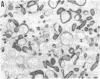Abstract
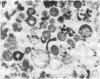
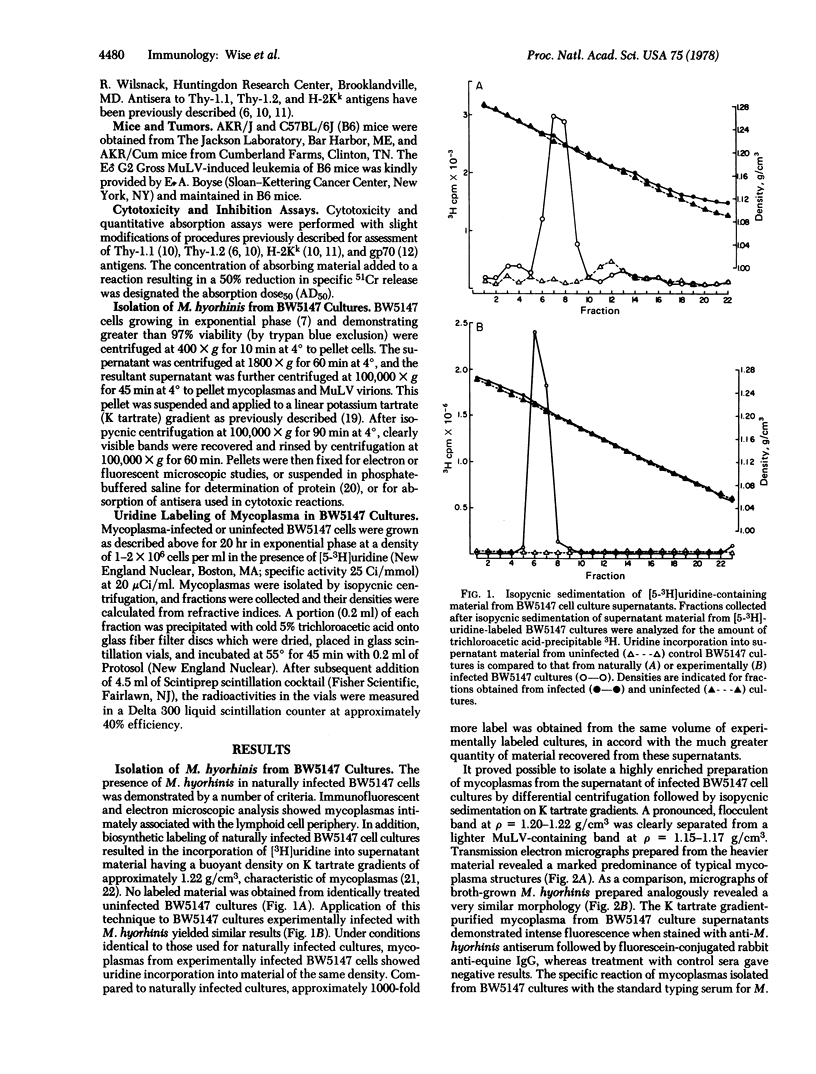
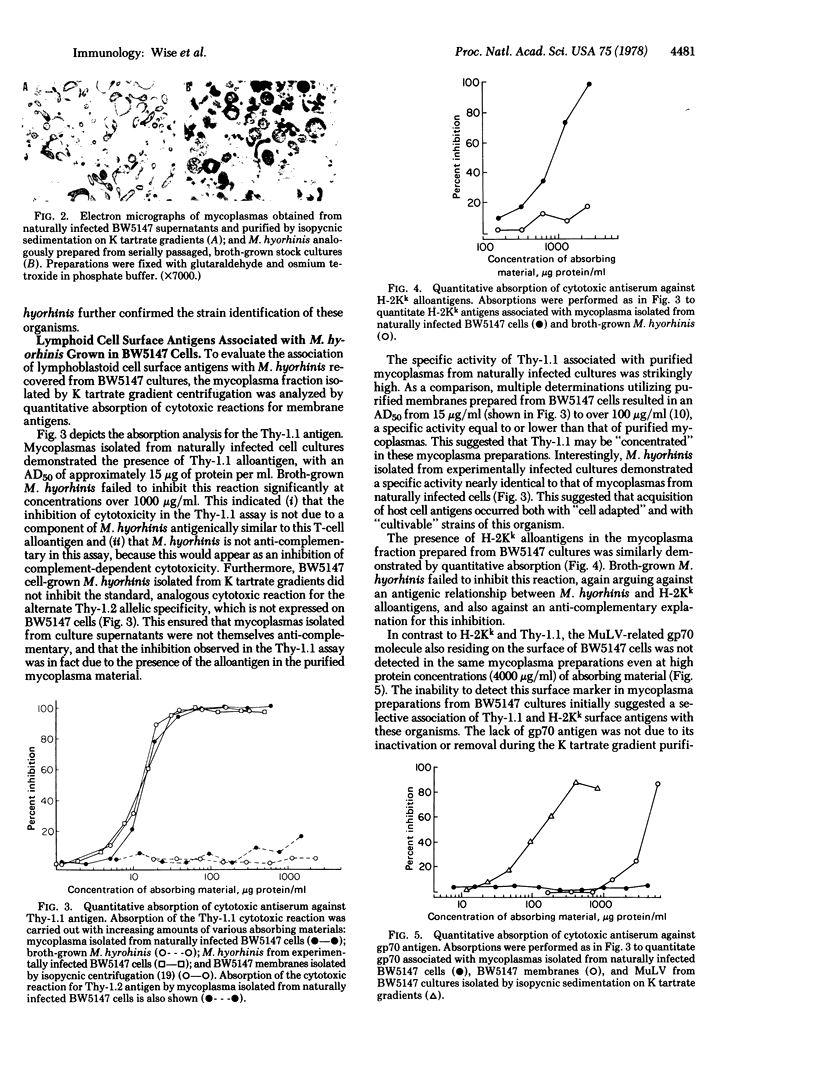
Mycoplasma hyorhinis, isolated by isopycnic centrifugation from supernatants of a persistently infected murine T lymphoblastoid cell line, demonstrated the presence of the Thy-1.1 differentiation alloantigen and H-2Kk histocompatibility antigens. The murine leukemia virus-related gp70 antigen also present on the surface of these lymphoblastoid cells was absent from mycoplasma preparations. Quantitative assessment of Thy-1.1 present in preparations of M. hyorhinis revealed a specific activity greater or equal to that of membrane preparations from lymphoblastoid cells, suggesting a marked accumulation of this T lymphoblastoid cells, suggesting a marked accumulation of this T lymphocyte antigen by membrane-associated mycoplasmas. The accumulation of the Thy-1.1 antigen in association with purified mycoplasmas was also demonstrated in lymphoblastoid cells experimentally infected with a defined culture of M. hyorhinis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Herberman R. B., Johnson P. A., Liu M., Sturm M. M. Wild-type gross leukemia virus: classification of soluble antigens (GSA). J Virol. 1972 Dec;10(6):1208–1219. doi: 10.1128/jvi.10.6.1208-1219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstad P. A., Henley S. L., Cox R. M., Lynn J. D., Acton R. T. Production of milligram quantities of H-2K and thy-1 alloantigens by large-scale mammalian cell culture. Proc Soc Exp Biol Med. 1977 Jul;155(3):296–300. doi: 10.3181/00379727-155-39793. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M., Ukena T. E., Yin H. H. Control of cell surface topography. Nature. 1974 Jan 4;247(5435):45–46. doi: 10.1038/247045a0. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Old L. J., Stockert E. An approach to the mapping of antigens on the cell surface. Proc Natl Acad Sci U S A. 1968 Jul;60(3):886–893. doi: 10.1073/pnas.60.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimuth W. W., Esselman W. J., Miller H. C. Release of thy-1.2 and thy-1.1 from lymphoblastoid cells: partial characterization and antigenicity of shed material. J Immunol. 1978 May;120(5):1651–1658. [PubMed] [Google Scholar]
- Freundt E. A. Present status of the medical importance of mycoplasmas. Pathol Microbiol (Basel) 1974;40(3):155–187. doi: 10.1159/000162521. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Henning R., Schrader J. W., Edelman G. M. Antiviral antibodies inhibit the lysis of tumour cells by anti-H--2 sera. Nature. 1976 Oct 21;263(5579):689–691. doi: 10.1038/263689a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masover G. K., Mischak R. P., Hayflick L. Some effects of growth medium composition on the antigenicity of a T-strain mycoplasma. Infect Immun. 1975 Mar;11(3):530–539. doi: 10.1128/iai.11.3.530-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Boyse E. A., Stockert E. The G (Gross) leukemia antigen. Cancer Res. 1965 Jul;25(6):813–819. [PubMed] [Google Scholar]
- Ross R. F., Dale S. E., Duncan J. R. Experimentally induced Mycoplasma hyorhinis arthritis of swine: immune response to 26th postinoculation week. Am J Vet Res. 1973 Mar;34(3):367–372. [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz H., Hunsmann G., Moenning V., Schäfer W. Properties of mouse leukemia viruses. XI. Immunoelectron microscopic studies on viral structural antigens on the cell surface. Virology. 1976 Jan;69(1):169–178. doi: 10.1016/0042-6822(76)90204-x. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Aaronson S. A., Rands E. Rapid detection of mycoplasma-infected cell cultures. Exp Cell Res. 1971 Mar;65(1):256–257. doi: 10.1016/s0014-4827(71)80077-0. [DOI] [PubMed] [Google Scholar]
- Yaguzhinskaya O. E. Detection of serum proteins in the electrophoretic patterns of total proteins of mycoplasma cells. J Hyg (Lond) 1976 Oct;77(2):189–198. doi: 10.1017/s002217240002461x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerner R. K., Acton R. T. Growth properties and alloantigenic expression of murine lymphoblastoid cell lines. J Exp Med. 1975 Aug 1;142(2):378–390. doi: 10.1084/jem.142.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerner R. K., Barstad P. A., Acton R. T. Isolation and characterizaiton of murine cell surface components. I. Purification of milligram quantities of Thy-1.1. J Exp Med. 1977 Oct 1;146(4):986–1000. doi: 10.1084/jem.146.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]