 1998;273:20007–20014. doi: 10.1074/jbc.273.32.20007. [DOI] [PubMed] [Google Scholar]
- 17.Morgan MT, Bennett MT, Drohat AC. Excision of 5-halogenated uracils by human thymine DNA glycosylase. Robust activity for DNA contexts other than CpG. J Biol Chem. 2007;282:27578–27586. doi: 10.1074/jbc.M704253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald ME, Drohat AC. Coordinating the initial steps of base excision repair. Apurinic/apyrimidinic endonuclease 1 actively stimulates thymine DNA glycosylase by disrupting the product complex. J Biol Chem. 2008;283:32680–32690. doi: 10.1074/jbc.M805504200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiti A, Morgan MT, Drohat AC. Role of two strictly conserved residues in nucleotide flipping and N-glycosylic bond cleavage by human thymine DNA glycosylase. J Biol Chem. 2009;284:36680–36688. doi: 10.1074/jbc.M109.062356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters TR, Swann PF. Thymine-DNA glycosylase and G to A transition mutations at CpG sites. Mutat Res. 2000;462:137–147. doi: 10.1016/s1383-5742(00)00031-4. [DOI] [PubMed] [Google Scholar]
- 21.Boiteux S, Guillet M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair (Amst) 2004;3:1–12. doi: 10.1016/j.dnarep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Rideout WM, 3rd, Coetzee GA, Olumi AF, Jones PA. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990;249:1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- 23.Cooper DN, Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988;78:151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- 24.Pfeifer GP, Besaratinia A. Mutational spectra of human cancer. Hum Genet. 2009;125:493–506. doi: 10.1007/s00439-009-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett MT, et al. Specificity of human thymine DNA glycosylase depends on N-glycosidic bond stability. J Am Chem Soc. 2006;128:12510–12519. doi: 10.1021/ja0634829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan MT, Maiti A, Fitzgerald ME, Drohat AC. Stoichiometry and affinity for thymine DNA glycosylase binding to specific and nonspecific DNA. Nucleic Acids Res. 2011;39:2319–2329. doi: 10.1093/nar/gkq1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schärer OD, Kawate T, Gallinari P, Jiricny J, Verdine GL. Investigation of the mechanisms of DNA binding of the human G/T glycosylase using designed inhibitors. Proc Natl Acad Sci USA. 1997;94:4878–4883. doi: 10.1073/pnas.94.10.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallinari P, Jiricny J. A new class of uracil-DNA glycosylases related to human thymine-DNA glycosylase. Nature. 1996;383:735–738. doi: 10.1038/383735a0. [DOI] [PubMed] [Google Scholar]
- 29.Maiti A, Morgan MT, Pozharski E, Drohat AC. Crystal structure of human thymine DNA glycosylase bound to DNA elucidates sequence-specific mismatch recognition. Proc Natl Acad Sci USA. 2008;105:8890–8895. doi: 10.1073/pnas.0711061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pozharski E. Percentile-based spread: A more accurate way to compare crystallographic models. Acta Crystallogr D Biol Crystallogr. 2010;66:970–978. doi: 10.1107/S0907444910027927. [DOI] [PubMed] [Google Scholar]
- 31.Parikh SS, et al. Uracil-DNA glycosylase-DNA substrate and product structures: Conformational strain promotes catalytic efficiency by coupled stereoelectronic effects. Proc Natl Acad Sci USA. 2000;97:5083–5088. doi: 10.1073/pnas.97.10.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fromme JC, Banerjee A, Verdine GL. DNA glycosylase recognition and catalysis. Curr Opin Struct Biol. 2004;14:43–49. doi: 10.1016/j.sbi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Barrett TE, et al. Crystal structure of a thwarted mismatch glycosylase DNA repair complex. EMBO J. 1999;18:6599–6609. doi: 10.1093/emboj/18.23.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett TE, et al. Crystal structure of a G:T/U mismatch-specific DNA glycosylase: Mismatch recognition by complementary-strand interactions. Cell. 1998;92:117–129. doi: 10.1016/s0092-8674(00)80904-6. [DOI] [PubMed] [Google Scholar]
- 35.Hardeland U, Bentele M, Jiricny J, Schär P. Separating substrate recognition from base hydrolysis in human thymine DNA glycosylase by mutational analysis. J Biol Chem. 2000;275:33449–33456. doi: 10.1074/jbc.M005095200. [DOI] [PubMed] [Google Scholar]
- 36.Bianchet MA, et al. Electrostatic guidance of glycosyl cation migration along the reaction coordinate of uracil DNA glycosylase. Biochemistry. 2003;42:12455–12460. doi: 10.1021/bi035372+. [DOI] [PubMed] [Google Scholar]
- 37.Werner RM, Stivers JT. Kinetic isotope effect studies of the reaction catalyzed by uracil DNA glycosylase: Evidence for an oxocarbenium ion-uracil anion intermediate. Biochemistry. 2000;39:14054–14064. doi: 10.1021/bi0018178. [DOI] [PubMed] [Google Scholar]
- 38.McCann JAB, Berti PJ. Transition-state analysis of the DNA repair enzyme MutY. J Am Chem Soc. 2008;130:5789–5797. doi: 10.1021/ja711363s. [DOI] [PubMed] [Google Scholar]
- 39.Drohat AC, Stivers JT. NMR evidence for an unusually low N1 pKa for uracil bound to uracil DNA glycosylase: Implications for catalysis. J Am Chem Soc. 2000;122:1840–1841. [Google Scholar]
- 40.Dinner AR, Blackburn GM, Karplus M. Uracil-DNA glycosylase acts by substrate autocatalysis. Nature. 2001;413:752–755. doi: 10.1038/35099587. [DOI] [PubMed] [Google Scholar]
- 41.Kwon K, Jiang YL, Stivers JT. Rational engineering of a DNA glycosylase specific for an unnatural cytosine:pyrene base pair. Chem Biol. 2003;10:351–359. doi: 10.1016/s1074-5521(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 42.Wibley JE, Waters TR, Haushalter K, Verdine GL, Pearl LH. Structure and specificity of the vertebrate anti-mutator uracil-DNA glycosylase SMUG1. Mol Cell. 2003;11:1647–1659. doi: 10.1016/s1097-2765(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 43.Baba D, et al. Crystal structure of thymine DNA glycosylase conjugated to SUMO-1. Nature. 2005;435:979–982. doi: 10.1038/nature03634. [DOI] [PubMed] [Google Scholar]
- 44.Baba D, et al. Crystal structure of SUMO-3-modified thymine-DNA glycosylase. J Mol Biol. 2006;359:137–147. doi: 10.1016/j.jmb.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 45.Horton JR, et al. Caught in the act: Visualization of an intermediate in the DNA base-flipping pathway induced by HhaI methyltransferase. Nucleic Acids Res. 2004;32:3877–3886. doi: 10.1093/nar/gkh701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang N, Banavali NK, MacKerell AD., Jr Protein-facilitated base flipping in DNA by cytosine-5-methyltransferase. Proc Natl Acad Sci USA. 2003;100:68–73. doi: 10.1073/pnas.0135427100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Priyakumar UD, MacKerell AD., Jr Computational approaches for investigating base flipping in oligonucleotides. Chem Rev. 2006;106:489–505. doi: 10.1021/cr040475z. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee A, Yang W, Karplus M, Verdine GL. Structure of a repair enzyme interrogating undamaged DNA elucidates recognition of damaged DNA. Nature. 2005;434:612–618. doi: 10.1038/nature03458. [DOI] [PubMed] [Google Scholar]
- 49.Parker JB, et al. Enzymatic capture of an extrahelical thymine in the search for uracil in DNA. Nature. 2007;449:433–437. doi: 10.1038/nature06131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman JI, Stivers JT. Detection of damaged DNA bases by DNA glycosylase enzymes. Biochemistry. 2010;49:4957–4967. doi: 10.1021/bi100593a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Brien PJ, Ellenberger T. Dissecting the broad substrate specificity of human 3-methyladenine-DNA glycosylase. J Biol Chem. 2004;279:9750–9757. doi: 10.1074/jbc.M312232200. [DOI] [PubMed] [Google Scholar]
- 52.Connor EE, Wyatt MD. Active-site clashes prevent the human 3-methyladenine DNA glycosylase from improperly removing bases. Chem Biol. 2002;9:1033–1041. doi: 10.1016/s1074-5521(02)00215-6. [DOI] [PubMed] [Google Scholar]
- 53.Kavli B, et al. Excision of cytosine and thymine from DNA by mutants of human uracil-DNA glycosylase. EMBO J. 1996;15:3442–3447. [PMC free article] [PubMed] [Google Scholar]
- 54.Brooks BR, et al. CHARMM: The biomolecular simulation program. J Comput Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacKerell AD, Jr, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 57.Mackerell AD, Jr, Feig M, Brooks CL., 3rd Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem. 2004;25:1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- 58.MacKerell AD, Banavali NK. All-atom empirical force field for nucleic acids: II. Application to molecular dynamics simulations of DNA and RNA in solution. J Comput Chem. 2000;21:105–120. [Google Scholar]
- 59.Zhang L, et al. Thymine DNA glycosylase specifically recognizes 5-carboxylcytosine-modified DNA. Nat Chem Biol. 2012;8:328–330. doi: 10.1038/nchembio.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.